INTRODUCTION
High-performance liquid chromatography (HPLC) is an influential technique for separating and comparing components to a greater extent than other techniques, which is uniquely held as an inevitable technique in the pharmaceutical domain. Reverse-phase chromatography (RP-HPLC) is a sub-technique that consists of both stationary and liquid phases where the components interact between phases, thus separating into possible individual components based on their interaction differences (Ahuja, 2007; Bailey, 1976).
Brivaracetam is an additional drug for the treatment of epilepsy (Khaleghi, 2017). Epilepsy is defined as an intellectual disarray that causes an individual to have persistent seizures at 16 years of age and to grow up with epilepsy. This is an unending medicinal disarray (typically consequential in erratic, malicious, repeated seizures which affect a range of physical and psychological functions), affecting more than 50 million people across the globe annually. BRIVIACT is used in combination with other antiepileptic drugs to treat the same type of seizures (Matagne et al., 2008; Rogawski, 2016).
To the best of our knowledge, there are no effective HPLC methods for the simultaneous quantification of assays and isomers of the Brivaracetam drug substance. Such a capable method to determine the assay free from isomers (Liao et al., 2020; Qiu et al., 2016) is more authenticated to estimate the content of active ingredients (Abram et al., 2019; Blackstone et al., 2014). Even the few reported methods are also limited to either lesser sensitivity (Baksam et al., 2020; Bhamare et al., 2021; Jessica et al., 2021; Mali and Mhaske, 2016; Mansour et al., 2021; Pankaj et al., 2020; Vasanth and Rajkamal, 2018; Zhu et al., 2017) or the need of sophisticated sensitive instruments (i.e., ultra-performance liquid chromatography) than HPLC (Iqbal et al., 2017; Mohamed et al., 2020; Vishweshwar et al., 2018). It is essential to develop an economic, simple, and single method to address all the issues with easy accessibility. Accordingly, the present research focused on developing/validating a highly sensitive/robust RP chiral HPLC approach to determine the Brivaracetam isomers (Fig. 1) from the Brivaracetam drug in the presence of related substances and its degradants.
MATERIALS AND METHODS
Chemicals
Analytical reagent grade ammonium bicarbonate, ammonium formate, ammonium acetate, and chromatography solvents were procured from Honeywell (Hyderabad). Milli-Q water equipment was used as the water source to prepare all solutions. The active ingredients and related components were procured from authenticated sources.
Instrumentation
The system used for the method development and validation in this study was Agilent 1260 Infinity II HPLC, with a low pressure pump, a degasser, an ultraviolet detector, autosampler, column thermostat, and a controller with Empower3 operating system from Waters. The same conditions were used for the MS study in the Liquid chromatography and mass spectrometry (LCMS) system using Agilent 6470B triple quadrupole LCMS, controlled by MassHunter workstation software.
 | Figure 1. Brivaracetam and its isomers (Note: Related compounds 1–3 are process related impurities of Brivaracetam, which are not provided here). [Click here to view] |
Chromatographic conditions
The required resolution was successfully attained using CHIRALPAK IG-3 (250 mm × 4.6 mm × 3 μm) with a 10 mM (NH4)HCO3 buffer: CH3CN (1:1) %v/v with a constant separation mode. The eluent was driven as 1 ml/minute in a constant flow mode with column thermostat at 25°C and detection wavelength at 210 nm. The analyte solution was introduced using the 100 μl loop with the accurate volume of 10 μl. The DS 1–2, enantiomer, and drug were identified on Agilent 6470B triple quadrupole LCMS. The MS parameters (ESI +ve) were capillary voltage (2.0 kV), cone voltage (15.0 V), extractor voltage (2.0 V), source and desolvation temperatures (150°C and 700°C, respectively), and gas flow (600 l/hour). The developed method was validated by the International Council for Harmonization (ICH, 2005) guidelines.
Related components and isomers’ preparation
5 mg each of the related compounds 1–3, DS 1–2, and enantiomer was added to separate 5 ml standard flasks with the diluents to prepare stock-1 solutions. 1 ml of each stock-1 solutions was diluted to 50 ml to obtain stock-2 solutions. Then, 5 ml of each stock-2 solutions was diluted to 100 ml (the resulting solutions contained 0.1% each of related compounds 1–3, DS 1–2, and En). 5 mg of the Brivaracetam racemate was diluted to 5 ml for the purpose of method development.
Standard solution preparation
10 mg of the Brivaracetam standard was diluted to 10 ml, which is known as the assay standard solution (standard stock-1). 1 ml of standard stock-1 was diluted to 50 ml (standard stock-2); furthermore, 5 ml of this was diluted to 100 ml (the resulting solution contained 0.1% Brivaracetam, with a sample strength of 1.0 mg/ml).
Test solution preparation for assay and isomer contents
About 10 mg of the test sample was dissolved and diluted to 10 ml with diluents.
RESULTS AND DISCUSSION
Method development and optimization
Selection of the phases
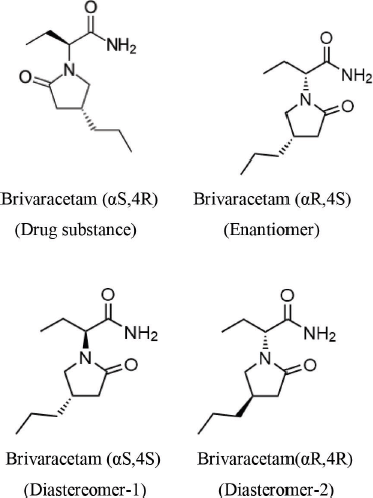
Choice of the separation method is vital as all components are isomers; therefore, RP was chosen for simple operation. For the separation of chiral and isomeric compounds, chiral phase will be the best choice and RP separation will provide a range of selectivity and easy handling. Columns tested in the study include CHIRALPAK IC, CHIRALPAK IG-3, and CHIRALCEL OJ-3R, with various organic modifiers. The initial experimental data are shown in Table 1, which indicate the importance of stationary phase selection because the selectivity of each analyte really depends on the stationary phase.
The method development was initiated with the volatile 10 mM ammonium bicarbonate, ammonium acetate, and ammonium formate as buffers, and acetonitrile and methanol as organic modifiers. Based on the preliminary trials, it was concluded that CHIRALPAK IG-3 with 10 mM ammonium bicarbonate as the buffer and acetonitrile as the organic modifier in 50% v/v provided favorable separation for all the isomers, as shown in Figure 2.
Method optimization
The method was optimized with the selective separation of all the isomers and Brivaracetam from other related compounds and unknown impurities using 10 mM ammonium bicarbonate buffer with acetonitrile as the organic modifier in the isocratic condition on CHIRALPAK IG-3. The optimization comprised buffer strength, thermostat, and mobile phase flow. The stationary phase was recognized based on the trials and column compatibility according to the separation requirements and required system suitability criteria. The method was further optimized with three individual factors at two stages (viz. buffer at 10 and 30 mM; thermostat at 20°C and 30°C; and flow at 0.8 and 1.2 ml/minute).
Finally, the modest buffer concentration of 10 mM with a 1.0 ml/minute flow rate was opted as the best condition to obtain the finest resolution with quality peaks. Column thermostat is another important parameter that did not show any notable effect on the selectivity, and hence kept at 25°C. The final chromatograms are shown in Figures 3–6 and the data of the spiked sample chromatogram are provided in Table 2.
 | Table 1. Stationary phase and mobile phase screening [Click here to view] |
Forced degradation and specificity
The sample was exposed to solid state stress, such as thermal degradation (7 days at 70°C), humidity (7 days at 98% RH/25°C), aerial oxidation exposure (7 days open), and photolytic degradation (UV 200 Watt-hour/m2, 1.2 million lux hours), and it was observed that there was no interference at the desired peak. The exposed conditions did not show any major degradation. Peak purity (purity angle < purity threshold) of the exposed samples was verified in the Photo diode array detector (PDA) detector, whereas the peak homogeneity was verified in the mass spectrometer. In MS, three Brivaracetam (BRVC) samples and one batch sample (spiked with all the isomers and related compounds) were analyzed under the same HPLC conditions with the PDA detector to check the quality of the isomers. All the isomers were confirmed and demonstrated that there are no other impurities co-eluting on the isomer peaks. Because the desired peaks are pure and homogeneous, the method is specific. The absence of interference at the retention time of any isomers from its related compounds and degradation products is witnessed by the data. The peak purity was examined by PDA (purity angle is lesser than the threshold), and the chromatogram and data are shown in Figure 7 and Table 3. The mass spectra with m/z values of sodium adduct are shown in Figures 8–12.
Method validation
According to the ICH guidelines, the developed RP chiral method (to estimate the isomers and Brivaracetam in the Brivaracetam drug) was validated. System suitability, specificity, system precision, method precision, intermediate precision, recovery, robustness, linearity, LOQ, LOQ precision, LOD, and range were determined.
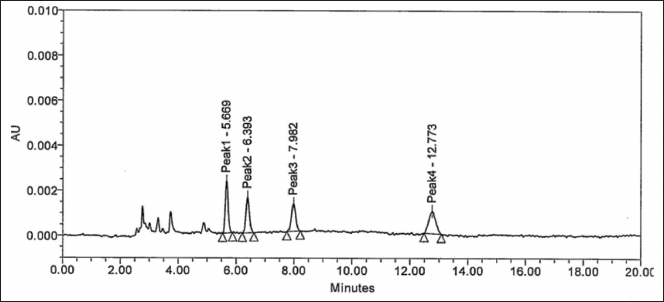 | Figure 2. Reverse phase chiral separation of all isomers and Brivaracetam. Elution order is, Peak-1: Enantiomer (En), Peak-2: Diastereomer-2 (DS-2), Peak-3: Diastereomer-1 (DS-1), Peak-4: Drug (Brivaracetam). [Click here to view] |
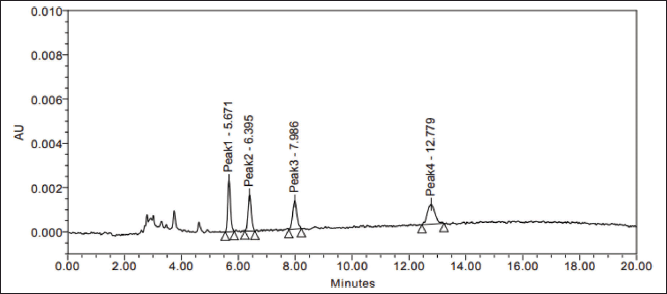 | Figure 3. Standard impurity mixture at 1.0 μg/ml level. [Click here to view] |
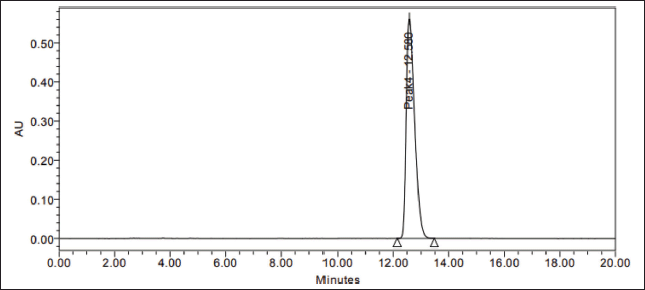 | Figure 4. Assay standard chromatogram. [Click here to view] |
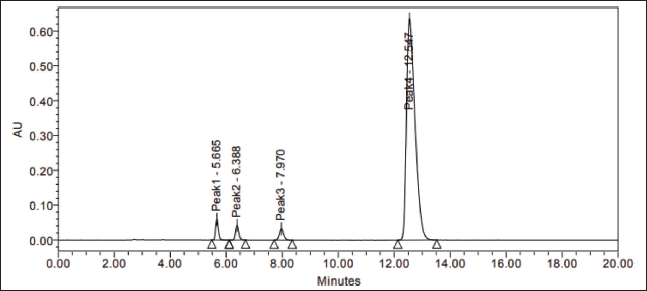 | Figure 5. Spiked sample chromatogram. [Click here to view] |
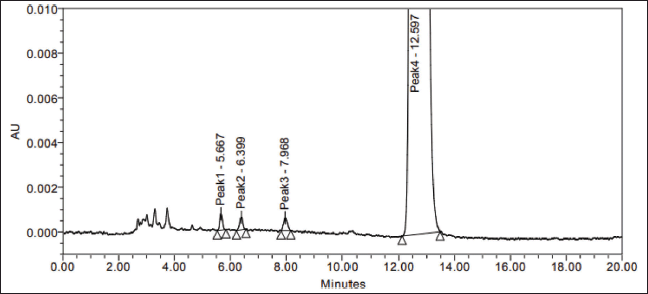 | Figure 6. LOQ level isomers spiked sample chromatogram. [Click here to view] |
Specificity
Method specificity was determined by spiking all the related substances in the sample by forced degradation study. In addition, peak homogeneity was confirmed using the LCMS study of the same spiked and unspiked samples by confirming m/z pureness of all those peaks.
Linearity and range
The linearity was measured by six levels, i.e., LOQ to 150% level of limit concentration (1 μg/ml) for DS-1, DS-2, En, and Brivaracetam. The linearity study solutions were prepared from LOQ to 150% of specification level for isomers and drug in the isomer quantification, whereas for the assay quantification, 80–120% of the test concentration solutions were injected to demonstrate the method linearity and range. The correlation was drawn for each analyte concentration against its response.
Precision
Precision was ascertained by determining the repeatability and ruggedness of recoveries (DS-1, DS-2, and En). Repeatability of the method was carried out by the spiked test sample preparations six times with all the components of the drug substance. For the assay, six replicate preparations were made and the % Related standard deviation (RSD) as an evaluation parameter was checked. The ruggedness was also estimated by performing intermediate precision with similar spiked sample preparations on different days under similar conditions and atmosphere.
Accuracy
Accuracy was verified at four concentrations (viz., LOQ, 50%, 100%, and 150%) by spiking all the components (DS-1, DS-2, and En) of Brivaracetam at each level, and calculating the amount recovered and standard deviation at each level in %recovery. The accuracy results were found to be 98–102%, which lies within the acceptable criterion of ±2%.
 | Table 2. Data of the spiked sample chromatogram. [Click here to view] |
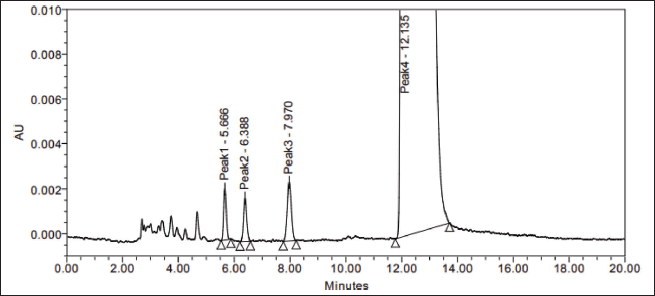 | Figure 7. Spiked sample LC chromatogram data for peak purity. [Click here to view] |
 | Table 3. Data of the spiked sample LC chromatogram. [Click here to view] |
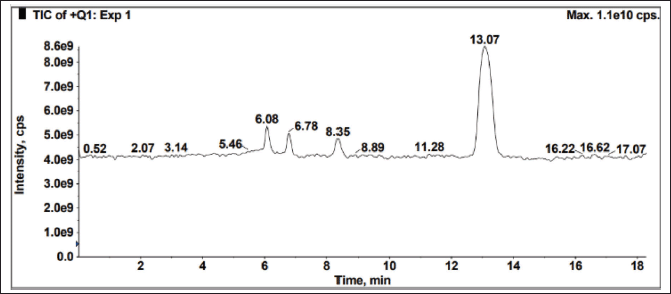 | Figure 8. Spiked sample LCMS spectral data Total ion current (TIC) for peak homogeneity. [Click here to view] |
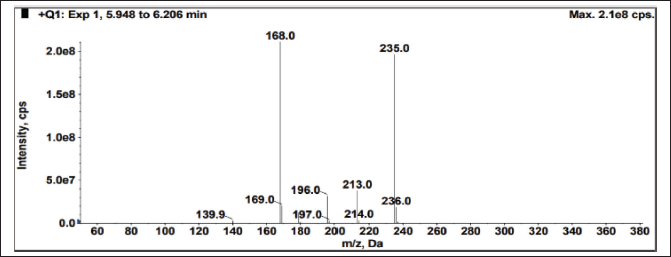 | Figure 9. Mass spectrum of Enantiomer (En) from spiked sample. [Click here to view] |
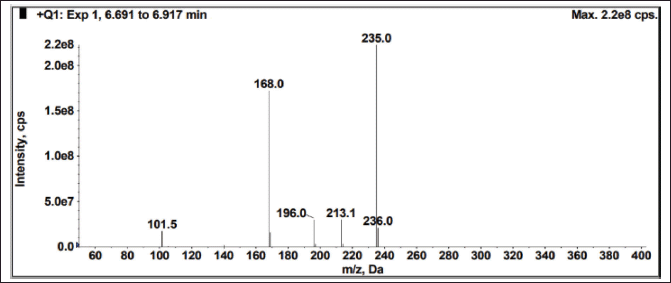 | Figure 10. Mass spectrum of diasteromer-2 (DS-2) from spiked sample. [Click here to view] |
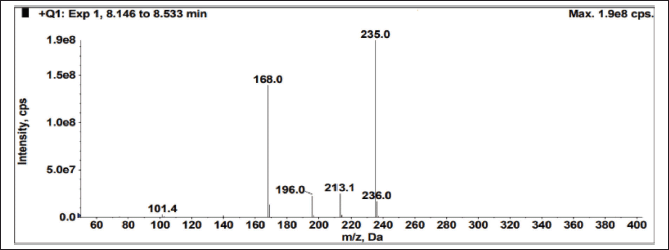 | Figure 11. Mass spectrum of diasteromer-1 (DS-1) from spiked sample. [Click here to view] |
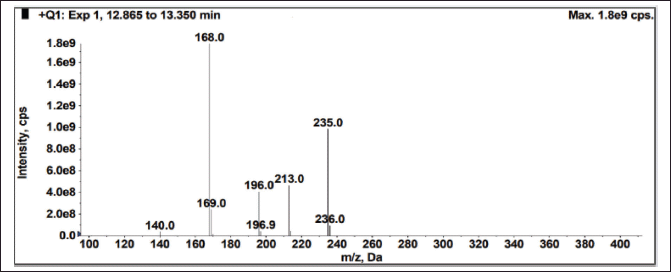 | Figure 12. Mass spectrum of drug (Brivaracetam) from spiked sample. [Click here to view] |
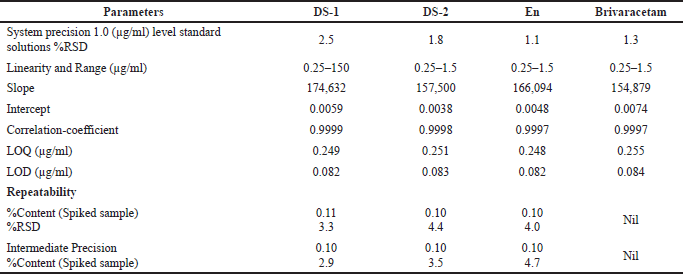 | Table 4. Validation data of the proposed method for stereoisomers determination. [Click here to view] |
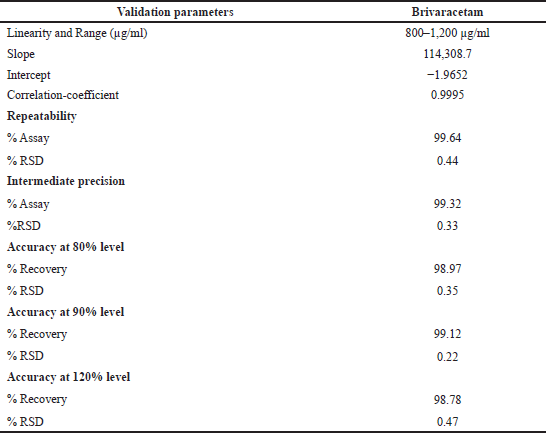 | Table 5. Validation of the proposed method for Brivaracetam determination. [Click here to view] |
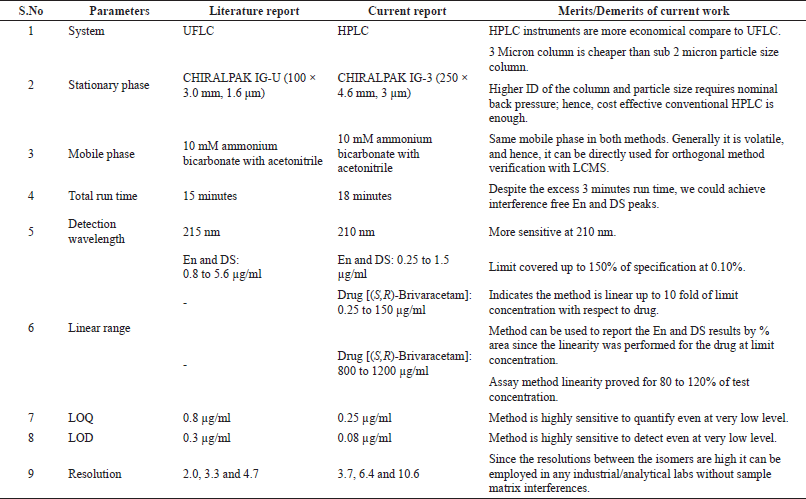 | Table 6. Detailed comparison between the current and literature methods. [Click here to view] |
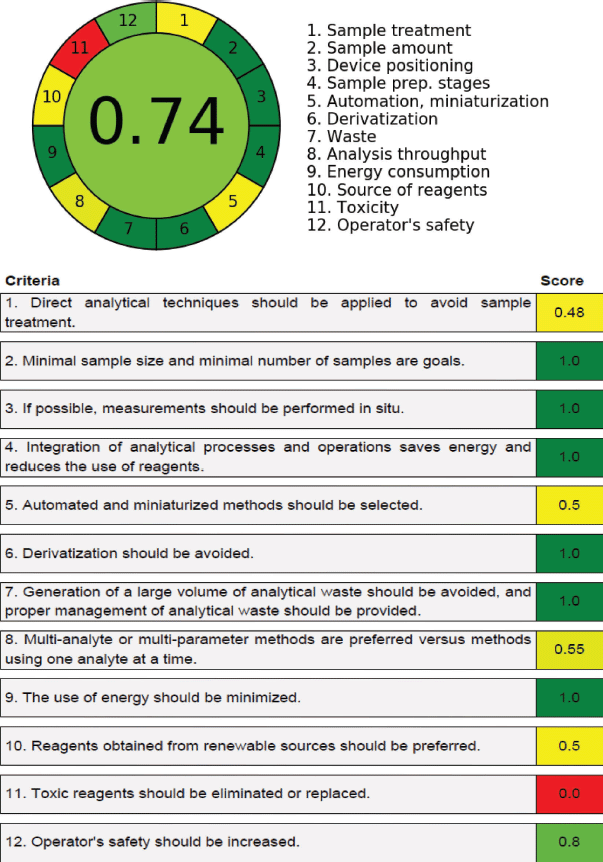 | Figure 13. Greenness chart for the developed HPLC method using AGREE tool. The overall value of 0.74 in the 0 to 1 scale indicates the green analytical procedure of this method. The 12 analytical principles (criteria) and their scores (out of 1) are reproduced in the second chart. [Click here to view] |
Robustness
Robustness was executed by deliberate changes in the strength of the buffer (10–20 mM), flow (0.8–1.2 ml/minute), and column thermostat (20–30°C) on the optimized method condition. Resolution, tailing of main peak and isomers, and %assay variation between control and modified conditions were used as tools to verify the robustness parameters. The observed results are considered to be acceptable since they are within the allowed limits.
Overall, the adopted approach of method development is based on the consideration of all possible variables (buffer, column chemistry, operating temperature, and organic modifiers and additives selection) to separate the components (DS-1, DS-2, En, and Brivaracetam) effectively from the degradants, related compounds, and unknown impurities using a PDA detector, thus achieving the desired resolution and sensitivity by optimizing all variables of the method.
Based on the validation data (highest sensitivity of 0.25 μg/ml quantification level and 0.08 μg/ml detection level with highest resolutions of 3.7, 6.4, and 10.6), it is clear that this method has higher sensitivity and better resolution than the other reported methods; in addition, the existing reports do not deal with the quantification of isomers and drug substance in single chromatographic conditions as projected by this study. The validation data are presented in Tables 4 and 5, whereas the advantages of this method are compared with a recent analogous method (Baksam et al., 2020) in Table 6 for better comprehension.
Thus, the present study offers a robust and simultaneous RP chiral chromatographic method for the separation of all the components (DS-1, DS-2, En, and Brivaracetam) and assay of Brivaracetam from the degradants, related compounds, and unknown impurities.
Assessment of greenness character of the current method using AGREE tool
AGREE is an analytical tool (Gamal et al., 2021; Pena-Pereira et al., 2020) that can be applied on any analytical procedure to evaluate the environmental compatibility of the developed analytical methods. This tool was constructed based on 12 (SIGNIFICANCE) factors of green analytical chemistry that are related to sample preparation, derivatization, simplicity, automation, solvents toxicity, quantity of samples used for testing, number of components determined in single analysis, consumption of energy per analysis, and analysis time, and the outcome is transformed into a 0–1 scale. The current HPLC method was executed for greenness character using this tool and was found to have a 0.74 score (Fig. 13), which indicates that the developed method is environmentally benign.
CONCLUSION
The proposed and validated RP chiral HPLC method for the simultaneous estimation of assay, isomers, and related impurities has high sensitivity, good resolution, and reliance on simpler HPLC equipment. This method has some other potential advantages, such as shorter run time (18 minutes), with the usage of volatile buffer, good sensitivity, and greener approach. This may be the first HPLC method with an appreciable chromatographic resolution of all the four components from the degradants, related compounds, and other impurities; and hence, may be considered for the direct application in quality control analysis and counterfeit pharmaceutical drug investigations.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abram M, Jakubiec M, Kami?ski K. Chirality as an important factor for the development of new antiepileptic drugs. Chem Med Chem, 2019; 14(20):1744–61. CrossRef
Ahuja S. Overview of HPLC method development for pharmaceuticals. Sep Sci Technol, 2007; 8:1–11. CrossRef
Bailey F. Applications of high-performance liquid chromatography in the pharmaceutical industry. J Chromatogr A, 1976; 122:73–84. CrossRef
Baksam V, Saritha N, Pocha VR, Chakka VB, Ummadi RR, Kumar P. Development of an effective novel validated stability indicating HPLC method for the resolution of brivaracetam stereoisomeric impurities. Chirality, 2020; 32(9):1208-19. CrossRef
Bhamare P, Dubey R, Upmanyu N, Umadoss Pothuvan. A simple HPLC method for in-vitro dissolution study of brivaracetam in pharmaceutical dosage form. Asian J Pharm Anal, 2021; 11(1):1–8. CrossRef
Blackstone EA, Fuhr JP Jr, Pociask S. The health and economic effects of counterfeit drugs. Am Health Drug Benefits, 2014; 7(4):216–24.
Gamal M, Naguib IA, Panda DS, Abdallah FF.Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal Methods, 2021; 13(3):369–80. CrossRef
ICH Q2 (R1): Validation of analytical procedures: text and methodology. International Council for Harmonisation, Geneva, Switzerland, 2005. [Online]. Available via https://www.ich.org/page/quality-guidelines (Accessed 21 January 2021).
Iqbal M, Ezzeldin E, Al-Rashood KA. UPLC–MS/MS assay for identification and quantification of brivaracetam in plasma sample application to pharmacokinetic study in rats. J Chromatogr B, 2017; 1060:63–70. CrossRef
Jessica DA, Cabral LM, Sosua VP. Stability indicating methods for determination of third generation antiepileptic drugs and their related substances. Crit Rev Anal Chem, 2021; 1–13; DOI: 10.1080/10408347.2021.1890544. CrossRef
Khaleghi F. A novel adjunctive therapy for partial-onset seizures. P T, 2017; 42(2):92–6.
Liao S, Chen H , Wang G, Wu S, Yang Z, Luo W, Gao X, Qin J, Li HC, Wang ZG. Identification, characterization, synthesis and strategy for minimization of potential impurities observed in the synthesis of brivaracetam. Tetrahedron, 2020; 76(26):131273. CrossRef
Mali NV, Mhaske DV. HPLC studies on degradation behaviour of brivaracetam and development of validated stability indicating HPLC assay method. IJSRM Human, 2016; 4(3):43–57.
Mansour NM, Sherbiny DTE, Fawzia A. Ibrahim, Subbagh HIE. Development of an inexpensive sensitive and green HPLC method for the simultaneous determination of brivaracetam, piracetam and carbamazepine application to pharmaceuticals and human plasma. Microchem J, 2021; 163:105863.Matagne A, Margineanu DG, Kenda B, Michel P, Klitgaard H. Anticonvulsive and antiepileptic properties of brivaracetam (ucb 34714) a high-affinity ligand for the synaptic vesicle protein SV2A. Br J Pharmacol, 2008; 154(8):1662–71. CrossRef
Mohamed S, Riva R, Contin M. Development and validation of an UHPLC-MS/MS assay for the therapeutic monitoring of brivaracetam plasma concentrations in patients with epilepsy. Ther Drug Monit, 2020; 42(3):445–51. CrossRef
Pankaj B, Umadoss P, Upmanyua N, Dubey R. Identification, isolation, structural characterisation synthesis and in silico toxicity prediction of the alkaline hydrolytic degradation product of Brivaracetam by using LC-PDA preparative HPLC LC/HENI/LTQ FTIR and 1H NMR. Anal Methods, 2020; 12:1868–81. CrossRef
Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE-Analytical GREEnness metric approach and software. Anal Chem, 2020; 92:10076–82. CrossRef
Qiu S, Tehrani KA, Sergeyev S, Bultinck P, Herrebout W, Mathieu B. Stereochemistry of the brivaracetam diastereoisomers. Chirality, 2016; 28(3):215–25. CrossRef
Rogawski MA. A new SV2A ligand for epilepsy. Cell, 2016; 167(3):587. CrossRef
Vasanth DA, Rajkamal B. A validated LC-MS/MS method for pharmacokinetic study of brivaracetam in healthy rabbits. Int J Pharm Pharm Sci, 2018; 10(2):24–9. CrossRef
Vishweshwar V, Babu JM, Muralikrishna R. Development and validation of stability-indicating UPLC method for the determination of brivaracetam its related impurities and degradation products. Int J Pharm Sci Res, 2018; 9(6):2315–27.
Zhu LN, Chen D, Chen T, Xu D, Chen SH, Liu L. The adverse event profile of brivaracetam A meta-analysis of randomized controlled trials. J Seizure, 2017; 45:7–16. CrossRef