Development and validation of dissolution procedures
Bhavesh Vaghela, Rajan Kayastha, Nayana Bhatt, Nimish Pathak, Dashrath Rathod
Pages: 50-56

Quantitative analysis of valsartan in tablets formulations by High Performance Thin-Layer Chromatography
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 76-78

A validated stability indicating HPLC method for the determination of Valsartan in tablet dosage forms
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 97-99

High performance thin layer chromatographic method for estimation of deflazacort in tablet
Patel Satish A, Patel Natvarlal J.
Pages: 94-98

Development and validation of spectrophotometric method for simultaneous estimation of metoprolol succinate and olmesartan medoxomil in tablet
Vachhani Kevin H, Patel Satish A
Pages: 112-115

Development and validation of spectrofluorimetric method for estimation of deflazacort in tablets
Patel Satish A., Patel Natvarlal J.
Pages: 127-131

Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Domperidone and Naproxen in Tablet Dosage Form
Md. Shozan Mondal, Md. Ahsanul Haque, Mohammad Safiqul Islam, S.M. Ashraful Islam
Pages: 145-148

UPLC method development and validation for Cefditoren Pivoxil in active pharmaceutical Ingredient
Ram Garg, Navneet Singh, Kona S. Srinivas, Binayak Deb, Ayaz Ahmed
Pages: 149-153

Development and validation of selective UV spectrophotometric analytical method for budesonide pure sample
Shakuntala, Pushp Bharti, Neetu Sachan, Phool Chandra, Kavita GahloMallikarjuna Gouda M, Ramakrishna Shabaraya. A, Shantakumar S.M, Somashekar Shyale. S, Putta Rajesh kumar
Pages: 158-161

RP HPLC Method for the determination of Tamsulosin in bulk and Pharmaceutical Formulations
Manish Kumar Thimmaraju, Venkat Rao, Hemanth .K, P. Siddartha Kumar
Pages: 177-180

Comparative in vitro dissolution study of Aceclofenac Marketed Tablets in Two Different Dissolution Media by Validated Analytical Method
S.M. Ashraful Islam, Sharmi Islam, Mohammad Shahriar, Irin Dewan
Pages: 87-92

Analytical Method Development and Validation of Anti-HIV Drug Abacavir Sulphate
Pradeep Nagisetty, S.M.Shanta Kumar, Putta Rajesh Kumar
Pages: 85-89

Process validation of Amoxicillin and Clavulanic acid
Sandhya chaurasia, Hemendra Kumar Sharma, Nishi Prakash Jain, Anshuli Sharma
DOI: 10.7324/JAPS.2012.2412Pages: 82-86

Q-Absorbance Ratio Spectrophotometric Method for the Simultaneous Estimation of Rifampicin and Piperine in their Combined Capsule Dosage
Jenil C. Khamar, Satish A. Patel
DOI: 10.7324/JAPS.2012.2416Pages: 137-141

Development and validation of a rapid liquid chromatographic method for the analysis of Ketorolac Tromethamine and its related production impurities
O' Connor N., Geary M., Wharton M., Curtin L
DOI: 10.7324/JAPS.2012.2524Pages: 15-21

Simultaneous Estimation of Finasteride andTamsulosin Hydrochloride in Combined DosageForms by RP-HPLC-PDA Method
M. Sindhura, K. Raghavi, R. Prashanthi, Buchi N. Nalluri
DOI: 10.7324/JAPS.2012.2626Pages: 203-209

Simultaneous Estimation of Ramipril and Amlodipine in Bulk and tablet Dosage form by RP-HPLC Method
Praveen S. Rajput, Amanjot Kaur, Navdeep Kaur Gill, Karan Mittal, Ganti Subrahmanya Sarma
DOI: 10.7324/JAPS.2012.2724Pages: 160-165

Development and Validation of RP-HPLC Method for Simultaneous Estimation of Enalapril Maleate and Amlodipine Besylate in Combined Dosage form
Bharat G. Chaudhari
DOI: 10.7324/JAPS.2012.2911Pages: 054-057

Derivative Spectrophotometric Methods for the Determination of Zolpidem Tartrate in Tablets
M. Mathrusri Annapurna, B. Swathi, M. Siri Chandra and K. Tulasi
DOI: 10.7324/JAPS.2012.21117Pages: 096-099

Stability Indicating HPLC Method for the Determination of Hydrochlorothiazide in Pharmaceutical Dosage form
Sachin Bhagwate and N. J. Gaikwad
DOI: 10.7324/JAPS.2013.30215Pages: 088-092

Selective High Performance Liquid Chromatographic Determination of Amodiaquine and Artesunate in bulk and pharmaceutical formulation
P. S. Jain, A. J. Chaudhari and S. J. Surana
DOI: 10.7324/JAPS.2013.30313Pages: 066-070

A RP-HPLC Method Development and Validation for the Estimation of Gliclazide in bulk and Pharmaceutical Dosage Forms
B.V.V Ravi kumar, A.K. Patnaik, Saroj Kumar Raul, Nagireddy Neelakanta Rao
DOI: 10.7324/JAPS.2013.3410Pages: 059-062

RP-HPLC Method Development and Validation of Gallic acid in Polyherbal Tablet Formulation
Kamal Kardani, Nilesh Gurav, Bhavna Solanki, Prateek Patel, Bhavna Patel
DOI: 10.7324/JAPS.2013.3508Pages: 037-042

Validation of a Stability-Indicating Assay of Amprolium Hydrochloride in Water Soluble Powder Formulation using Hydrophilic Interaction Liquid Chromatography
Mashhour Ghanem and Saleh Abu-Lafi
DOI: 10.7324/JAPS.2013.31009Pages: 051-058

Simple Spectrophotometric Method for Estimation of Raltegravir Potassium in Bulk and Pharmaceutical Formulations
Girija B. Bhavar, Sanjay S. Pekamwar, Kiran B. Aher, Sanjay R. Chaudhari
DOI: 10.7324/JAPS.2013.31026Pages: 147-150

Development and Validation of a Stability Indicating Spectrofluorimetric Method for the Determination of Lanzoprazole via its Degradation Product
Ghaleb Oriquat, Afaf Osman, Mohammad Abdul-Azim and Sawsan Abuhamdah
DOI: 10.7324/JAPS.2014.40410Pages: 057-061

Fast and simple multi-element determination of essential and toxic metals in whole blood with quadrupole ICP-MS
Carmen Silvia Kira, Alice Momoyo Sakuma, Nelson da Cruz Gouveia
DOI: 10.7324/JAPS.2014.40507Pages: 039-045

Simultaneous estimation of Cefpodoxime proxetil and Ofloxacin In tablet dosage form using RP-HPLC
Annadi Chiranjeevi and Medidi Srinivas
DOI: 10.7324/JAPS.2014.40508Pages: 046-050

A rapid and simple high performance thin layer chromatographic method for simultaneous analysis of β-sitosterol-D-glucoside, gallic acid, withaferin A and withanolide A in Ashvagandharishta
Prashant D Bhondave, Sachin E Potawale, Satish Y Gabhe, Abhay M Harsulkar
DOI: 10.7324/JAPS.2014.40714Pages: 082-087

A comparative estimation of quercetin content from Cuscuta reflexa Roxb.using validated HPTLC and HPLC techniques
Sunita Shailajan, Harshvardhan Joshi, Bhavesh Tiwari
DOI: 10.7324/JAPS.2014.40721Pages: 123-128

In silico designing of siRNA targeting PB 1 gene of Influenza A virus and in vitro validation
Bhawana Jain, Amita Jain, Om Prakash, Ajay Kr Singh, Tanushree Dangi, Mastan Singh, Kaleshwar P Singh
DOI: 10.7324/JAPS.2014.40808Pages: 042-047

An approach for validated RP-HPLC method for the analysis of paclitaxel in rat plasma
Nandhakumar Sathyamoorthy, Vijayalakshmi Rajendran, Naveena V.S.H, Magharla Dasaratha Dhanaraju
DOI: 10.7324/JAPS.2014.40913Pages: 073-076

Chromatographic Separation and in Vitro Dissolution Assessment of Tenofovir disoproxil fumarate, Emtricitabine and Nevirapine in a Fixed Dose Combination of Antiretrovirals
Kalpana Jayapalu, Himaja Malipeddi, Anbarasu Chinnasamy
DOI: 10.7324/JAPS.2014.41113Pages: 076-080

RP-HPLC-PDA method development and validation for the analysis of Tadalafil in bulk, pharmaceutical dosage forms and in-vitro dissolution samples
Aziz Unnisa, Yogesh Babu, Santosh kumar suggu, Siva Chaitanya
DOI: 10.7324/JAPS.2014.41213Pages: 072-076

Validated UV-Visible Spectrophotometric method for simultaneous estimation of Cefixime and Moxifloxacin in Pharmaceutical Dosage Form
S S Pekamwar, T M Kalyankar, B V Tambe, S J Wadher
DOI: 10.7324/JAPS.2015.50107Pages: 037-041

RP-HPLC Method for the Simultaneous Estimation of Ambroxol Hydrochloride and Fexofenadine Hydrochloride In bulk and in a Tablet Mixture
Prathyusha Buchupalli, Srinivas Medidi
DOI: 10.7324/JAPS.2015.50211Pages: 074-080

Stability Indicating RP-HPLC Assay Method for Estimation of Dronedarone Hydrochloride in Tablet
Atul T Hemke, Shivshanker Kukade, Rajesh T Lohiya, Krishna R Gupta
DOI: 10.7324/JAPS.2015.50516Pages: 083-088

A Simplified Liquid Chromatography-Mass Spectrometry Method for Simultaneous Determination of Pyrimethamine, Sulphadoxine and Artesunate in Human Plasma
S M Sandhya, P S Shijikumar
DOI: 10.7324/JAPS.2015.50618Pages: 109-114

Method Development and Validation using UV Spectrophotometry for Nigella sativa Oil Microparticles Quantification
Ahmad Fahmi Harun Ismail, Abd Almonem Doolaanea, Farahidah Mohamed, NurIzzati Mansor, Mohd Affendi Mohd Shafri, Fathin Athirah Yusof
DOI: 10.7324/JAPS.2015.50915Pages: 082-088

A simple spectrophotometric quantitative determination of Cilostazol in bulk and pharmaceutical dosage forms using DNPH reagent
Siddappa Anjanappa Kuruba, Prashant Chandrakant Hanamshetty
DOI: 10.7324/JAPS.2015.501220Pages: 117-121

Quinine-Loaded Polymeric Nanoparticles: Validation of a simple HPLC-PDA Method to Determine Drug Entrapment and Evaluation of its Photostability
Luana Roberta Michels, Lisiane Bajerski, Tamara Ramos Maciel, Letícia Marques Colomé, Sandra Elisa Haas
DOI: 10.7324/JAPS.2016.60202Pages: 009-015

Simultaneous estimation of Esomeprazole and Tadalafil in pharmaceutical formulations using High Performance Liquid Chromatography
Mohammed Hamad, Ahmed Al-Sharqawi, Wael Abu Dayyih, Eyad Mallah, Tawfiq Arafat
DOI: 10.7324/JAPS.2016.60407Pages: 052-059

Development and Validation of a Stability-Indicating High Performance Thin Layer Chromatography (HPTLC) Method for estimation of Canagliflozin in bulk and Pharmaceutical Dosage Form
Ishpreet Kaur, Sharad Wakode, Harsharan Pal Singh
DOI: 10.7324/JAPS.2016.60508Pages: 051-057

Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Sumatriptan in Bulk and Pharmaceutical Dosage Form
M. Srinidhi, Md. Mushabbar Basha, V. Raj Kumar, J. Rajendra Kumar
DOI: 10.7324/JAPS.2016.60604Pages: 020-025

A Simple and Sensitive HPLC/UV Method for Determination of Meloxicam in Human Plasma for Bioavailability and Bioequivalence Studies
Laila H. Emara, Maha F. Emam, Nesrin F. Taha, Hala M. Raslan, Ahmed A. El-Ashmawy
DOI: 10.7324/JAPS.2016.60702Pages: 012-019

Development of Validated Stability Indicating RP-HPLC-PDA Method for Camptothecin Analysis
Buchi N. Nalluri, Saisrianusha Valluru, Chandrapriyanka Bonthu
DOI: 10.7324/JAPS.2016.60921Pages: 140-146

Development and validation of a new RP-HPLC method for the estimation of dutasteride in bulk and pharmaceutical formulations
Poonguzhali Subramanian, P. S. Rajinikanth
DOI: 10.7324/JAPS.2016.601207Pages: 047-055

Development and Validation of Content Uniformity Analytical Procedure of Glipizide Extended Release Tablet
Ilma Nugrahani, Indhah Fatmawati, Slamet Ibrahim
DOI: 10.7324/JAPS.2016.601228Pages: 192-196

Application of modern RP-HPLC technique for the quantitation of betulinic acid from traditional drug Symplocos racemosa Roxb.
Sunita Shailajan, Sasikumar Menon, Dipti Singh, Gauri Swar, Suhina Bhosale
DOI: 10.7324/JAPS.2017.70321Pages: 129-134

Development and validation of colorimetric method for the quantitative analysis of kanamycin in bulk and pharmaceutical formulation
Malik A. Hussien, Mohamed E. Adam, Shaza W. Shantier, Elrasheid A.E. Garalnabi and Elrasheed A. Gadkariem
DOI: 10.7324/JAPS.2017.70424Pages: 163-167

Computational, structural and functional aspects of hypothetical protein of Aspergillus flavus Pheromone Receptor Pre-A (PRP-A)
Maneesh Kumar, Sindhuprava Rana, Harish Kumar, Pratik Kumar, Manas Ranjan Dikhit, Rani Mansuri, Jainendra Kumar, Ganesh Chandra Sahoo
DOI: 10.7324/JAPS.2017.70714Pages: 089-097

Evaluation of Cardiospermum halicacabum leaf compounds against human DihydroOrotate Dehydrogenase: a target for Rheumatoid Arthritis using Structure based Drug Designing
Priya Swaminathan, Lilly Saleena
DOI: 10.7324/JAPS.2017.70808Pages: 048-061

Development and Validation of UV-Spectroscopic Method for Estimation of Niacin in Bulk and Pharmaceutical Dosage Form
Indranil Chanda, Ripunjoy Bordoloi, Debarupa D. Chakraborty, Prithviraj Chakraborty, Smriti Rekha Chanda Das
DOI: 10.7324/JAPS.2017.70911Pages: 081-084

A new rapid Stability indicating RP-PDA-UPLC method for the estimation of Assay of Pemetrexed disodium-An anti-Lung cancer drug from lyophilized parenteral formulation
Vamsi Krishna Galla, V. Archana, Rajeswari Jinka
DOI: 10.7324/JAPS.2017.71019Pages: 131-137

Development and validation of high-performance liquid chromatography method for simultaneous determination of acyclovir and curcumin in polymeric microparticles
Jéssica Brandão Reolon, Maicon Brustolin, Sandra Elisa Haas, Eduardo André Bender, Marcelo Donadel Malesuik, Letícia Marques Colomé
DOI: 10.7324/JAPS.2018.8120Pages: 136-141

Validation and quantitative analysis of cadmium, chromium, copper, nickel, and lead in snake fruit by Inductively Coupled Plasma-Atomic Emission Spectroscopy
M. Tan, Sudjadi, Astuti, Rohman
DOI: 10.7324/JAPS.2018.8206Pages: 044-048

Sensitive Analytical Liquid Chromatography-Tandem Mass Spectroscopy Method for the Estimation of Dexlansoprazole in Pharmaceutical Formulations
Rinchi Bora, S.T. Narenderan, B. Babu, S.N. Meyyanathan, Abel Jacob George, M. Kalaivani
DOI: 10.7324/JAPS.2018.8706Pages: 033-036

Screening of two glucocorticoids in non-prescription skin whitening creams purchased via internet in Iraq by HPLC method
Mohanad Naji Sahib
DOI: 10.7324/JAPS.2018.8713Pages: 078-084

Validation of a simple isocratic HPLC-UV method for rifampicin and isoniazid quantification in human plasma
Laura Carolina Luciani-Giacobbe, María Laura Guzman, Rubén Hilario Manzo, María Eugenia Olivera
DOI: 10.7324/JAPS.2018.8715Pages: 093-099

Separation, Quantification and Control of Enatiomers of the Key Starting Material of Dextromethorphan Hydrobromide
Ajit Anerao, Vishal Solase, Amol More, Nitin Pradhan
DOI: 10.7324/JAPS.2018.8805Pages: 032-038

Development and Validation Method for Simultaneous Analysis of Retinoic Acid, Hydroquinone and Corticosteroid in Cream Formula by High-Performance Liquid Chromatography
Elvi Rahmayuni, Harmita Harmita, Herman Suryadi
DOI: 10.7324/JAPS.2018.8913Pages: 087-092

A new quantitative reverse phase high-performance liquid chromatographic method for the quantification of Rilpivirine hydrochloride in bulk and dosage form
Sonam Patel, Krishnaveni Nagappan, Gouru Santhosh Reddy
DOI: 10.7324/JAPS.2018.81122Pages: 157-162

Efficient validated method of UPLC-MS/MS to determine curcumin in rat plasma and ovarium
Wenny Trias Ramadanty, Wawaimuli Arozal, Melva Louisa, Vivian Soetikno, Sigit Purbadi, Priyanto Priyanto
DOI: 10.7324/JAPS.2019.90109Pages: 058-065

RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation
Suhair S. Al-Nimry, Bashar M. Altaani, Razan H. Haddad
DOI: 10.7324/JAPS.2019.90211Pages: 079-086
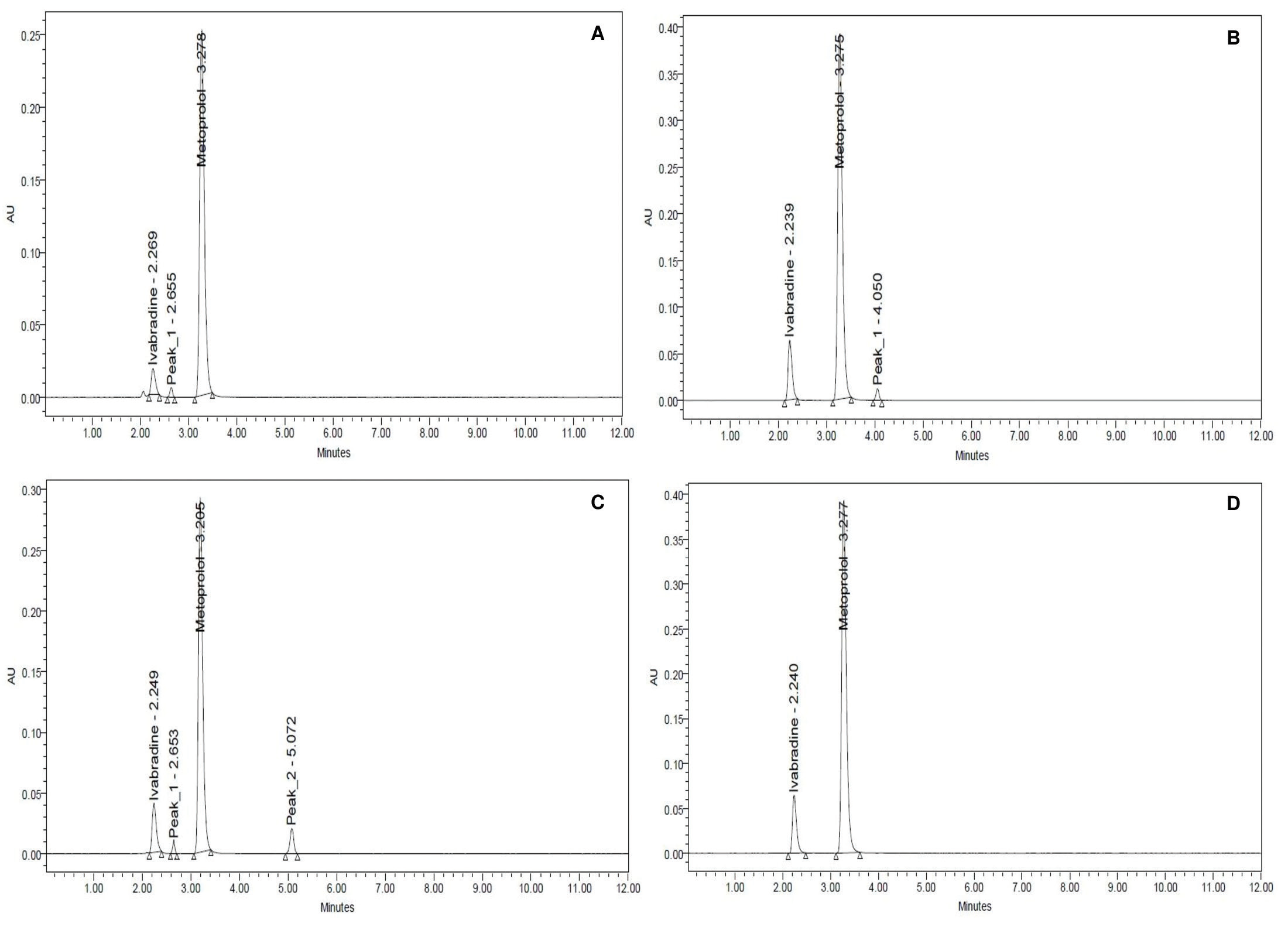
Stability indicating RP-HPLC method for the simultaneous estimation of ivabradine and metoprolol in bulk and tablet formulation
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90418Pages: 137-144
A novel analytical liquid chromatography-tandem mass spectrometry method for the estimation of Gemfibrozil in bulk and pharmaceutical formulations
S. Vishnupriya, S. T. Narenderan, K. Vishnu, B. Babu, S. N. Meyyanathan
DOI: 10.7324/JAPS.2019.90512Pages: 097-101

Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: Analytical quality by design approach
Dilipkumar Suryawanshi, Durgesh Kumar Jha, Umesh Shinde, Purnima D. Amin
DOI: 10.7324/JAPS.2019.90604Pages: 021-032

Development of validated stability indicating RP-HPLC method for the estimation of glecaprevir and pibrentasvir in bulk and pharmaceutical dosage form
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90607Pages: 052-060

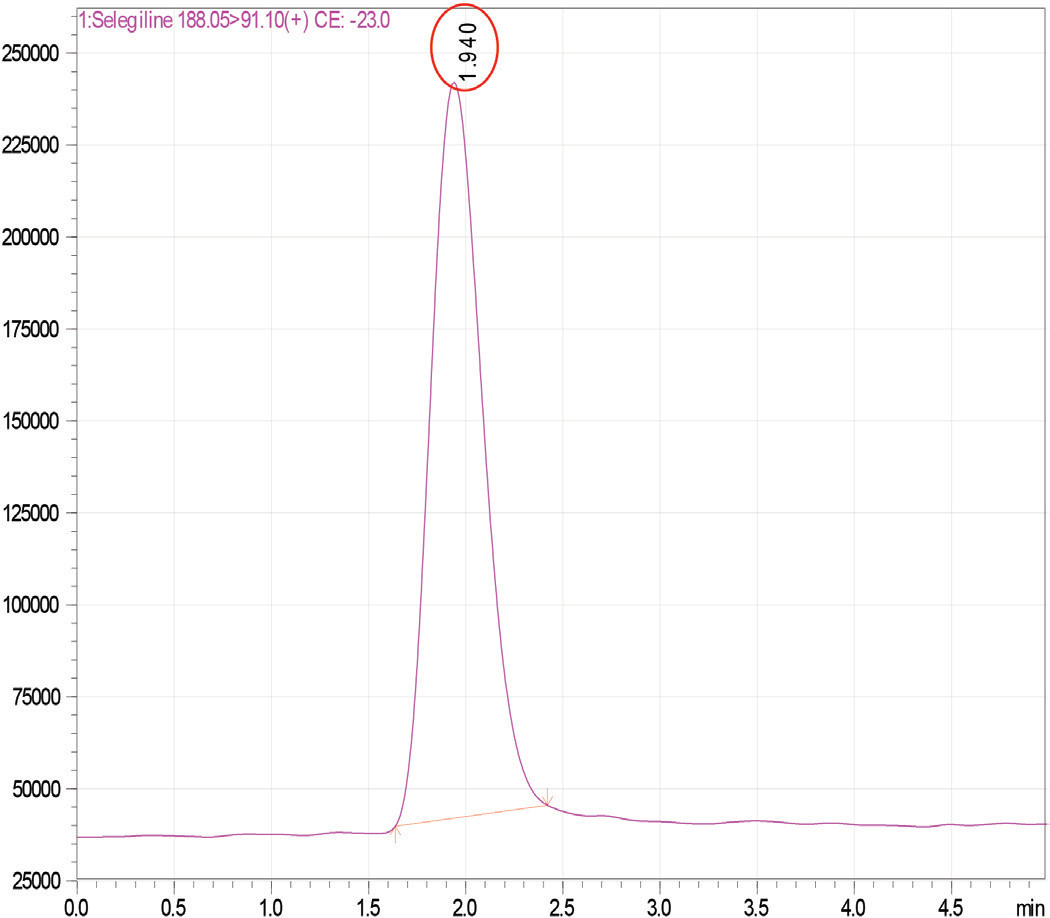
A validated LC-ESI-MS/MS method for the quantification of Selegiline HCl in bulk and pharmaceutical formulation
R. Sangamithra, Krishna Veni Nagappan, Sailaja Mukkamala, Anoop Karthika, S. T. Narenderan, Kowmudi Gullapalli
DOI: 10.7324/JAPS.2019.90715Pages: 106-110
Development and validation of stability indicating LC-MS/MS Technique for the quantification of tapentadol in biological matrices: Application to bioavailability study in healthy rabbits
Phanindra Adluri, Yamsani Shravan Kumar
DOI: 10.7324/JAPS.2019.91009Pages: 068-074

Validation of methodology for assay, pharmaceutical equivalence, and comparative dissolution profile for tablets containing amlodipine besylate
Renata Micheli Martinez, Jenifer Freitas da Silva, Larissa Regina Jorge, Rhye Lessa Ishikawa, Ana Paula Novelli, Talita Laiane Cardoso Cezar, Sandra Regina Georgetti, Marcela Maria Baracat, Rúbia Casagrande
DOI: 10.7324/JAPS.2019.91112Pages: 093-100

A novel analytical liquid chromatography–tandem mass spectrometry method for the estimation of Ribavirin in bulk and pharmaceutical formulation
Prachi Sharma, Narenderan S. T, Meyyanathan S. N, Sangamithra R, Mohire Sourabh Sanjay, Babu B, Kalaivani M
DOI: 10.7324/JAPS.2020.101013Pages: 096-100

Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107
Comparison of UV-spectrophotometric and RP-HPLC methods for estimation of deflazacort in solid dosage form
Manisha Puranik, Samta Shambharkar, Shantanu Nimbalkar, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2020.10711Pages: 082-088

Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138

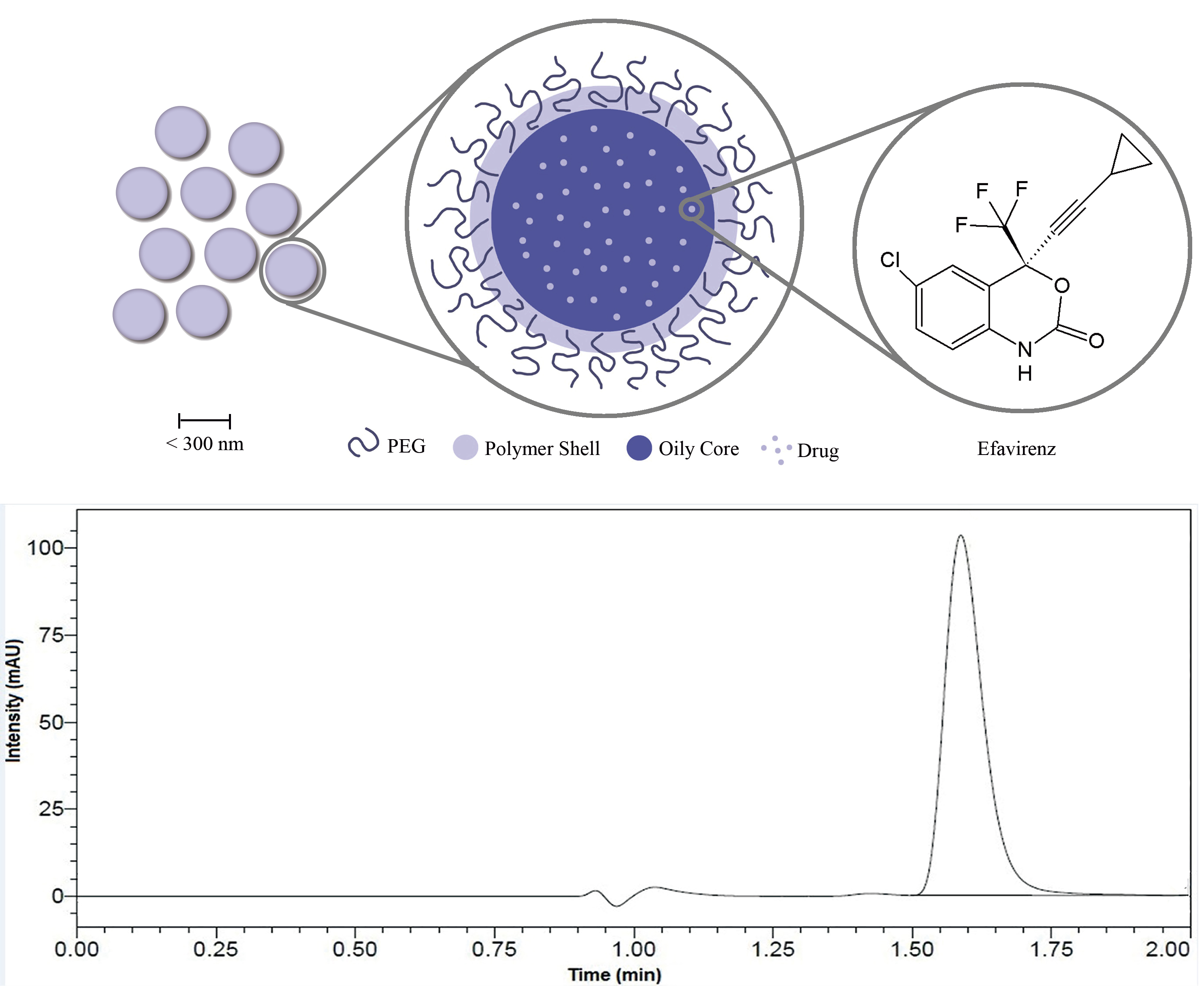
Efavirenz-loaded polymeric nanocapsules: Formulation, development, and validation of an RP-UHPLC-DAD method for drug quantification, determination of encapsulation efficiency, stability study, and dissolution profile
Amanda Martinez Lyra, Juliana Parente Menezes Ribeiro, Jessica Mendes Nadal, Sinvaldo Baglie, Traudi Klein, Andressa Novatski, Paulo Vitor Farago
DOI: 10.7324/JAPS.2021.110212Pages: 093-101
Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053

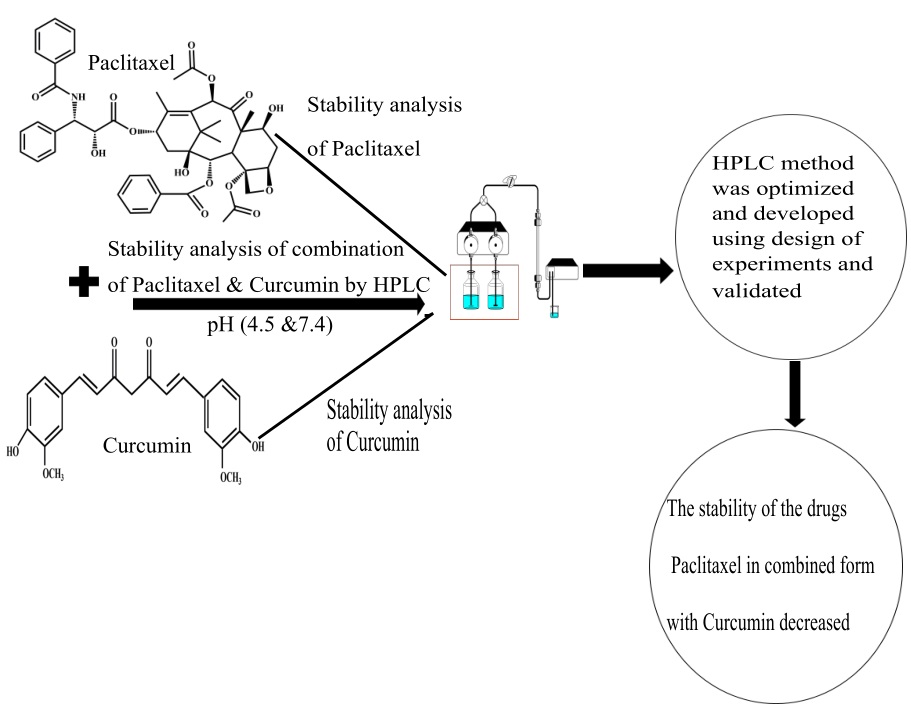
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
A green analytical method for the determination of glucosamine using FTIR spectrophotometry
Lodoe Choezom, Ravandur Shivanna Chandan, Gurupadayya Bannimath
DOI: 10.7324/JAPS.2021.110615Pages: 125-131

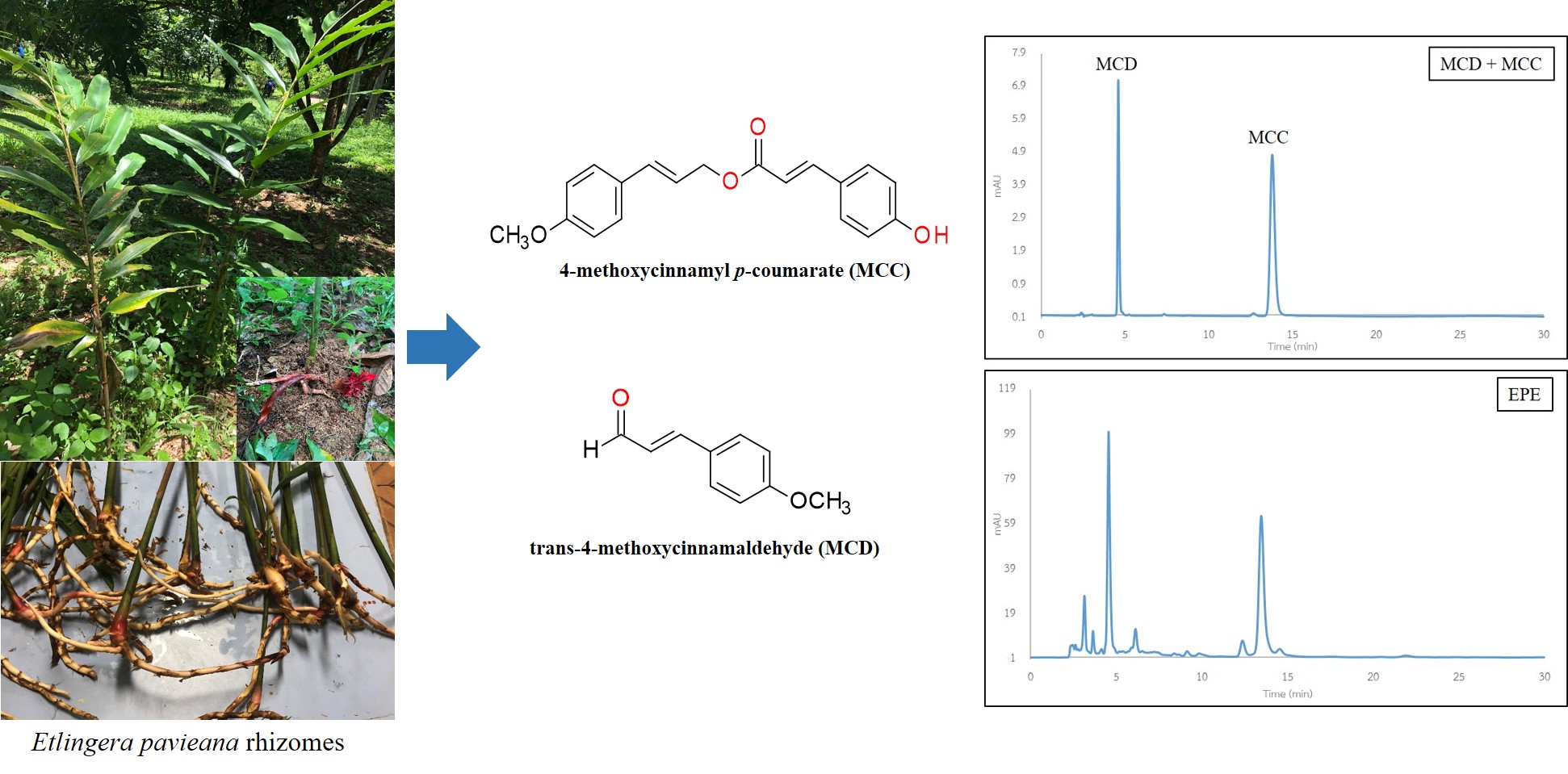
Validation of quantitative RP-HPLC-DAD method and extraction optimization of 4-methoxycinnamyl p-coumarate and trans-4- methoxycinnamaldehyde in Etlingera pavieana rhizomes
Klaokwan Srisook, Chartchai Malapong, Petchrat Sawai, Ekaruth Srisook
DOI: 10.7324/JAPS.2021.1101005Pages: 029-034
New validated Reverse Phase Ultra Performance Liquid Chromatography method for drospirenone and estetrol in Active Pharmaceutical Ingredient and tablet form and its stress studies
Rafi Syed, Rambabu Kantipudi
DOI: 10.7324/JAPS.2021.1101015Pages: 106-112
Development and validation of A-SOAP notes: Assessment of efficiency in documenting patient therapeutic records
Pooja Sudarsan, Aishwarya Gowda Murulya Balakrishna, Jerlin Anusha Rajasingh Asir, Deepan Balu, Sadagoban Gopal Krishnamoorthy, Swathi Swaroopa Borra
DOI: 10.7324/JAPS.2021.1101001Pages: 001-006

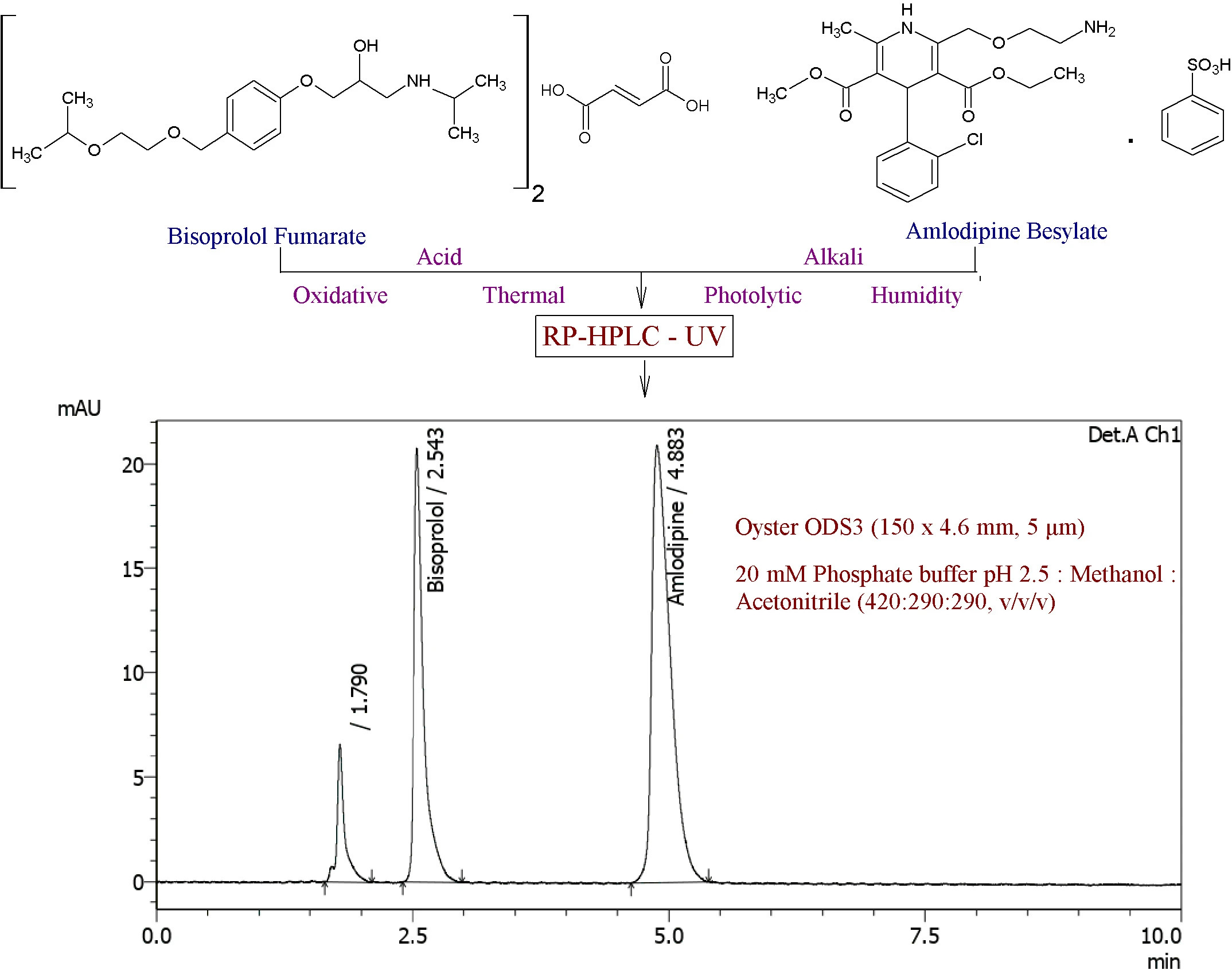
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form
Rameshwar Bhausaheb Gholve, Sanjay Sudhakar Pekamwar, Tukaram Mohanrao Kalyankar
DOI: 10.7324/JAPS.2021.1101211Pages: 121–134
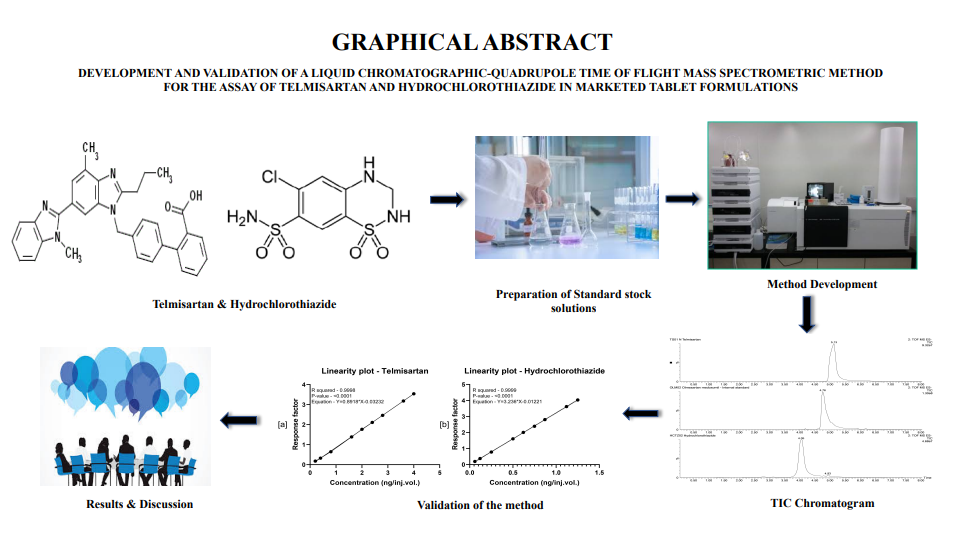
Development and validation of a liquid chromatographic-quadrupole time of flight mass spectrometric method for the assay of telmisartan and hydrochlorothiazide in marketed tablet formulations
Vignesh Madhavan, Kowmudi Gullapalli, Anoop Karthika, Ramalingam Peraman, Krishnaveni Nagappan
DOI: 10.7324/JAPS.2021.120207Pages: 066-074
Development and validation of a stability indicating related substances of Opicapone by reverse phase high performance liquid chromatography and its degradation
Ramachandran Dittakavi, Neeharika Tirumalasetty
DOI: 10.7324/JAPS.2021.120219Pages: 179-186

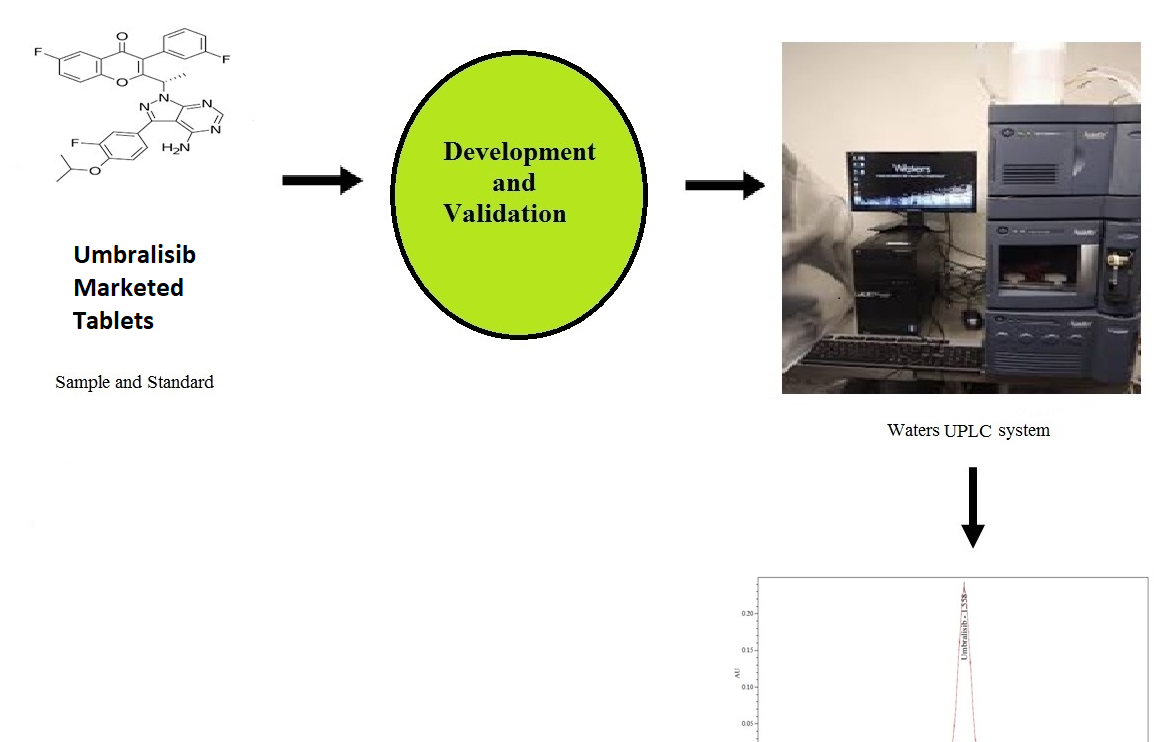
Determination of Umbralisib using reverse phase ultra performance liquid chromatography in bulk and pharmaceutical dosage form
Ramadevi Potturi, Rambabu Kantipudi
DOI: 10.7324/JAPS.2021.120218Pages: 172-178
Determination of dioecy in Hippophae salicifolia by evaluating gallic acid content through a validated HPTLC method
Ishita A. Basera, Preeti D. Verma, Vijay P. Bhatt, Mamta B. Shah
DOI: 10.7324/JAPS.2022.120308Pages: 082–086

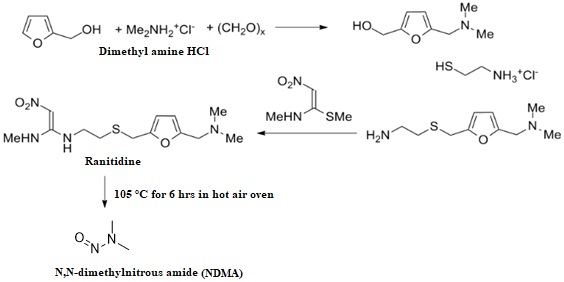
Novel stability indicating LC-MS method for N-Nitroso dimethyl amine genotoxic impurity quantification in ranitidine drug substance and drug product
Ganpisetti Srinivasa Rao, Dharamasoth Ramadevi, B. M. Rao, Nagaraju Rajana, K. Basavaiah
DOI: 10.7324/JAPS.2022.120711Pages: 106-114
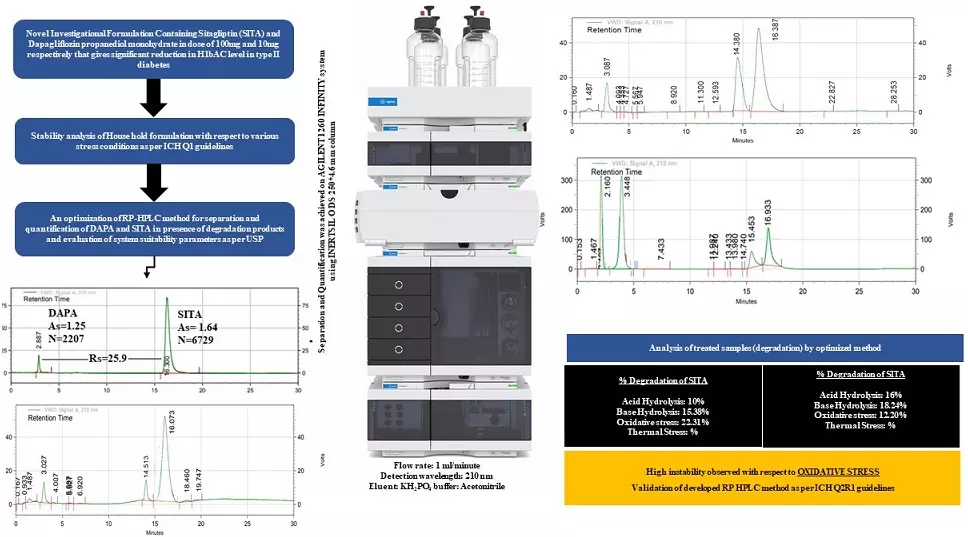
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155
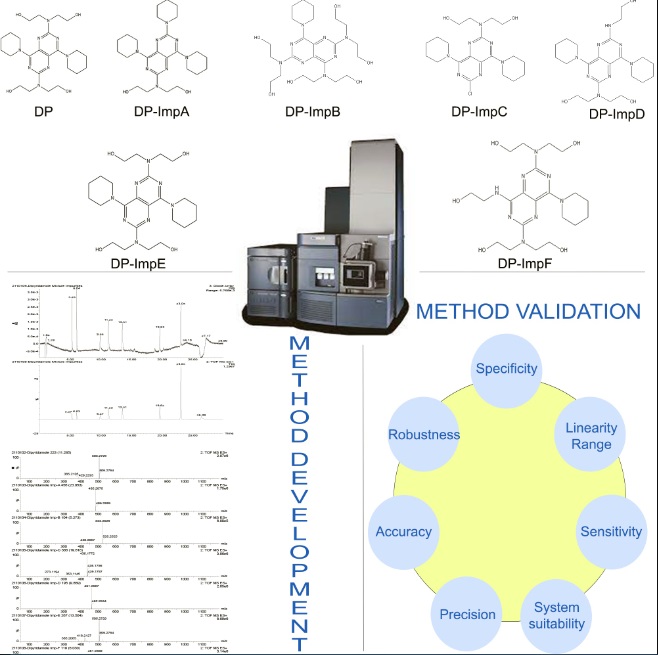
UPLC-Q-TOF-MS method development and validation for simultaneous analysis of dipyridamole and its related impurities
T. Menaka, Ramya Kuber
DOI: 10.7324/JAPS.2023.130120Pages: 201-211
Simultaneous determination of Brivaracetam and its isomers in the Brivaracetam drug by RP-HPLC
Palaniappan Ilayaraja, Murugan Maniavannan, Paramasivam Parthiban
DOI: 10.7324/JAPS.2022.120915Pages: 127-138

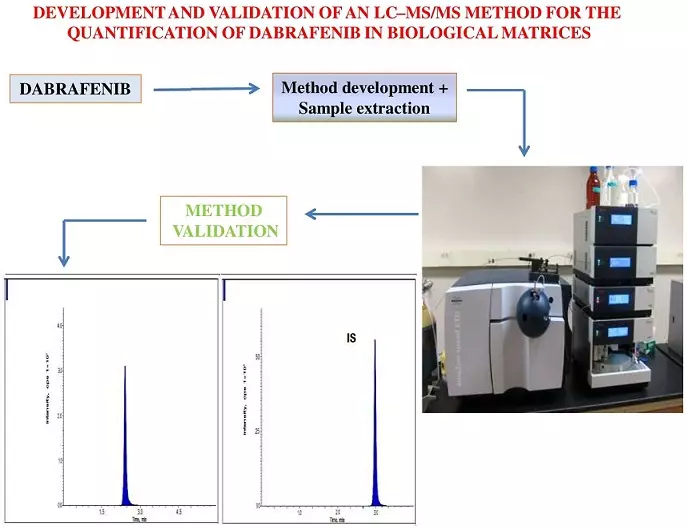
An LC–MS/MS quantification method development and validation for the dabrafenib in biological matrices
Gella Sai Uday Kiran, Sandhya Pasikanti, Shankar Cheruku, DVRN Bhikshapathi, Mamatha Palanati
DOI: 10.7324/JAPS.2023.130117Pages: 180-186
Simultaneous determination of chloroquine and colchicine co-nanoencapsulated by HPLC-DAD
Tamara Ramos Maciel, Camila de Oliveira Pacheco, Pietra Fonseca Ramos, Ana Claudia Funguetto Ribeiro, Renata Bem dos Santos, Sandra Elisa Haas
DOI: 10.7324/JAPS.2023.130212Pages: 106-112

Development and validation of LC-MS/MS method for alpelisib quantification in human plasma: Application to pharmacokinetics in healthy rabbits
Tandrima Majumder, Shiva Kumar Gubbiyappa
DOI: 10.7324/JAPS.2023.75269Pages: 089-096

A green chemistry approach to establish a conductometric technique for quantifying Metformin HCl in pharmaceutical samples and its greenness assessment using an analytical greenness metric calculator
Shailendra S. Suryawanshi, Mahesh S. Palled
DOI: 10.7324/JAPS.2023.145086Pages: 238-244

A stability-indicating reverse phase-HPLC method development and validation for newly approved drug, Belzutifan in bulk and pharmaceutical dosage forms
Dumpala Sravya, Banoth Ramya Kuber
DOI: 10.7324/JAPS.2023.114351Pages: 233-240
A novel chiral HPLC and LC-MS/MS method development for the triazole antifungal compound
R. Sangamithra, S. N. Meyyanathan, B. Babu
DOI: 10.7324/JAPS.2023.118532Pages: 001-008
_.jpg)
Validation steps and parameters of bioanalytical methods using in clinical studies: A narrative review
Mohammed Abdessadek, Mourad Kharbach, Hayat Ben-Saghroune, Belkassem El Amraoui, Youssef Khabbal
DOI: 10.7324/JAPS.2023.109956Pages: 068-083
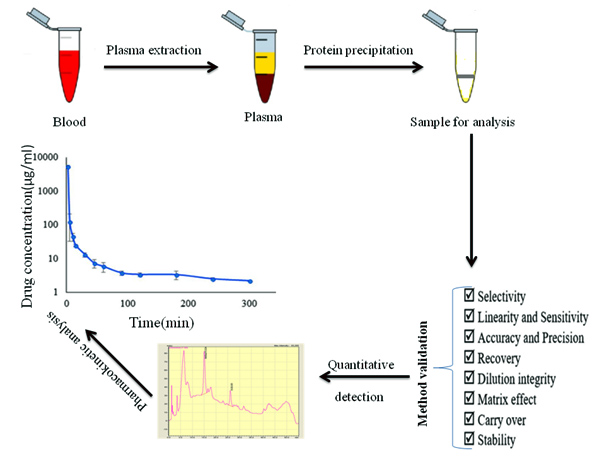
Development of volumetric absorptive microsampling for analysis phenytoin levels and its application to monitoring therapy in epilepsy patients
Rizka Mardhiani, Yahdiana Harahap, Winnugroho Wiratman, Fitri Octaviana, Irwandi Jaswir
DOI: 10.7324/JAPS.2023.125948Pages: 075-081

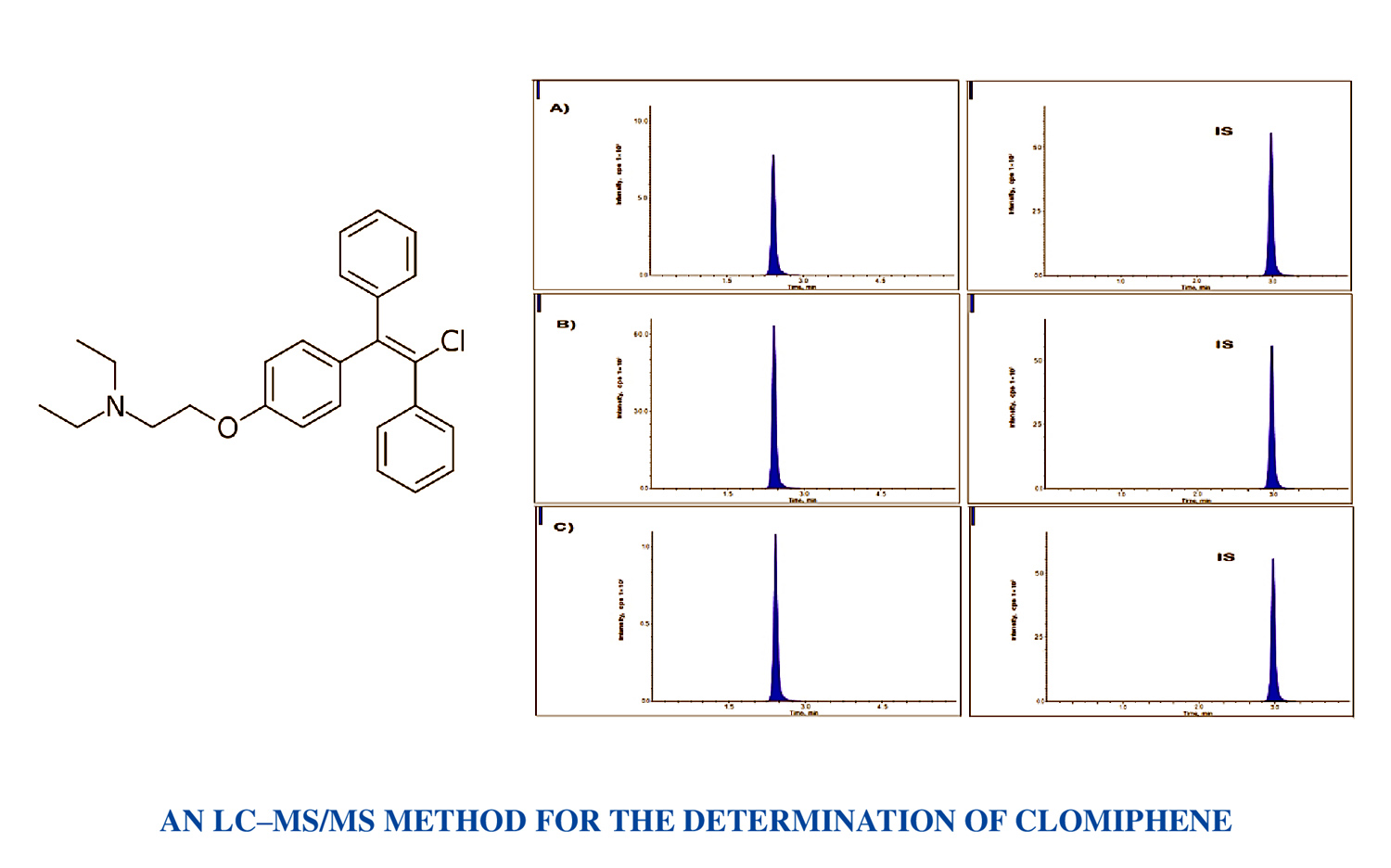
An LC–MS/MS method development and validation for the determination of clomiphene in biological matrices
Vinayaga Sundaram Krishnan, Bhikshapathi Darna
DOI: 10.7324/JAPS.2023.144778Pages: 184-189
Bioanalytical and validation high-performance liquid chromatography method for simultaneous quantification cefotaxime and ciprofloxacin in human plasma
Luh Putu Mirah Kusuma Dewi, Djoko Wahyono, Ika Puspitasari, Rizka Humardewayanti, Endang Lukitaningsih
DOI: 10.7324/JAPS.2024.153492Pages: 221-229
Measuring dopamine in rodent brain tissue by using UHPLC-Xevo-TQD triple quadrupole mass spectrometry: A comparative influence of selegiline nanoemulsion administered intranasally over solution on upregulation of dopamine level
Shobhit Kumar, Karan Wadhwa, Rakesh Pahwa, Sanjula Baboota, Javed Ali, Rehan Abdur Rub, Govind Singh, Ozair Alam
DOI: 10.7324/JAPS.2024.134987Pages: 232-238
_.jpg)
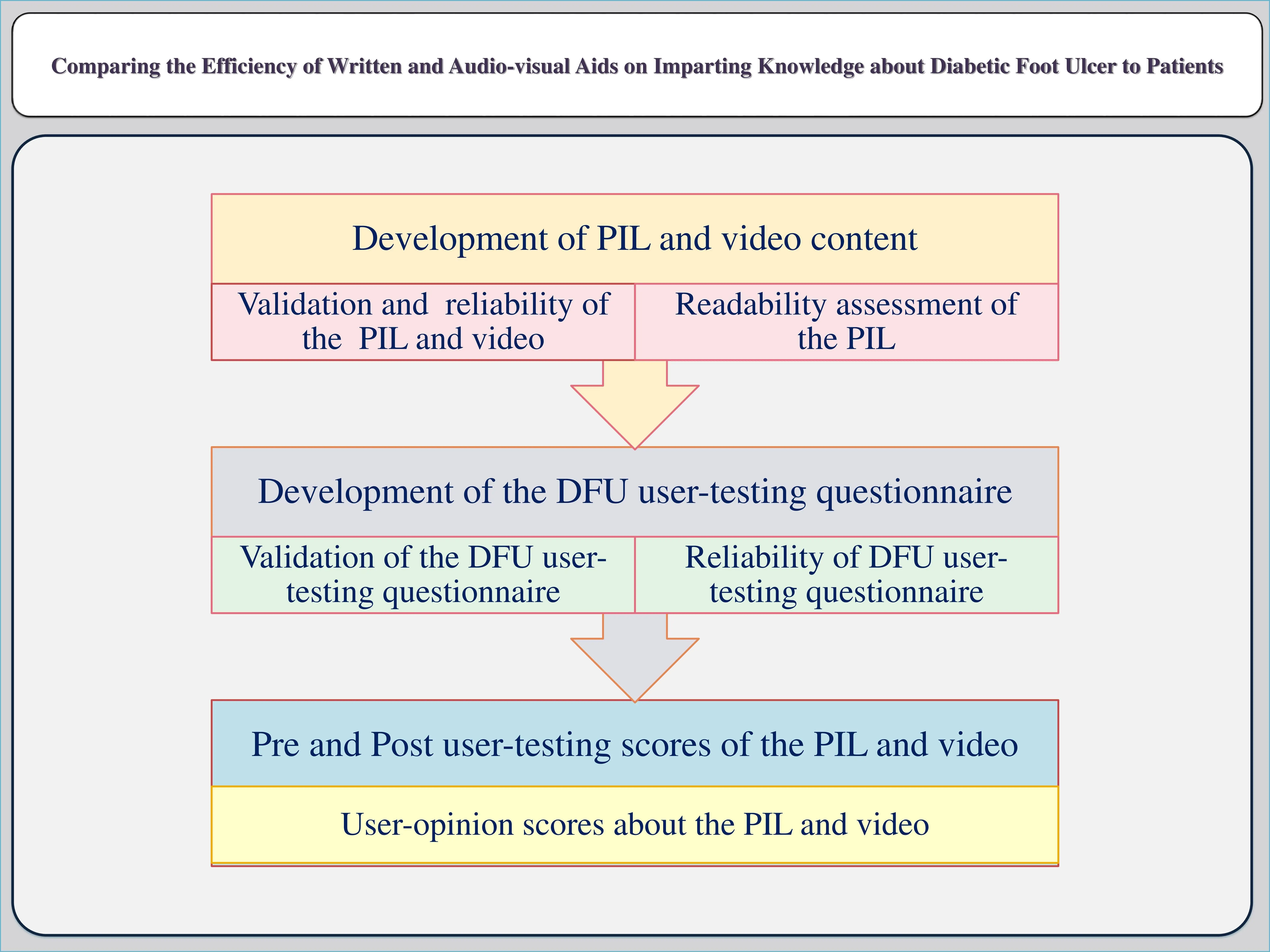
Comparing the efficiency of written and audio-visual aids on imparting knowledge about diabetic foot ulcer to patients
Barma Naga Raju, Uday Venkat Mateti, Rajashekar Mohan, Caren D’Souza, Chakrakodi Shashidhara Shastry, Neevan D’Souza
DOI: 10.7324/JAPS.2024.147324Pages: 255-260
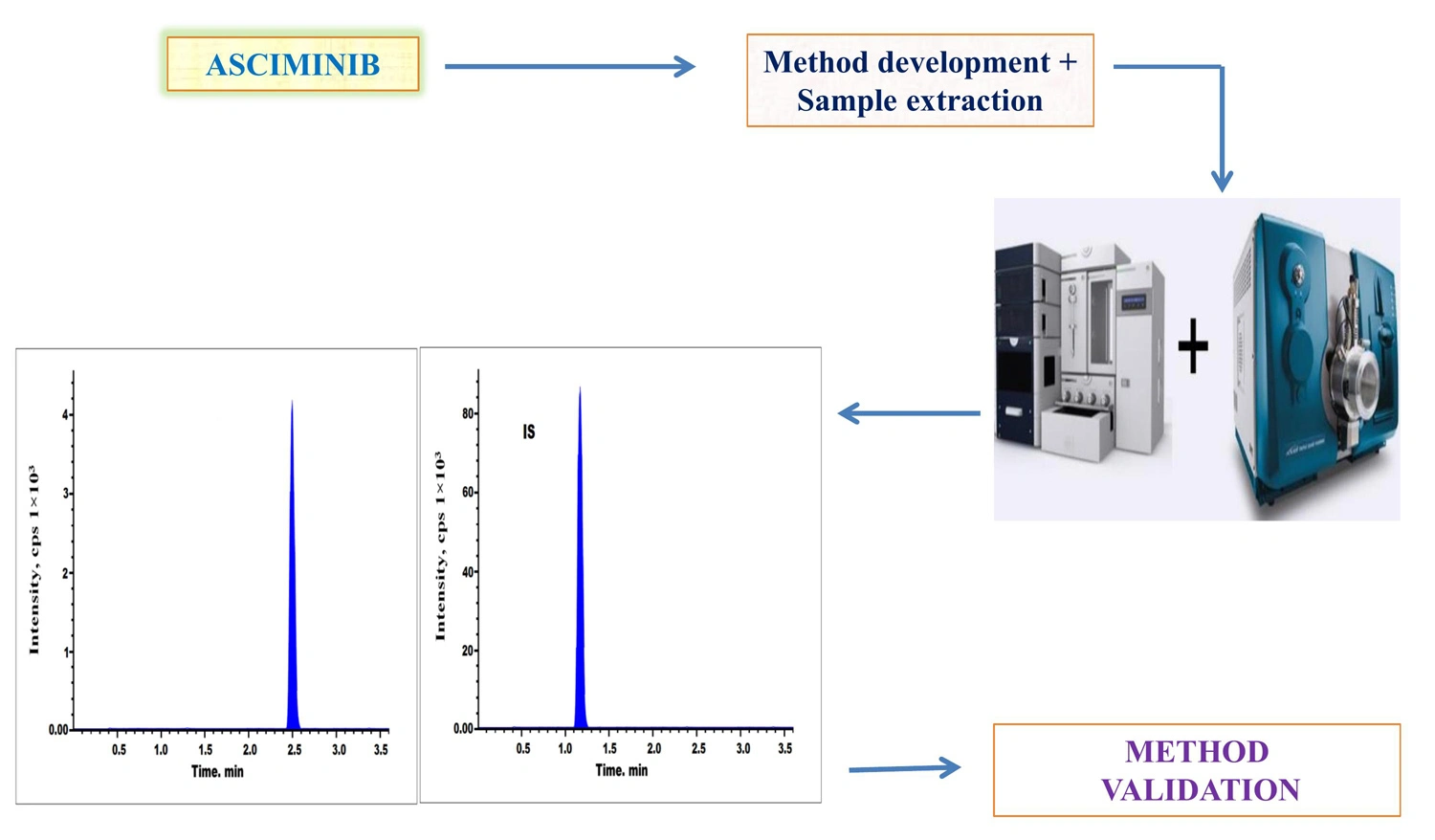
Quantification of asciminib by LC-MS/MS method in human plasma: Validation and stability studies
Hema, Naresh Panigrahi
DOI: 10.7324/JAPS.2024.163326Pages:
QbD-based RP-HPLC method development for quantitative computation of phase III composition comprising apixaban and clopidogrel
Rashmi Shukla, Ankit Chaudhari, Pinak Patel, Krunal Detholia
DOI: 10.7324/JAPS.2024.181311Pages: 085-093

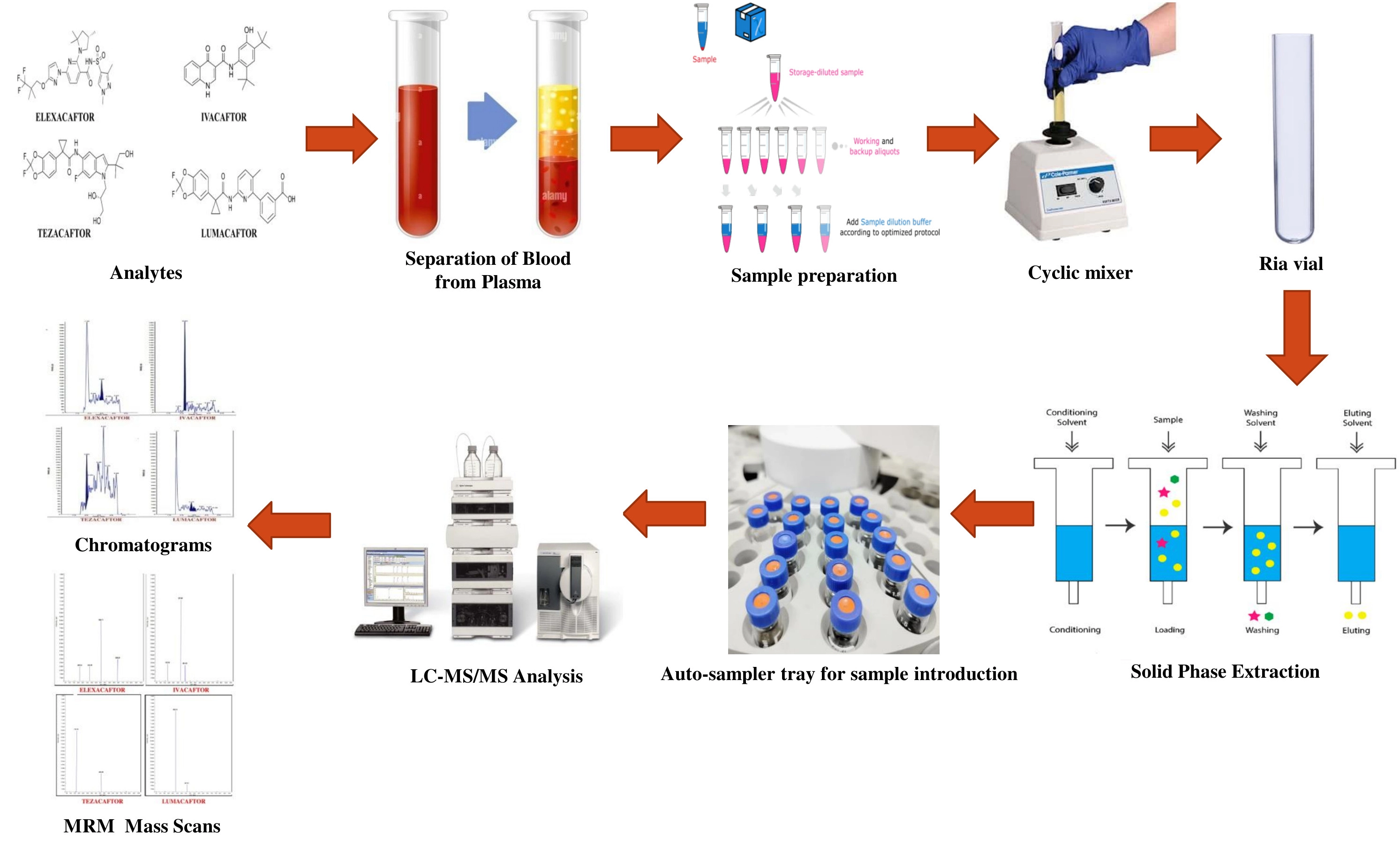
Triplet analysis: Quantifying ivacaftor, tezacaftor, and elexacaftor in plasma with mass spectrometry
Ravikanth Inturi, Srikanth Inturi, David Raju Medepalli, Venkata Basaveswara Rao Mandava
DOI: 10.7324/JAPS.2024.182963Pages: 276-288
Development of an LC-MS/MS technique and its validation for the determination of infigratinib in human K2EDTA plasma; Pharmacokinetics in healthy rabbits
Kunala Anusha, Gummadi Sowjanya
DOI: 10.7324/JAPS.2024.171011Pages: 148-155
_.webp)
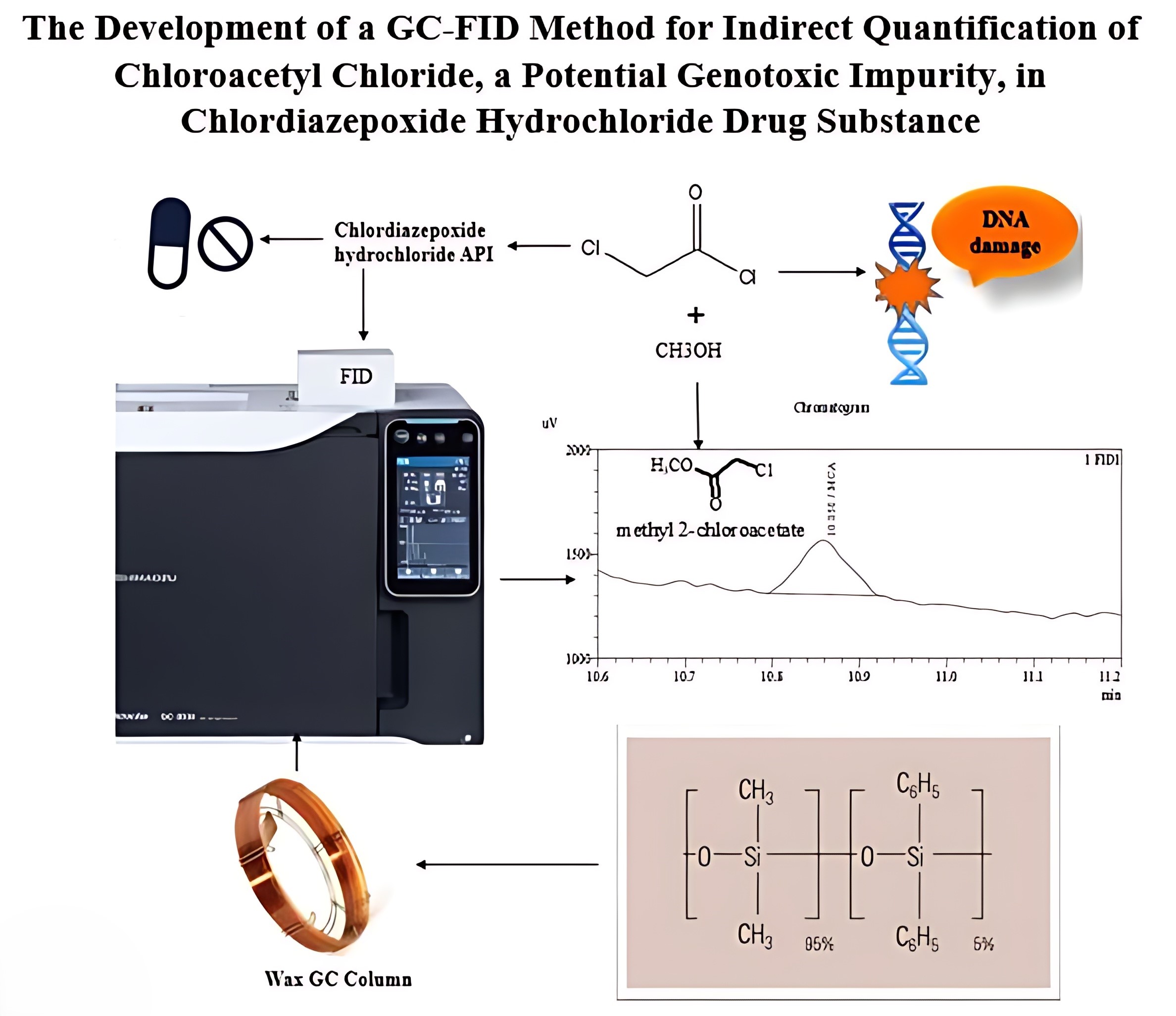
The development of a GC-FID method for indirect quantification of chloroacetyl chloride, a potential genotoxic impurity, in chlordiazepoxide hydrochloride drug substance
Srinivas Birudukota, Bhaskar Mangalapu, Ramesha Andagar Ramakrishna, Swagata Halder, Venkata Narayana Palakollu
DOI: 10.7324/JAPS.2024.182017Pages: 196-207
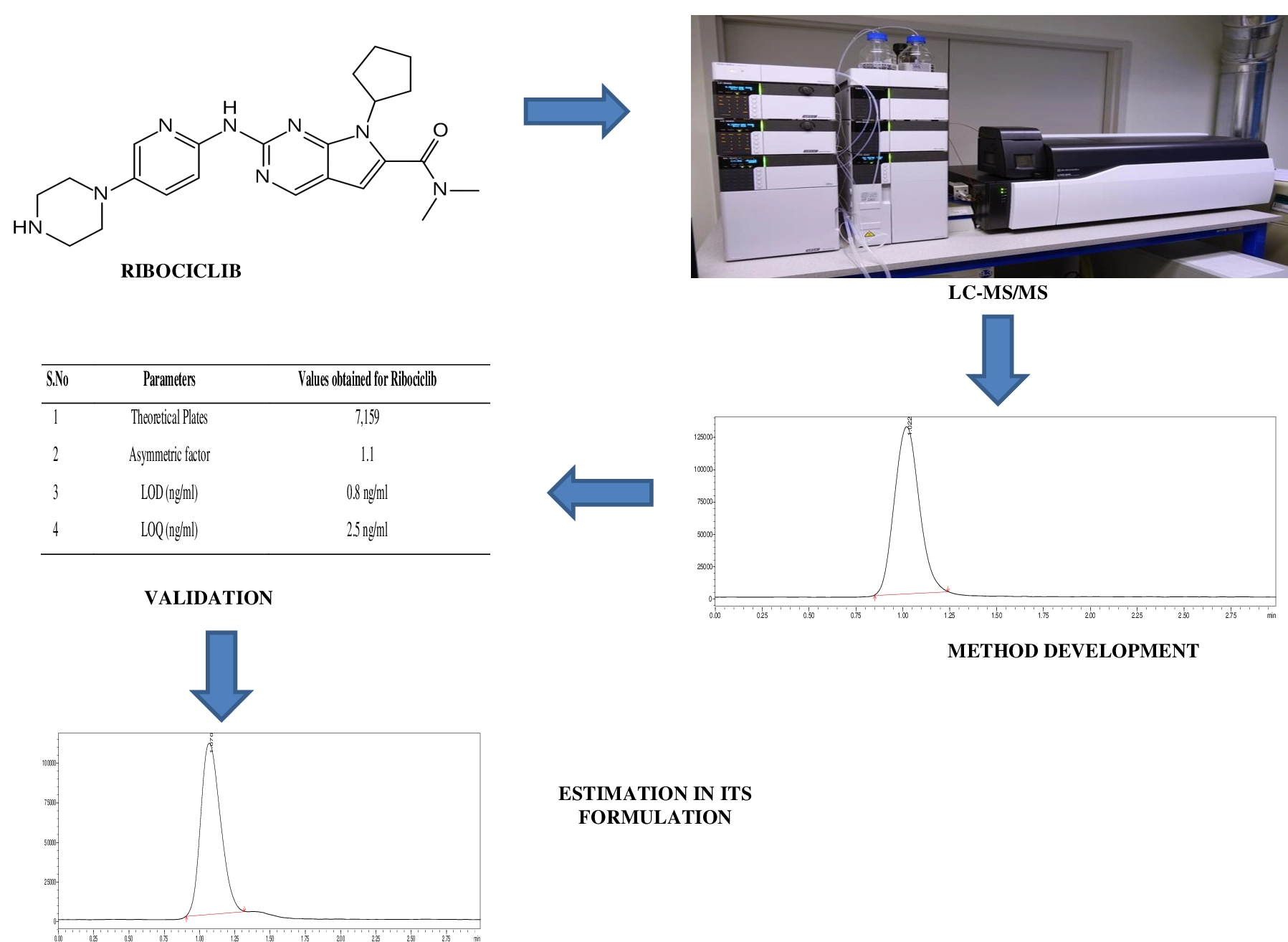
A sensitive liquid chromatography tandem mass spectrometric method development and validation for ribociclib and its formulation
J. Ramesh, B. Babu, R. Sangamithra, D. Anandha Jothi, S.N. Meyyanathan, B. Gowramma
DOI: 10.7324/JAPS.2024.178562Pages: 227-232
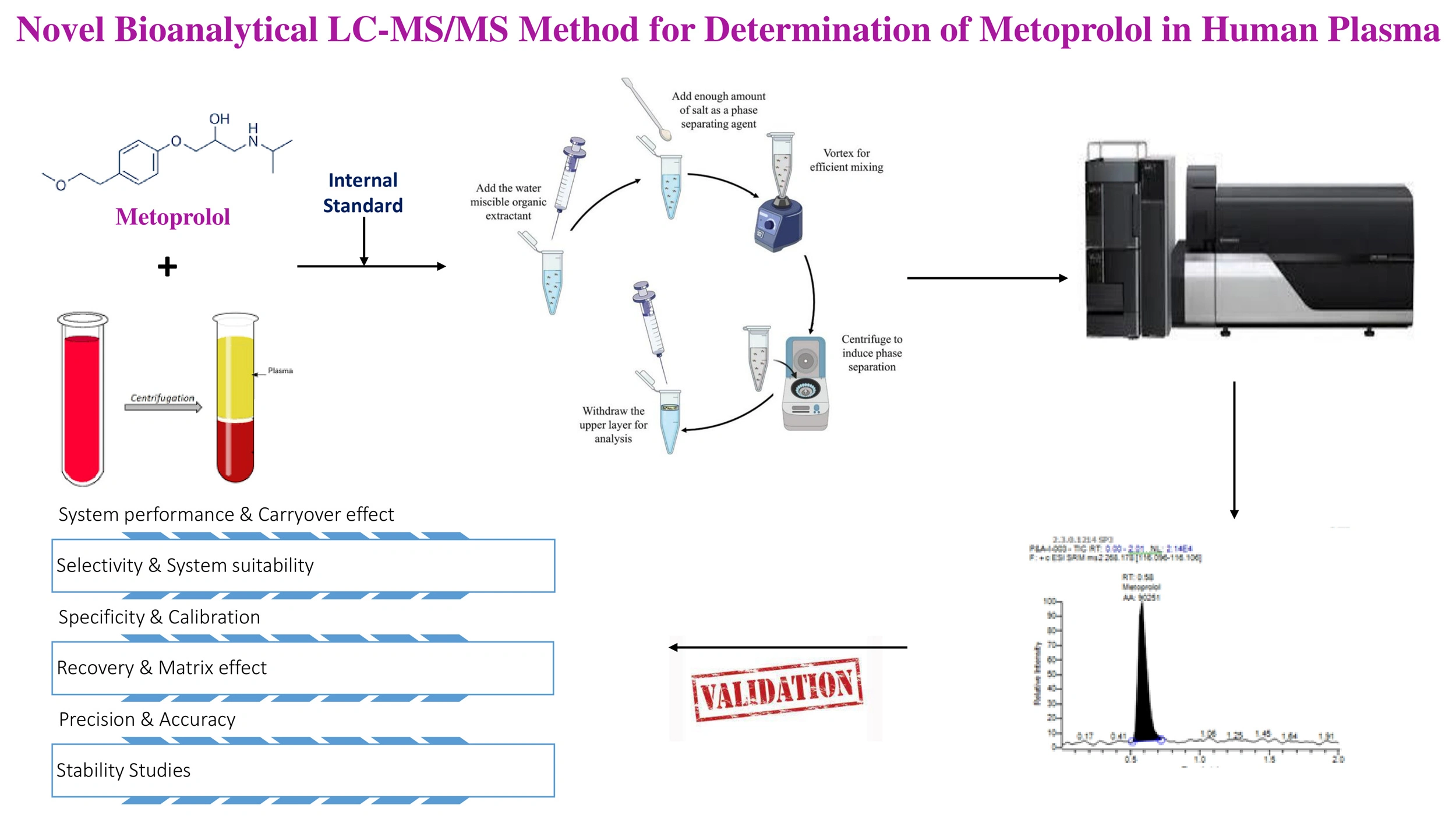
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138
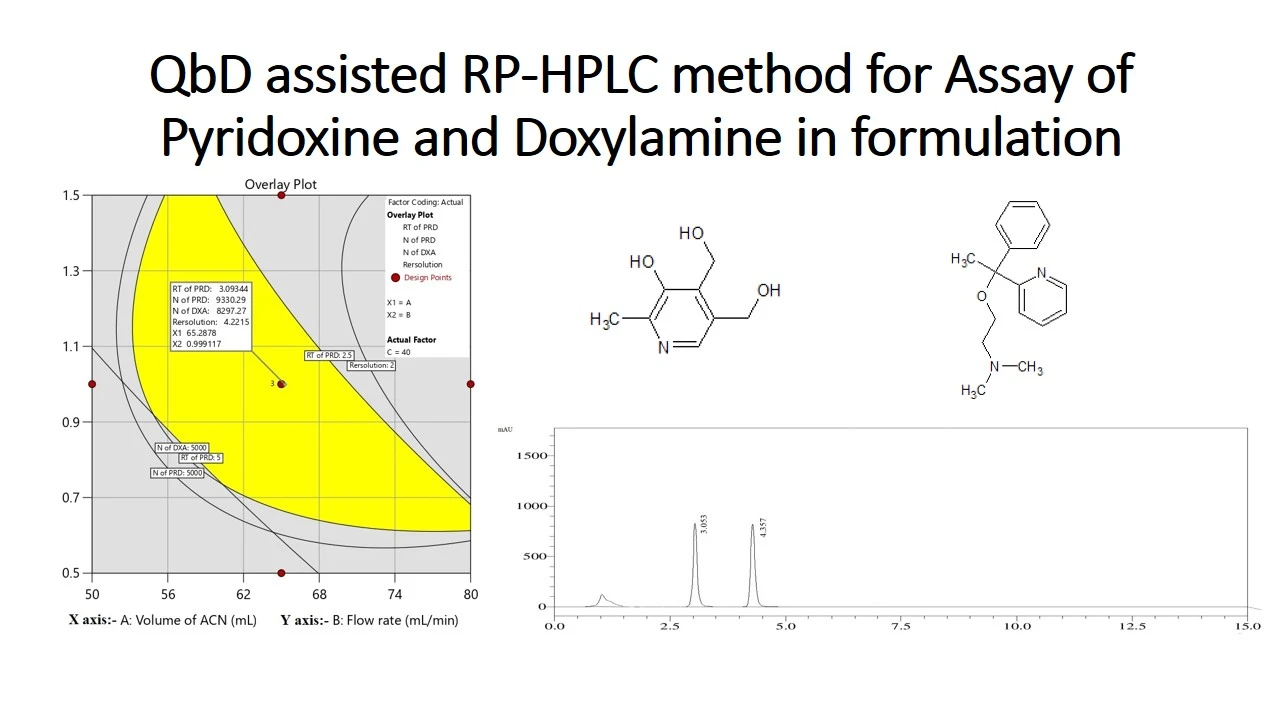
QbD assisted RP-HPLC method for determination of Pyridoxine and Doxylamine in pharmaceutical formulation using central composite design
Gangu Naidu Challa, Daniel Raju Kunda, Sheik Jakir Hussain Mustaq, Nagabharathi Marni, Srilekhya Ketha, Urmila Gorle, Shravitha Jakkula, Bhagavan Rajesh Babu Koppisetty
DOI: 10.7324/JAPS.2025.205442Pages: 072-083
Development of a validated RP-HPLC/PDA method for the quantitative estimation of tepotinib in tablet dosage form
Sumalatha Chepyala, Srinivas Medidi, Jitender Kumar Malik
DOI: 10.7324/JAPS.2024.184366Pages: 064-071
_.jpg)
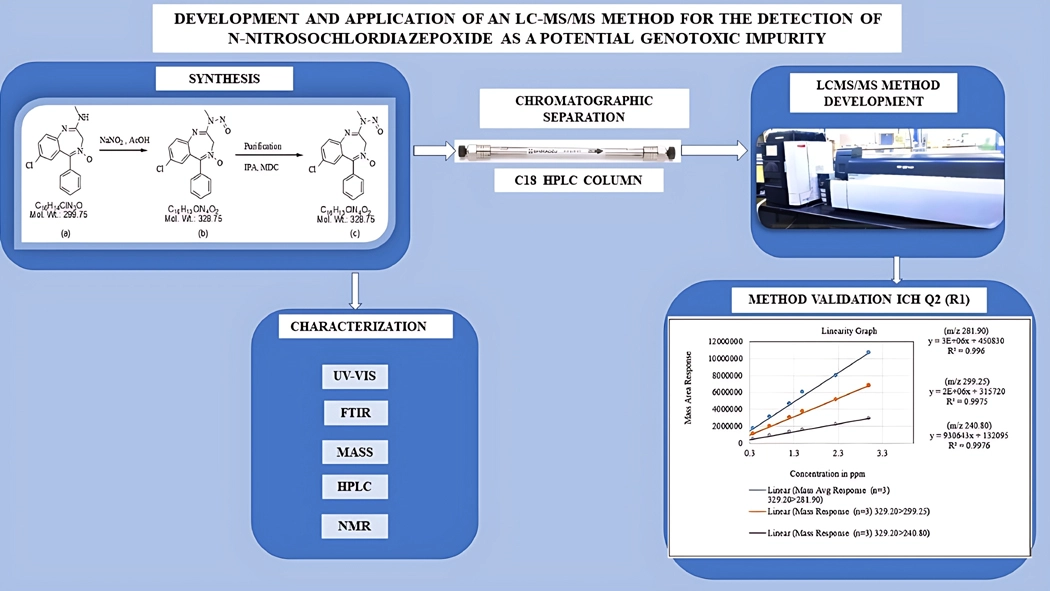
Development and application of an LC-MS/MS method for the detection of N-nitrosochlordiazepoxide as a potential genotoxic impurity
Srinivas Birudukota, Bhaskar Mangalapu, Ramesha Andagar Ramakrishna, Swagata Halder
DOI: 10.7324/JAPS.2025.199616Pages: 242-253
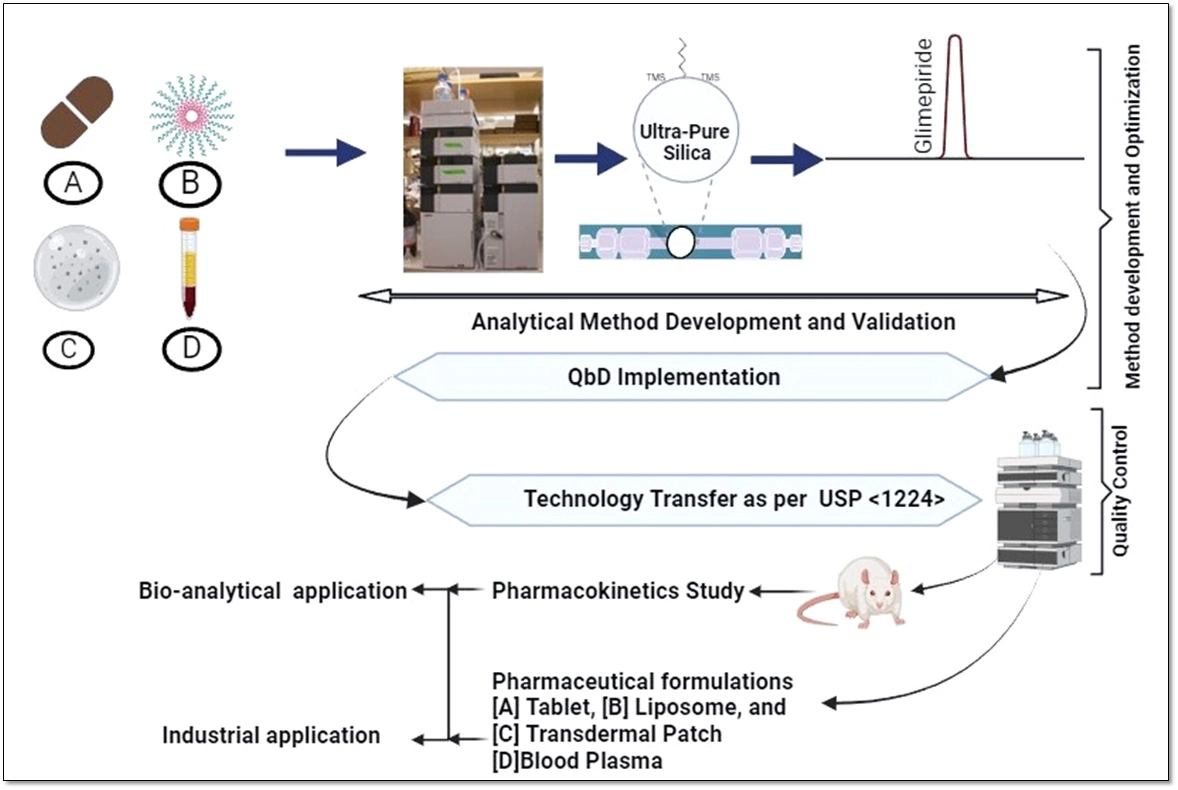
Development of a quality by design based hybrid RP-HPLC method for Glimepiride: Bioanalytical and industrial applications
Abhiram Kumar, Chhavi Dhiman, Madhaw Kumar, N. Kannappan, Deepak Kumar, Manish Kumar Chourasia, Kumar Pranav Narayan
DOI: 10.7324/JAPS.2025.214654Pages: 102-115
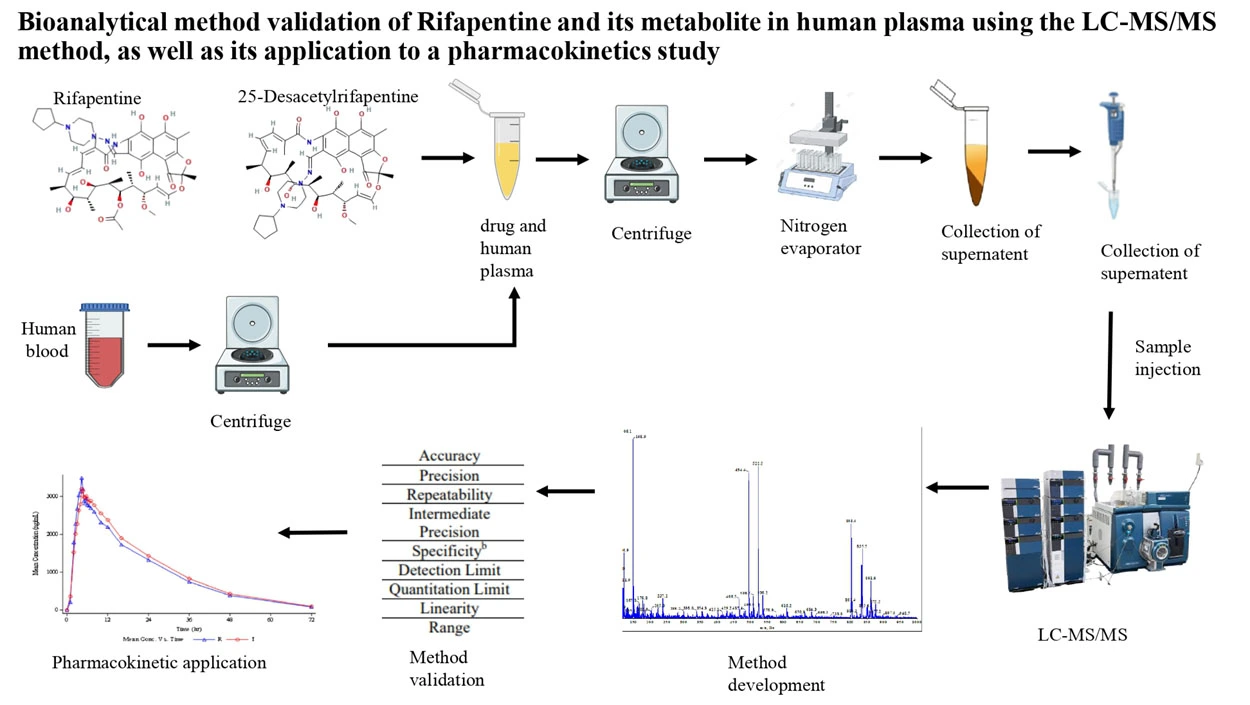
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201
Development and validation of an LC-MS/MS method for pharmacokinetic assessment of tucatinib in rat plasma
Bandaru Venkata Ramarao, Anand Solomon Kamalakaran
DOI: 10.7324/JAPS.2025.202389Pages: 225-233
_.jpg)
Advancing relugolix analysis: A comparative study and AQbD-driven method optimization with stability testing
Priyanka Nagar, Arvind Kumar Sharma, Robin Kumar, Chhaya Chauhan, Rini Singhal, Minakshi Garg
DOI: 10.7324/JAPS.2025.239585Pages: 055-070
_.jpg)
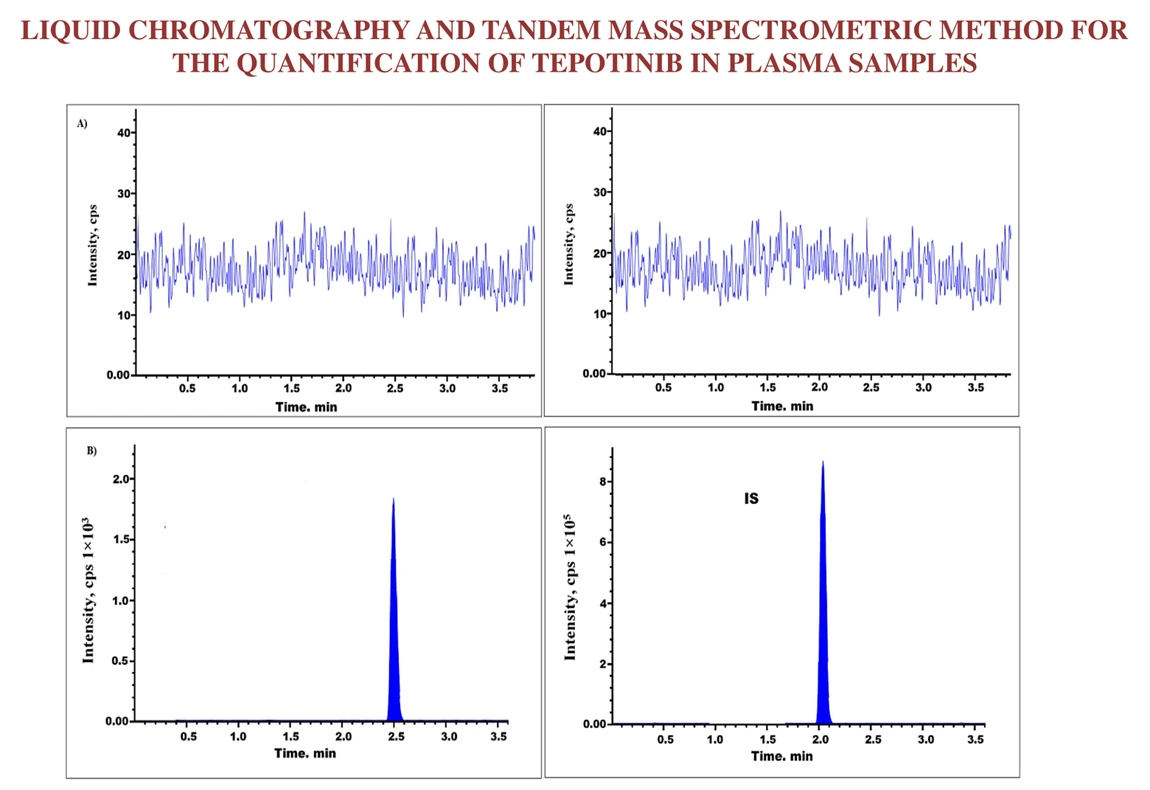
Liquid chromatography and tandem mass spectrometric method for the quantification of Tepotinib in plasma samples
Arjun Siliveri, Kavitha Pingili
DOI: 10.7324/JAPS.2025.206883Pages: 178-184
Development of a validated LC-ESI-MS/MS method for the quantification of tucatinib in plasma samples
Sunil Dattatray Pawar, Deepak D. Kayande, Kokila Pawar, Mazahar Farooqui, Anil Shankarwar, Amol Deshpande, Sunil Shankarwar
DOI: 10.7324/JAPS.2025.213916Pages:
