INTRODUCTION
Fungal infections affect over a billion individuals, resulting in more than 1.5 million deaths annually. Despite the preventable nature of most fungal-related fatalities, public health authorities have largely overlooked this issue. Individuals with underlying medical conditions such as asthma, acquired immunodeficiency syndrome, cancer, organ transplantation, and corticosteroid treatments are particularly susceptible to severe fungal infections [1]. Antifungal treatments are categorized into polyenes, azoles, allylamines, and echinocandins to combat these infections. Azoles are commonly prescribed for aspergillosis; however, the emergence of antifungal resistance poses a significant challenge in the treatment of Candida and aspergillosis [2]. Voriconazole (VRC), which is a synthetic derivative of fluconazole, is a second-generation triazole drug that received approval from the US Food and Drug Administration in May 2002. This antifungal medication is administered systemically for the treatment of filamentous fungal infections [3].
VRC is a broad-spectrum antifungal medication that acts by inhibiting the cytochrome P450-dependent 14-lanosterol demethylation process, which is crucial for the synthesis of cell membrane ergosterol. It exhibits effectiveness against fluconazole-resistant Candida species and is commonly prescribed for the treatment of aspergillosis and candidiasis infections in various body parts such as the abdomen, kidney, bladder wall, wounds, and skin [4–7]. However, the available commercial forms of VRC for fungal infections primarily include oral pills, oral solutions, and intravenous injections. Although oral VRC has a bioavailability of 96%, it is generally not recommended for topical infections due to adverse effects such as visual disturbances, skin rashes, drug interactions, elevated hepatic enzyme levels, abdominal pain, nausea, and vomiting [8]. Furthermore, VRC has limited water solubility and is susceptible to extensive first-pass metabolism. Consequently, there has been growing interest in exploring topical delivery methods to overcome these limitations [9]. Currently, no topical VRC preparation is available in the market. However, extensive research has been conducted on topical delivery, the majority of which focuses on ocular delivery, while there have been very few reports on skin delivery [10]. One of the challenges in topical delivery is the need for the drug to penetrate through the stratum corneum, a crucial barrier that determines the drug’s ability to reach the basal layer of the epidermis and exert its pharmacological effects. To overcome the barrier properties of the stratum corneum, researchers have explored the use of nanocarrier-based techniques to enhance drug delivery in the topical region [11–13]. While conventional vesicles primarily function as drug carriers without any inherent biological activity, the development of vesicles that possess both carrier properties and biological activity or targeting capabilities would offer distinct advantages. Ascorbic acid, a potent antioxidant found in human plasma and cell membranes, is well-known for its antioxidant properties. Its esters, such as ascorbyl palmitate, exhibit amphiphilic characteristics and retain the antioxidant properties of ascorbic acid. Ascorbyl palmitate is widely recognized as a skin-whitening agent that promotes collagen synthesis and enhances skin elasticity. Due to its lipophilic nature, ascorbyl palmitate improves skin penetration [14,15]. Moreover, it has been shown to have skin-whitening effects by reducing skin pigmentation. Ascorbyl palmitate can form vesicles called aspasomes, which combine the carrier properties of vesicles with the added benefits of ascorbyl palmitate, making them a promising approach in drug delivery systems [16].
A formulation optimization process was conducted using a 32 factorial design. Based on the design of experiments, a suggested batch was formulated and subjected to analysis for various parameters. The formulated batch was then evaluated for its antifungal activity specifically against Candida albicans. Aspasomal formulations have the potential to effectively reduce pigmentation associated with fungal infections.
MATERIALS AND METHODS
Materials
VRC was obtained as the gift sample from MSN Laboratories Pvt. Ltd., Hyderabad. Ascorbyl palmitate was obtained from Loba Chemie Pvt. Ltd., Mumbai. Cholesterol was obtained from HiMedia Laboratories Pvt. Ltd., Mumbai. Dicetyl phosphate was obtained from Avanti Polar Lipids, INC. Methanol and Chloroform were obtained from Molychem Mumbai, Carbopol 934 Lubrizol Advanced Materials India Pvt. Ltd., India.
Strain and animals
Candida albicans strains were obtained from Dr. Prabhakar Kore Basic Science Research Center, Belagavi, Karnataka. Wistar rats of either sex (weighing 180–220 g) were provided by the KLE College of Pharmacy, Belagavi, Karnataka.
PREPARATION OF ASPASOMES
Aspasomes were prepared using the thin film hydration method. A mixture of lipids, including ascorbyl palmitate, cholesterol, and dicetyl phosphate, along with 25 mg of VRC, was dissolved in a 10 ml organic solvent mixture of chloroform and methanol (9:1 ratio) in a rounded bottom flask. The flask was placed in a rotary evaporator under reduced pressure at a temperature of 50°C, with a fixed rotation speed of 80 rpm. This process allowed the organic solvent to evaporate in around 5 to 10 minutes, resulting in a dry thin film on the inner wall of the flask. The film was then hydrated by adding 10 ml of phosphate buffer saline (PBS) (pH 7.4). To convert the multilamellar vesicles into small unilamellar vesicles, the vesicular suspension underwent probe sonication using an ultrasonic probe sonicator for 5 minutes at 20 kHz and at a temperature of 4°C. The prepared aspasomal formulations were stored in vials at refrigerated temperature until further use [16,17].
Experimental design
The optimization of the aspasomal formulation was carried out by 32 factorial designs (Design Expert 13.00). The formulation variables, ascorbyl palmitate concentration (A), and cholesterol (B) were selected as independent variables. The vesicle size (VS) (Y1) and entrapment efficiency (%EE) (Y2) were selected as the dependent variables. Based on the factorial design, 9 formulations were prepared. The formulations were coded as F1, F2, and F3…F9. Optimization refers to the evaluation of how responses are affected when two factors are simultaneously altered [16]. To optimize multiple responses simultaneously, a desirability function was employed. The combinations of values for X1 and X2 (independent variables) that resulted in the highest desirability value were chosen as the optimal requirements for formulating the VRC-loaded aspasomal dispersion.
To validate the response surface methodology design, triplicate batches of the optimized VRC-loaded aspasomal dispersion were prepared based on the desirability function parameters for VS and %EE. A statistical comparison between the observed and predicted values for VS and %EE was performed to assess the accuracy of the regression models obtained in this study. The results showed a negligible discrepancy between the observed and predicted responses, indicating that the regression models are well-fitted and reliable [18–20].
CHARACTERIZATION OF THE PREPARED ASPASOMES
Determination of VS
The size of the vesicles was analyzed using a particle size analyzer, specifically the Malvern Zetasizer. A 0.5 ml sample of the aspasomal formulation was diluted with 10 ml of deionized water and filtered through a polytetrafluroethylene (PTFE) syringe filter (Millex® Syringe Filter). The dynamic light scattering technique was used to measure the size of the vesicles. The results were reported as the mean value ± standard deviation (SD) of three replicate measurements, conducted at a temperature of 25°C ± 0.5°C.
 | Table 1. Variables utilized in 32 factorial design for VRC aspasomes formulation. [Click here to view] |
Determination of %EE
To determine the percentage of %EE of the VRC-loaded aspasomal dispersion, an indirect method was employed. One milliliter of the freshly prepared aspasomal dispersion containing VRC from each batch was subjected to centrifugation at 15,000 rpm and 4°C for 30 minutes using a KUBOTA-7000 high-capacity refrigerated centrifuge (Japan). After centrifugation, a 0.1 ml sample was taken from the supernatant and appropriately diluted with 10 ml of methanol. The resulting solution was then filtered through a PTFE syringe filter (Millex® Syringe Filter) and analyzed spectrophotometrically at 256 nm using a SHIMADZU UV-1800 spectrophotometer (Japan), with a blank aspasomal dispersion prepared similarly. This method allowed the determination of the encapsulation efficiency of the VRC within the aspasomal formulation [21].
The %EE of aspasomes was calculated by this Eq. (1).
Aspasomes morphology
The surface morphology of the optimized aspasomes was investigated using high-resolution transmission electron microscopy (HR-TEM) with a camera attachment (JSM-2100PLUS JEOL, Japan). The aspasomal dispersion was placed on a copper grid and allowed to settle for 10 minutes. After thorough drying, the samples were stained with a 2% w/v aqueous solution of uranyl acetate for image analysis.
Preparation of VRC-loaded aspasomal gel
The optimized formulation of VRC-loaded aspasomal gel was prepared using Carbopol 934. The gel was formulated by directly incorporating 1% w/v Carbopol 934 into the optimized batch of aspasomal formulation. Continuous stirring with a magnetic stirrer was carried out for 4 hours. Then, 1% methylparaben was added as a preservative. Triethanolamine was added to neutralize the pH of the gel. For comparative purposes, plain gel formulations of VRC were prepared [22,23].
The gel formulations were subjected to evaluation based on the following parameters.
Drug content
To determine the drug content, a precisely measured amount (0.4 g) of gel, containing 2 mg of VRC, was dissolved in 10 ml of methanol. The solution was then examined using a UV-Vis Spectrophotometer at a wavelength of 256 nm. The drug content of the aspasomal gel formulation was analyzed three times for accuracy.
Viscosity
The viscosity of the aspasomal gel formulations was assessed using a Brookfield viscometer. The gel was placed into the viscometer’s sample holder, and a spindle was inserted into it. The spindle was rotated at a speed of 100 rpm for 90 seconds. All the rheological experiments were conducted at room temperature. The viscosity measurement was performed three times for accuracy.
pH
The pH of the aspasomal gel formulation was determined using a pH meter. A precisely measured amount of gel (5 g) was dissolved in 50 ml of distilled water using a glass stirrer and the pH meter probe was immersed into the solution. The recorded pH values were obtained in triplicate for accuracy [24].
Spreadability
The glass slide method was used to assess the spreadability of the aspasomal gel (in triplicates). A 1 cm diameter circle was drawn in the center of the glass slide into which 0.5 g of sample was placed. A sandwich configuration was formed by placing another glass slide over the aspasomal gel. The upper plate was loaded with 25 g weight for 10 seconds and the increase in diameter was measured. Noted values were substituted in Eq. (2) to calculate spreadability [25].
In vitro diffusion study of aspasomal gel
The in vitro release of the drug was evaluated using a Franz diffusion cell with a dialysis membrane having a diffusion area of 1.77 cm2. The receptor medium used was phosphate buffer at pH 7.4 and the temperature was maintained at 37°C ± 0.5°C throughout the experiment. The dialysis membrane was pre-soaked in phosphate buffer pH 7.4 for 24 hours before the start of the experiment. The donor and receptor compartments were separated by placing a dialysis membrane between them. In the donor compartment, plain VRC gel and aspasomal gel containing an equivalent of 2 mg of VRC were placed without occlusion. The experiment was conducted for 24 hours. At predetermined time intervals (1, 2, 3, 4, 5, 6, 8, 12, and 24 hours), 0.5 ml of the sample was removed from the receptor compartment and promptly substituted with an equivalent volume of fresh receptor medium to uphold sink conditions. The withdrawn samples were analyzed at a wavelength of 256 nm using a UV-visible spectrophotometer. The cumulative drug release was calculated and a graph of % cumulative drug release versus time was plotted.
Ex vivo skin permeation study
The ex vivo drug permeation study of the prepared aspasomal gel was conducted using the skin of Wistar rats weighing 25 ± 5 g. The Franz diffusion cell with a diffusion surface area of 1.77 cm2 was used, and the receptor chamber contained PBS at pH 7.4, with a volume of 12 ml. The temperature was maintained at 37.0 °C ± 0.5 °C and the system was stirred at 50 rpm during the experiment [26,27]. The study was carried out for 12 hours. At predetermined time intervals (1, 2, 3, 4, 5, 6, 8, and 12 hours), 0.5 ml of the sample was withdrawn from the receptor chamber and immediately an equal volume of fresh receptor medium was added to maintain sink conditions. The withdrawn samples were analyzed at a wavelength of 256 nm using a UV spectrophotometer.
In vitro anti-fungal study
The anti-fungal study was performed using a fungi strain of C. albicans [28].
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
The MIC refers to the lowest concentration of an antifungal agent that prevents the growth of fungal yeast, while the MFC is the lowest concentration required to kill the fungal yeast. The MIC and MFC were determined using the Micro broth dilution method. In this method, (C. albicans) a fungal yeast, was inoculated into different concentrations of the antifungal drug and incubated for 24 hours. After incubation, the fungal viability was assessed by subculturing on agar media supplemented with a small amount of antibiotic (Ciprofloxacin 25 µl). To conduct the test, the optimized aspasomal formulation and the standard drug (VRC) were diluted into various concentrations ranging from 50 to 0.09 µg/ml in sterile Sabouraud dextrose broth. A loopful (10 µl) of C. albicans culture was inoculated into the Eppendorf tubes containing the different concentrations of VRC in Sabouraud dextrose broth. The tubes were then incubated at 37°C for 24 hours and the presence of growth or turbidity was observed. If growth was observed, a loopful of the broth from each test tube showing growth was inoculated onto Sabouraud dextrose agar plates, which were then incubated at 37°C for 24 hours. The plates were visually examined for the presence of fungal growth. By performing this procedure, the MIC and MFC values for VRC and the optimized aspasomal formulation were determined [29].
Zones of inhibition study
The in vitro antifungal activity of various formulations was assessed using the cup-plate method against C. albicans. To prepare the agar medium, 3.25 g of Sabouraud dextrose agar powder was dissolved in 50 ml of distilled water and then sterilized in an autoclave at 121°C and 15 lb. pressure for 20 minutes. The plates used for the experiment were sterilized in a hot air oven at 160°C for 1 hour before use. Subsequently, 25 ml of the Sabouraud dextrose agar solution was poured into each plate and allowed to solidify. After solidification, the plates were streaked with C. albicans suspension. Using a cork borer, four wells, each with a diameter of 8 mm, were made in the agar medium on each plate. Each well was filled with 25 µl of different substances: blank formulation (negative control), optimized formulation equivalent to 25 µg of the drug (test sample), marketed product equivalent to 25 µg of the drug (positive control), and another negative control containing PBS of pH 7.4. Following this, the plates were stored at room temperature for 24 hours, after which the zone of inhibition around each well was measured. This method allowed the assessment of the antifungal activity of the different formulations by observing the zones of inhibition formed around the wells containing the formulations [30].
Skin irritancy study
Ethical requirements for handling animals: Following written approval from CPCSEA and IAEC. (Reg. No.221/Po/Re/S/2000/CPCSEA in its meeting dated 03/12/2022), this study was initiated. All relevant experiments were carried out at Animal House, KLE College of Pharmacy, Belagavi. KLE Academy of Higher Education and Research Belagavi, Karnataka 590010. A skin irritancy study was performed to assess any potential skin irritation caused by the application of aspasomal gel on Wistar rats. The rats were divided into two groups, each containing six rats. In Group I, the rats were given aspasomal gel without the drug, while in Group II, the rats received the application of aspasomal gel containing the drug. To prepare for the test, the dorsal part of the rats was carefully shaved 24 hours before the study to avoid any peripheral damage. After application, visible changes in the skin, particularly erythema (redness), were observed and recorded at 24, 48, and 72 hours post-application. The severity of skin irritancy was classified into different scores: 0 indicated no erythema, 1 represented slight erythema with a light pink color, 2 denoted moderate erythema with a dark pink hue, 3 indicated moderate to severe erythema with light redness, and 4 represented severe erythema with extreme redness. This assessment allowed the researchers to evaluate and characterize the potential irritancy of aspasomal gel when applied to the skin of Wistar rats [31].
Statistical analysis
Using the Design-Expert® software (version 13 Stat-Ease Inc., USA), the VRC-loaded aspasomal formulation was optimized. The significance of the model and model terms was confirmed using the analysis of variance (ANOVA).
Stability studies
Stability studies of VRC-loaded aspasomal formulation were performed according to ICH guidelines for 90 days at two different temperatures 4°C ± 2°C and 25°C ± 2°C. VRC-loaded aspasomal formulation was evaluated for VS and %EE [32–34].
RESULTS
Determination of VS
The average VS of the prepared aspasomes formulation ranged between 180 and 404 nm (Table 2). The polynomial equation produced by the Design-Expert® Software demonstrates how independent variables have an impact on VS.
Vesicle size (VS) = +264.67+ 85.00*A+ 29.17*B.(3)
A and B stand for the concentration of ascorbyl palmitate and cholesterol (% w/w), respectively.
Figure 2 illustrates how ascorbyl palmitate (A) and cholesterol (B) affect VS. The graph and Eq. (3) demonstrate that the concentration of ascorbyl palmitate and cholesterol was directly proportional to VS. VS was increased as a result of the increased concentration of ascorbyl palmitate and cholesterol, which could potentially contribute to an augmented thickness of the bilayer [16].
 | Figure 1. Graph representing the mean VS of 151.36 nm for optimized formulation [24]. [Click here to view] |
 | Table 2. Results of the responses of experimental 32 factorial design for optimization of VRC-loaded aspasomes. [Click here to view] |
Formulation
Entrapment efficiency (%EE)
%EE is a crucial factor in determining the aspasomes nanoformulation’s ability to deliver drugs. The %EE was found to be in the range of 30.5%–83.77%, respectively, as shown in (Table 2). The polynomial equation illustrates how various independent variables affect the %EE of VRC.
Entrapment efficiency (%EE)= +64.27–1.16*A–5.79*B–4.43*AB–14.84*A2+7.88*B2. (4)
Both the graph (Fig. 3) and Eq. (4) provide evidence that the concentration of ascorbyl palmitate and cholesterol exhibited an inverse relationship with the percentage of encapsulation efficiency (%EE). The high values of %EE for the drug can be attributed to its lipophilic properties, which enable its successful integration within the hydrophobic bilayer of aspasomes. The stability of the formulation could be attributed to the effective concentration of cholesterol [16]. The above %EE study results indicate that higher concentrations of ascorbyl palmitate lead to elevated %EE. While increased levels of cholesterol result in reduced %EE.
All the formulation batches of aspasomes were analyzed using Design Expert® Software, fitting the obtained results to various polynomial models to identify the most suitable model. The findings indicated that the linear model was the best-fit model. The adjusted R2 and predicted R2 values showed reasonably close agreement for both response variables, with a difference of less than 0.2. The ANOVA results of the suggested linear model demonstrated adequate precision in terms of the signal-to-noise ratio. The precision values were determined as 11.955 for VS and 20.763 for percentage encapsulation efficiency (%EE), surpassing the desirable threshold of 4.
 | Figure 2. 3D response surface and contour plot for VS of aspasomes. [Click here to view] |
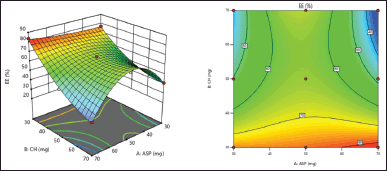 | Figure 3. 3D response surface and Contour plot for %EE of aspasomes. [Click here to view] |
Optimization of aspasomal formulation
To obtain the optimized formulation, a desirability function was employed, considering the goals of minimizing VS and maximizing the percentage of %EE. Through this approach, the optimal formulation was determined, which consisted of 36.940 mg of ascorbyl palmitate and 30.00 mg of cholesterol. This combination resulted in a maximum combined desirability value of 0.929. Figure 1
Evaluation of optimized aspasomal formulation
The optimized batch was evaluated in triplicates to ensure reliability and consistency. The average values for the VS, %EE, polydispersity index (PDI), and zeta potential were determined as 171.36 ± 2.3 nm, 80.77% ± 1.7%, 0.33 ± 0.05, and −63.37 ± 1.1 mV, respectively.
Morphology of aspasomes
HR-TEM (JSM-2100PLUS JEOL, Japan.) imaging was used to analyze and confirm the surface morphology and structure of aspasomes. The HR-TEM image (Fig. 4) showed that the VRC-loaded aspasomes were uniformly spherical in shape and VS measured in nm.
Evaluation of aspasomal gel
The optimized formulation of VRC-loaded aspasomal gel was prepared using Carbopol 934. Analyzed the formulated aspasomal gel for drug content, spreadability, viscosity, and pH and the results obtained are displayed in Table 3.
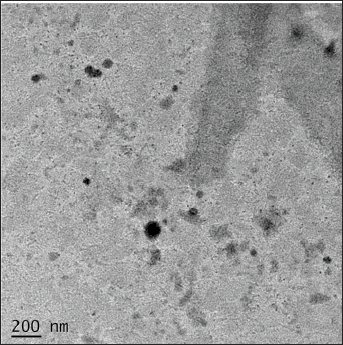 | Figure 4. HR-TEM image of aspasomes. [Click here to view] |
 | Table 3. Evaluation of aspasomal gel. [Click here to view] |
Diffusion study
The in vitro drug diffusion study of prepared aspasomal gel and plain VRC gel was performed on the Franz diffusion cell. The in vitro drug release profile of optimized ASP Gel v/s plain VRC gel is shown in Figure 5. The optimized ASP Gel and plain VRC gel are showing 94.098% and 79.29% of drug release at 24 hours. This study was performed in triplicates and the mean was considered.
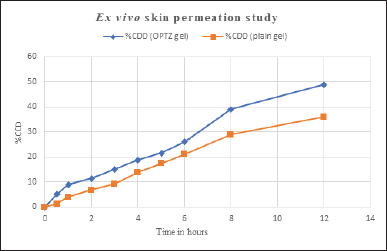
Ex vivo skin permeation study
A permeation study was conducted for 12 hours to compare the drug permeation from the optimized aspasomal gel and plain gel. The results suggested that the aspasomal gel exhibited a drug permeation rate of 48.87%, whereas the plain gel showed a lower permeation rate of 35.91% (Fig. 6). The permeation profiles demonstrate that the aspasomal
gel facilitated a notably greater amount of VRC permeation through rat skin compared to the plain VRC gel. This finding underscores the effectiveness of the prepared aspasomes. The improved permeation can be attributed to the structure and small size of the aspasomes, which played a crucial role in enhancing drug permeation. In addition, the optimal ratio of cholesterol to surfactant contributed to increased penetration by enhancing the elasticity of the lipid vesicles. The study’s findings suggest that these vesicles can partition within the lipid layers of the skin due to their lipophilic nature. Furthermore, their amphiphilic properties provide a stable environment for VRC, promoting its effective delivery [31].
In vitro anti-fungal study
MIC and MFC
The MIC and MFC were evaluated on Sabouraud dextrose agar plates by streaking previously diluted Sabouraud dextrose broth with different concentrations of VRC and C. albicans culture. The lowest concentration of a drug (VRC) that inhibited the growth of fungal colonies on agar plates after 24 hours of incubation is termed the MIC. The concentration that did not show fungal colonies growing on agar plates was considered the MFC. Both the formulation and the standard drug inhibited fungal growth at a concentration of 3.125 µg/ml, and no fungal growth of colonies was observed at a concentration of 6.25 µg/ml for both the formulation and the standard drug. As a result, the MIC is 3.125 µg/ml and the MFC is 6.25 µg/ml.
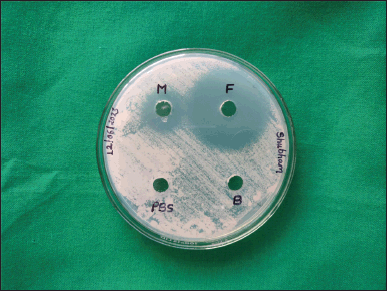
Zones of inhibition study
The in vitro antifungal activity of the optimized aspasomal formulation was evaluated using the cup plate method against C. albicans. The graphical representation in Figure 7. illustrates the zones of inhibition for the optimized formulation (F), a commercially available fluconazole gel (M), blank formulation (B), and PBS (pH 7.4). The results indicate that both the optimized aspasomal formulation and the marketed formulation exhibited notable zones of inhibition in comparison to the blank formulation and PBS (pH 7.4). The observed zones of inhibition diameters are presented in Table 4. The results indicate that the optimized formulation of VRC-loaded aspasomes exhibited a larger anti-fungal activity area (3.0 cm) compared to the marketed formulation, blank formulation, and negative control. These findings demonstrate the sensitivity of the optimized aspasomal formulation toward C. albicans and confirm its effective anti-fungal activity against the pathogen.
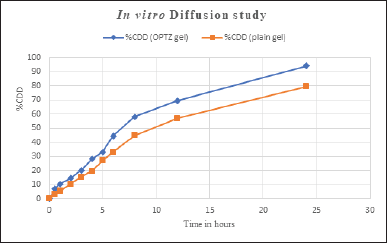 | Figure 5. In vitro diffusion study of aspasomal gel. [Click here to view] |
 | Figure 6. Ex vivo permeation study of aspasomal gel. [Click here to view] |
 | Table 4. In vitro antifungal study (Zone inhibition) [Click here to view] |
 | Figure 7. In vitro antifungal study (zone of inhibition). [Click here to view] |
 | Table 5. Evaluation of skin irritation based on the Draize scoring system. [Click here to view] |
 | Figure 8. In vivo skin irritancy study. [Click here to view] |
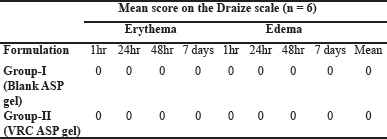
Skin irritancy study
The possibility of skin irritation triggered by the topical application of aspasomal gel was visually evaluated on rat skin. Both the VRC-loaded aspasomal gel and the blank aspasomal gel showed no signs of erythema (redness) with all erythema scores recorded as 0 (Table 5). In addition, no edema (swelling) was observed in any of the animals (Fig. 8). Based on these observations, it can be concluded that the aspasomes are safe and non-irritating to rat skin.
Stability study
The %EE and VS were evaluated after day 1, 30 days, and 90 days of different storage conditions. The impact of different storage conditions on the VS and %EE (%EE) of aspasomal vesicles was investigated. The findings indicated that the optimized aspasomal formulation exhibited no significant changes in VS and %EE when stored at room temperature or in a refrigerated environment for one month. However, after three months of storage, the %EE of the aspasomal formulation decreased from 83.43% to 74.64% at room temperature and 82.06% under refrigerated conditions. In addition, the VS of the aspasomal formulation increased from 151.36 to 169.47 nm at room temperature and to 154.01 nm when refrigerated (Table 6). Notably, the changes in %EE and VS were not significant when stored under refrigerated conditions. These results suggest that the optimized aspasomal formulation exhibits greater stability when stored at refrigerated temperature (below 20°C) compared to room temperature [35].
 | Table 6. Outcomes of the Stability Analysis [Click here to view] |
DISCUSSIONS
In this study, we explored the impact of varying concentrations of ascorbyl palmitate and cholesterol on the size of aspasomes, revealing their significant influence on VS. Formulation F7 (Table 2) demonstrated that an elevated concentration of ascorbyl palmitate led to an increase in VS, likely attributed to an enhanced bilayer thickness [16]. The lipophilic nature of VRC contributed to its successful integration within the hydrophobic bilayer of aspasomes, resulting in a high %EE. The concentration of ascorbyl palmitate and cholesterol showed an inverse relationship with %EE, suggesting the need for an optimal balance between the two components. The desirability function helped determine the optimal formulation by considering the goals of minimizing VS and maximizing %EE (Table 1) [19]. The identified formulation, with specific concentrations of ascorbyl palmitate and cholesterol, achieved the highest combined desirability value. The optimized formulation exhibited desirable characteristics, such as small VS, high %EE, low PDI, and suitable zeta potential. These properties are crucial for effective drug delivery and stability of the aspasomal formulation. The HR-TEM image confirmed that the VRC-loaded aspasomes had a uniform spherical shape, aligning with their intended structure and size as shown in Figure 4. The formulated aspasomal gel was evaluated for various parameters, including drug content, spreadability, viscosity, and pH, ensuring its suitability for topical application. The optimized aspasomal gel demonstrated a higher drug release rate than the plain VRC gel, indicating the enhanced delivery of VRC from the aspasomal formulation as shown in Figure 5.
The aspasomal gel showed notably increased drug permeation through rat skin in contrast to the plain gel (Fig. 6), attributed to the structure, small size of aspasomes, and the optimal ratio of cholesterol to surfactant (Dicetyl phosphate). The optimized aspasomal formulation and standard drug effectively inhibited fungal growth against C. albicans, as MIC and MFC values indicated. The optimized formulation exhibited a larger zone of inhibition compared to the marketed formulation, blank formulation, and negative control, confirming its anti-fungal activity. The absence of erythema and edema on rat skin after applying the aspasomal gel indicated its safety and non-irritating nature. The optimized aspasomal formulation showed stability in terms of VS and %EE when stored at room temperature or in a refrigerated environment for one month. After three months, a slight decrease in %EE and an increase in VS was observed, suggesting better stability under refrigerated conditions.
The results demonstrate the successful formulation and evaluation of optimized aspasomes for VRC delivery. The aspasomal gel exhibited enhanced drug permeation, effective anti-fungal activity, and satisfactory stability, making it a potential candidate for targeted and efficient drug delivery in fungal infections.
CONCLUSION
The successful preparation of VRC-loaded aspasomal gel was achieved by the thin film hydration method. Ascorbyl palmitate, an amphiphilic compound, formed active vesicles in combination with cholesterol and dicetyl phosphate. These aspasomes were incorporated into Carbopol 934 gel for convenient topical application. Drug diffusion and ex vivo skin permeation studies demonstrated significantly higher levels in the aspasomal gel over 24 and 12 hours, respectively, compared to the plain gel. Aspasomes, known for antioxidant properties, skin-whitening, and skin-smoothing capabilities, are suitable for treating fungal infections. An in vivo skin irritancy study confirmed the non-irritant nature of the aspasomal gel, with no signs of edema or erythema on rat skin. Compared to a marketed formulation, the aspasomal formulation exhibited enhanced in vitro anti-fungal activity against C. albicans. A three-month stability study affirmed the formulations’ stability under refrigerated conditions. Optimized VRC-loaded aspasomes offer a promising alternative for delivering drugs in treating skin fungal infections. This innovative drug delivery system shows potential compared to conventional methods.
ACKNOWLEDGMENT
The authors express their sincere gratitude to MSN Laboratories Pvt. Ltd. in Hyderabad for generously providing them with a gift sample of voriconazole for their research work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethic Committee (IAEC) of KLE College of Pharmacy, KLE Academy of Higher Education and Research Belagavi, Karnataka with approval number 221/Po/Re/S/2000/CPCSEA on dated 03/12/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCE
1. Bongomin F, Gago S, Oladele RO, Denning, DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi. 2017;3(4):57. CrossRef
2. Toyotome T, Hagiwara D, Takahashi H, Watanabe A, Kamei K. Emerging antifungal drug resistance in Aspergillus fumigatus and among other species of Aspergillus. Curr Fungal Infect Rep. 2018;12:105–11. CrossRef
3. Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. BioMed Res Int. 2013;5(8):258. CrossRef
4. Song SH, Lee KM, Kang JB, Lee SG, Kang MJ, Choi YW. Improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. Chem Pharm Bull. 2014;62(8):793–8. CrossRef
5. Sahu RK, Salem-Bekhit MM, Bhattacharjee B, Almoshari Y, Ikbal AMA, Alshamrani M, et al. Mucormycosis in Indian COVID-19 patients: insight into its pathogenesis, clinical manifestation, and management strategies. Antibiotics. 2021;10(9):1079. CrossRef
6. Mukherjee PK, Wang M. Antifungal drug resistance: significance and mechanisms. Antifungal Ther. 2010;7(5):63–86.
7. Hossain CM, Ryan LK, Gera M, Choudhuri S, Lyle N, Ali KA et al. Antifungals and drug resistance. Encyclopedia. 2022;2(4):1722–37.
8. Song CK, Balakrishnan P, Shim CK, Chung SJ, Chong S, Kim DD. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Coll Surf B Biointerfaces. 2012;92:299 –304. CrossRef
9. Johnson LB. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36(5):630–7. CrossRef
10. Waghule T, Rapalli VK, Singhvi G, Manchanda P, Hans N, Dubey SK, et al. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol. 2019;52:303–15. CrossRef
11. Pandit J, Garg M, Jain NK. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J Liposome Res. 2014;24(2):163–9. CrossRef
12. El Zaafarany GM, Awad GA, Holayel SM, Mortada ND. Role of edge activators and surface charge in developing ultradeformablevesicles with enhanced skin delivery. Int J Pharm. 2010;397(1–2):164–72. CrossRef
13. Zaid Alkilani A, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–70. CrossRef
14. Ravetti S, Clemente C, Brignone S, Hergert L, Allemandi D, Palma, S. Ascorbic acid in skin health. Cosmetics. 2019;6(4):58. CrossRef
15. Gosenca M, Obreza A, Pecar S, Gašperlin M. A new approach for increasing ascorbyl palmitate stability by addition of non-irritant co-antioxidant. AAPS PharmSciTech. 2010;11:1485–92. CrossRef
16. Shinde G, Desai P, Shelke S, Patel R, Bangale G, Kulkarni, D. Mometasone furoate-loaded aspasomal gel for topical treatment of psoriasis: formulation, optimization, in vitro and in vivo performance. J Dermatol Treat. 2022;33(2):885–96. CrossRef
17. Gopinath D, Ravi D, Rao BR, Apte SS, Renuka D, Rambhau D. Ascorbyl palmitate vesicles (aspasomes): formation, characterization, and applications. Int J Pharm. 2004;271(1–2):95–113. CrossRef
18. Khuri AI, Conlon M. Simultaneous optimization of multiple responses represented by polynomial regression functions. Technometrics. 1981;23(4):363–75.
19. Pal S, Gauri SK. A desirability functions-based approach for simultaneous optimization of quantitative and ordinal response variables in industrial processes. Int J Eng Sci Technol, 2018;10(1):76–87. CrossRef
20. Gohel MC, Parikh RK, Aghara PY, Nagori SA, Delvadia RR, Dabhi MR. Application of simplex lattice design and desirability function for the formulation development of mouth dissolving film of salbutamol sulphate. Current Drug Deliv. 2009;6(5):486–94. CrossRef
21. Patil RP, Pawara DD, Gudewar CS, Tekade AR. Nanostructured cubosomes in an in situ nasal gel system: an alternative approach for the controlled delivery of donepezil HCl to brain. J Liposome Res. 2019;29(3):264–73. CrossRef
22. Gull A, Ahmed S, Ahmad FJ, Nagaich U, Chandra A. Hydrogel thickened microemulsion; a local cargo for the co-delivery of cinnamaldehyde and berberine to treat acne vulgaris. J Drug Deliv Sci Technol. 2020;58:101835. CrossRef
23. Tanwar YS, Jain AK. Formulation and evaluation of topical diclofenac sodium gel using different gelling agent. Asian J Pharm Res Health Care, 2012;4(1):1–6.
24. Gaikwad VL, Yadav VD, Dhavale RP, Choudhari PB, Jadhav SD. Effect of carbopol 934 and 940 on fluconazole release from topical gel formulation: a factorial approach. J Current Pharm Res. 2012;2(2):487.
25. Roachman MF, Marviani T. Formulation of coenzyme Q10 liquid foundation with a variations linseed oil as the oil phase. Int Halal Sci Technol Conf. 2021;1(1):105–15. CrossRef
26. Aboul-Einien MH, Kandil SM, Abdou EM, Diab HM, Zaki MS. Ascorbic acid derivative-loaded modified aspasomes: formulation, in vitro, ex vivo and clinical evaluation for melasma treatment. J Liposome Res. 2020;30(1):54–67. CrossRef
27. Shah MK, Azad AK, Nawaz A, Ullah S, Latif MS, Rahman H, et al. Formulation development, characterization, and antifungal evaluation of chitosan NPs for topical delivery of voriconazole in vitro and ex vivo. Polymers. 2021;14(1):135. CrossRef
28. Premkumar J, Ramani P, Chandrasekar T, Natesan A, Premkumar P. Detection of species diversity in oral candida colonization and anti-fungal susceptibility among non-oral habit adult diabetic patients. J Nat Sci Biol Med. 2014;5(1):148. CrossRef
29. Owuama CI. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr J Microbiol Res. 2017;11(23):977–80. CrossRef
30. Nayak D, Tawale RM, Aranjani JM, Tippavajhala VK. Formulation, optimization, and evaluation of novel ultra-deformable vesicular drug delivery system for an anti-fungal drug. AAPS PharmSciTech. 2020;21:1–10. CrossRef
31. Khalil RM, Abdelbary A, Arini SK, Basha M, El-Hashemy HA, Farouk F. Development of tizanidine loaded aspasomes as transdermal delivery system: ex-vivo and in-vivo evaluation. J Liposome Res. 2021;31(1):19–29. CrossRef
32. Freitas C, Müller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Euro J Pharm Biopharm. 1999;47(2):125–32. CrossRef
33. Guideline IH. Stability testing of new drug substances and products. Q1A (R2), current step. 2003;4:1–24.
34. Frøkjaer S, Hjorth EL, Wørts O. Stability testing of liposomes during storage. In Liposome technology. Boca Raton, FL: CRC Press; 2019, pp. 235–45.
35. Du Plessis J, Ramachandran C, Weiner N, Müller DG. The influence of lipid composition and lamellarity of liposomes on the physical stability of liposomes upon storage. Int J Pharm. 1996;127(2):273–8. CrossRef