INTRODUCTION
One of the most frequent malignancies in women worldwide is breast cancer, it has a role in the 5 million annual fatalities caused by breast cancer. According to several epidemiological research studies across a wide range of demographic cohorts, breast cancer is a significant cause of cancer death and challenge in India. The most commonly diagnosed cancer among women is hormone-related breast cancer. According to the American Society of Clinical Oncology, approximately 6% of women have been diagnosed with de novo metastatic cancer [1]. For metastatic and advanced breast cancer, the well-known treatment is Endocrine therapy; however, almost all the patients develop resistance. Recent years have seen the introduction of a novel medication for patients with advanced breast cancer: a class of oral cyclin-dependent kinase 4 and cyclin-dependent kinase 6 inhibitors. Clinical trials have shown that using CDK 4/6 inhibitors in conjunction with endocrine therapy improves progression-free survival significantly. Along with endocrine therapy for hormone-sensitive advanced breast cancer, the Food and Drug Administration has approved three CDK 4/6 inhibitors—palbociclib, ribociclib, and abemaciclib [2]. Several new medications have recently hit the market to treat breast cancer. One such medication is ribociclib (Fig. 1), which is being used more frequently to treat hormone-related breast cancer in women, namely human epidermal growth factor receptor 2 [3,4].
A major target for cancer treatments, the cyclin-dependent kinase 4/6 pathway is commonly disturbed in cancer, favoring cell-cycle progression and ongoing growth. Activation of the CDK4/6 pathway has also been connected to hormone receptor-positive (HR+) breast cancer resistance to endocrine therapy. Ribociclib is a novel cyclin-dependent kinase inhibitor used in the medical treatment of postmenopausal women with breast cancer together with aromatase inhibitors (reducing estrogen levels) [5]. Ribociclib is associated with both clinically evident liver injury and a little increase in serum aminotransferase throughout treatment. Commercially, pills containing 200 mg of it are available. Antineoplastic medication Ribociclib inhibits the growth of cancer by preventing the action of CDK 4 and CDK 6.
As per the literature review in biological samples, various studies were carried out for the quantification of Ribociclib and its metabolites. Few bio-analytical methods have been developed in liquid chromatography tandem mass spectrometry (LC-MS/MS) such as analyzing the ribociclib in dried blood spot (DBS) with the mobile phase of ammonium acetate and methanol [6]. In women, with breast cancer treated with ribociclib. DBS samples were collected along with the plasma samples and analyzed using ammonium bicarbonate and methanol [7]. New green approach for developing ribociclib through capillary electrophoresis. Since it involves far smaller sample volumes and reagent usage and generates less waste than competing procedures, it provides simpler, more affordable, and environmentally friendly analysis. The term “green chemistry” refers to a method of creating, handling, evaluating, and utilizing chemicals in a way that minimizes risks to human health and the environment [8]. For a combination regimen, Sahu et al. developed a method to quantify new cyclin dependant kinase inhibitors in animal plasma and tissue homogenate with a mobile phase combination of ammonium bicarbonate and methanol [9]. On the other side, the method was developed to determine the total and free ribociclib in human blood plasma and brain tumor tissue samples [10,11]. A stability study has been carried out for ribociclib with an isocratic flow of acetonitrile and ammonium formate using high performance liquid chromatography [12].
From the above literature review, the reported methods are with gradient flow and it is for bio-analytical studies. In contrast to gradient elution, which employs multiple mobile phases and allows the mobile’s polarity to be gradually changed during the separation process, isocratic elution employs a single mobile phase composition with the same polarity. As a result, this study aims to optimize an isocratic flow method that will be simple, quick, and accurate for determining Ribociclib along with its formulation using LC-MS/MS.
METHODS AND MATERIALS
Reagents
The active pharmaceutical ingredient (API) ribociclib was purchased from clearsynth as the standard formulation of ribociclib from Novartis. The chemical ammonium acetate and the solvents methanol and acetonitrile (LC-MS/MS grade) were provided by standard deviation (SD) Fine Chemicals and Merck, both are Mumbai-based. Milli Q RO system (Millipore, Bedford) was utilized for purifying the water.
Instrumentation
The Shimadzu LC-MS/MS 8030 (Triple Quadrupole) is equipped with a variety of features: lab solution software, auto-sampler (SIL-20AC), photo diode array detector (SPD M20), pump (LC-20 AD), and ESI. The mass spectrometer was configured for positive ionization (M+H) using an electron spray ionization source. The ionization temperature was set at 300°C, the capillary voltage was set at 5,000 V, the nebulizer gas was set at 350 kPa, and the gas circulation rate was set at 15 l/min. The ionization source gas and collision cell were both nitrogen-based.
Chromatographic conditions
Acetonitrile and 10 mM buffer (90:10 v/v) was used as the mobile phase (flow rate: 0.7 ml/minute) with a Phenomenex C18 column (50 × 4.6 mm i.d., 3 µ) as the stationary phase. 10 µL of the prepared sample solution was injected using the column temperature of 25°C for 3 minutes. The m/z ratios were found using an LC-MS/MS and an ESI-coupled mass spectrometer. The mass spectrometry was operated in positive ionization mode (M+H)+ using multiple reaction monitoring for ribociclib, observing transitions from m/z 435. 0 (parent ion) to m/z 322.0 (daughter ion) (Figs. 2 and 3).
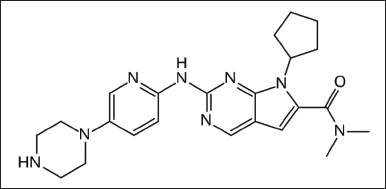 | Figure 1. Structure of ribociclib. [Click here to view] |
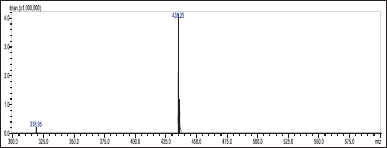 | Figure 2. Molecular spectra for ribociclib (molecular weight—434.5). [Click here to view] |
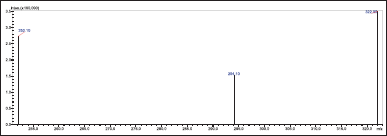 | Figure 3. Daughter ion spectra for ribociclib m/z 435.0 (parent ion) → m/z 322.0 (daughter ion). [Click here to view] |
Preparation of working standard solution
Ribociclib 1 mg/ml working standard was prepared by dissolving 10 mg of the drug in 10 ml of dimethyl sulfoxide in a volumetric flask. From the above stock solution, a working standard solution of 1 µg/ml was prepared. Using the working standard, a calibration standard that ranges from 5 to 100 ng/ml of Ribociclib was developed. Various quality control (QC) levels were prepared in the mobile phase: 10, 50, and 100 ng/ml.
METHOD VALIDATION FOR RIBOCICLIB
The optimized LC-MS/MS method was validated in terms of specificity, limit of quantification (LOQ), limit of detection (LOD), linearity, accuracy, precision, and robustness [13].
The recovery was used to assess the accuracy of the newly developed method. The degree to which a value found and a value that is recognized as a conventional true value or an acknowledged reference value agree is expressed as the accuracy of an analytical method. When several measurements are made from the same homogeneous material under specified conditions, the precision of an analytical technique is the degree of scatter, or closeness of agreement, between those measurements. The accuracy and precision of the recommended LC-MS/MS method were evaluated using three levels of QC samples with six replicates and the percentage relative standard deviation (RSD) was calculated. Specificity is the capacity to evaluate the analyte in the presence of potentially predicted components. Usually, these could consist of matrix, degradants, contaminants, and so on. These are frequently degradants and impurities. There was no interference found when three duplicates of standard solutions were used for this approach. The capacity of an analytical method to produce test findings that are exactly proportionate to the amount of analyte in the sample, within a specified range, is known as linearity. Using the regression equation (Y = mx + C), the linearity was calculated and the R2 value was observed. Six different ribociclib concentrations (ranging from 5 to 100 ng/ml) were examined. Signal-to-noise ratios (S/Ns) were found to be 3:1 and 10:1. The drug reaction should be threefold higher than the noise response for LOD and tenfold higher than the noise response for LOQ. The ability of an analytical procedure to withstand slight but intentional changes in method parameters is measured as its robustness. By adjusting experimental variables such as mobile phase, flow rate, and pH, among others, the robustness of the method was examined.
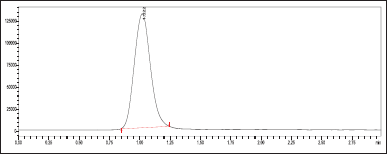 | Figure 4. MRM chromatogram for standard ribociclib. [Click here to view] |
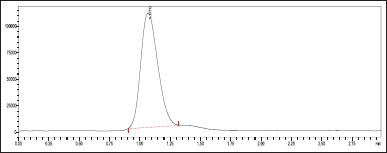 | Figure 5. MRM chromatogram for ribociclib in formulation. [Click here to view] |
 | Figure 6. Chromatogram of sample without any co-elution. [Click here to view] |
ESTIMATION OF RIBOCICLIB
The average weight of 20 tablets was determined, tablets were finely triturated and weighed. Furthermore, 50 ml of an organic solvent (acetonitrile) was added. The mixture was sonicated for 20 minutes. The solution was diluted with acetonitrile to a final volume of 100 ml, and it was filtered through a 0.45-micron filter. Following another dilution of the solution, the standard and test solutions were examined under ideal chromatographic circumstances (Table 5 and Fig. 5).
 | Table 1. Linearity for ribociclib. [Click here to view] |
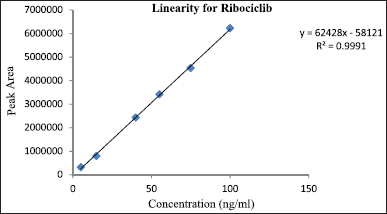 | Figure 7. Calibration curve for ribociclib. [Click here to view] |
 | Table 2. Accuracy and repeatability precision studies of ribociclib. [Click here to view] |
 | Table 3. System suitability parameters for ribociclib. [Click here to view] |
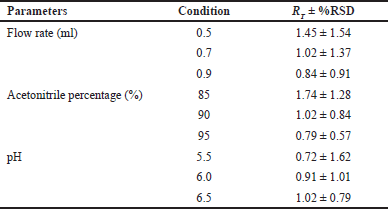 | Table 4. Robustness studies for ribociclib. [Click here to view] |
RESULTS AND DISCUSSION
In this investigation, ribociclib was developed, isolated, and validated using LC-MS/MS. Suitable and optimal conditions were used to create an easy-to-use, precise, and effective LC-MS/MS technique for ribociclib. During the screening phase of the drug: theoretical plates, tailing factor, peak area, and peak asymmetry factor were chosen as optimized conditions. During method development, the optimal chromatographic conditions were a Phenomenex C18 column (50 × 4.6 mm, 3 m) with acetonitrile and 10 mM ammonium acetate (90:10 v/v ratio) as mobile phase and a flow of 0.7 ml/min. The total run time was 3 minutes. The retention time (RT) of Ribociclib was observed to be 1.02 minutes (Fig. 4).
Specificity
There were no possible interference peaks observed during the ribociclib elution time (Fig. 6). As an outcome, the method turned out to be highly specific.
 | Table 5. Recovery studies for ribociclib. [Click here to view] |
Linearity
Ribociclib’s calibration curve was defined at six different concentrations, with an R2 value of 0.9991. Linearity concentrations of Ribociclib vary between 5 and 100 ng/ml (Table 1). The regression equation was found to be Y = 62428X − 58121 which implies by high R2 value (Fig. 7).
Precision and accuracy
To determine method accuracy, a recovery study was carried out and the ribociclib recovery percentage varied between 94.65% and 97.57% (Table 2). Repeatability precision was carried out using 3 QC sample preparations with six replicates, which were examined on the day of preparation (as intraday) and the next day (as interday). Ribociclib’s percentage RSD was found to be (1.16%–0.58%) for repeatability precision studies. These examined values are well within the acceptable limit of ±2% which is established by International Council for Harmonisation (ICH) Q2 (R2) guidelines (Table 2).
Limit of detection and quantification
The sensitivity of the analysis equipment has a significant impact on LOD and LOQ. For the same analyte, different instruments may produce varying LOD and LOQ values, even when using standard operating procedures. Accurate quantification of impurities and APIs is necessary in pharmaceutical manufacturing to ensure the potency and purity of therapeutic products. To ensure the safety and quality of the final product, low LOD and LOQ values make it possible to identify and quantify these chemicals at low concentrations. The performed LOD and LOQ were found to be 0.8 and 2.5 ng/ml for ribociclib of the proposed technique are established using the S/N.
System suitability
A system suitability study was performed in compliance with ICH standards for establishing the system suitability parameters, which are LOD, LOQ, tailing factor, and the theoretical plate (N). The findings have been determined and are well within the limits (Table 3).
Robustness
The flow rates (0.5, 0.7, and 0.9 ml/minute), pH (5.5, 6.0, and 6.5), and percentage Acetonitrile (85%, 90%, and 95%) were used in the robustness study. Individual %RSD was calculated for pH (1.62%–0.79%), flow rate (1.54%–0.91%), and acetonitrile percentage (1.28%–0.57%) for the selected conditions (Table 4). The examined values were compared and are well within the acceptable limit of ±2% which is established by ICH Q2(R2) guidelines.
CONCLUSION
A sensitive LC-MS/MS method for ribociclib has been optimized following ICH regulations. It has been found that the LC-MS/MS technique is robust, rapid, accurate, precise, and has great sensitivity and repeatability. Assessments have been made about the developed approach’s accuracy in commercial products. This is an approach to estimating ribociclib that is formulation-specific. When evaluating ribociclib, degradants do not interact or co-elute. The method utilizes a straightforward sample preparation procedure and enables rapid analysis. The reaction to linearity was linear across the operational range. The outcomes were adequate and within the parameters.
LIST OF ABBREVIATIONS
CDK: cyclin dependant kinase; ICH: International Council for Harmonisation; LC-MS/MS: liquid chromatography tandem mass spectrometry; LOD: limit of detection; LOQ: limit of quantification; QC: quality control; RSD: relative standard deviation.
ACKNOWLEDGMENT
The authors are grateful to Clearsynth, Mumbai, India, for providing a standard for this work, and the authors are grateful to Dr. M. Siva Selva Kumar for his kind assistance throughout the research process.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Wolff AC, Hammond ME, Allison KH, Harvey BE, McShane LM, Dowsett M. HER2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update summary. J Oncol Pract. 2018;14(7):437–41. CrossRef
2. Burke SM, Kamal M, Goey AK. Development and validation of a quantitative LC-MS/MS method for CDK4/6 inhibitors palbociclib, ribociclib, abemaciclib, and abemaciclib-m2 in human plasma. Ther Drug Monit. 2023;45(3):327–36. CrossRef
3. Posocco B, Buzzo M, Poetto AS, Orleni M, Gagno S, Zanchetta M, et al. Simultaneous quantification of palbociclib, ribociclib and letrozole in human plasma by a new LC-MS/MS method for clinical application. PLoS One. 2020;15(2):e0228822. CrossRef
4. Turkovic L, Bockor L, Ekpenyong O, Silovski T, Lovric M, Crnkovic S, et al. Development and validation of a novel LC-MS/MS method for the simultaneous determination of abemaciclib, palbociclib, ribociclib, anastrozole, letrozole, and fulvestrant in plasma samples: a prerequisite for personalized breast cancer treatment. Pharmaceuticals. 2022;15(5):614. CrossRef
5. Drug Information. [Online]. Available from: http://www.drugs.com (Accessed 10 July 2023).
6. Braal CL, Lam MH, Rienks T, van Tilborg CJ, Heuts W, Heijns JB, et al. Quantification of ribociclib in dried blood spots by LC–MS/MS: method development and clinical validation. J. Pharm. Biomed. Anal. 2021;201:114118. CrossRef
7. Martínez-Chávez A, Rosing H, Hillebrand M, Tibben M, Schinkel AH, Beijnen JH. Development and validation of a bioanalytical method for the quantification of the CDK4/6 inhibitors abemaciclib, palbociclib, and ribociclib in human and mouse matrices using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2019;411:5331–45. CrossRef
8. Mlinaric Z, Turkovic L, Begovic I, Nigovic B, Sertic M. Rapid capillary electrophoresis method for simultaneous determination of abemaciclib, ribociclib, and palbociclib in pharmaceutical dosage forms: a green approach. Mol. 2022;27(21):7603. CrossRef
9. Sahu AK, Jadav T, Rajput N, Sharma MK, Sengupta P. Bioanalysis by LC-MS/MS and preclinical pharmacokinetic interaction study of ribociclib and oleanolic acid. Bioanalysis. 2022;14(15):1051–65. CrossRef
10. Bao X, Wu J, Sanai N, Li J. Determination of total and unbound ribociclib in human plasma and brain tumor tissues using liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019;166:197–204. CrossRef
11. Kala A, Patel YT, Davis A, Stewart CF. Development and validation of LC–MS/MS methods for the measurement of ribociclib, a CDK4/6 inhibitor, in mouse plasma and Ringer’s solution and its application to a cerebral microdialysis study. J. Chromatogr. B. 2017;1057:110–7. CrossRef
12. Sahu AK, Goswami A, Kate AS, Sengupta P. Identification and structural characterization of potential degraded impurities of ribociclib by time of flight-tandem mass spectrometry, and their toxicity prediction. J. Pharm. Biomed. Anal. 2021;197:113933. CrossRef
13. Harron DW. Technical requirements for registration of pharmaceuticals for human use: the ICH process. Textb Pharm Med. 2013;18:447–60. CrossRef