INTRODUCTION
In many countries, especially tropical countries, infection by pathogenic bacteria requires special attention. Therapeutic drug monitoring (TDM) of antibiotics in treating infections is an important aspect of achieving therapeutic goals. TDM carried out by determining the correct dose and administration time can support the achievement of the effectiveness of therapy. It is known that the concentration of the drug in the plasma will determine the amount of drug that reaches the receptor and gives effect. In some cases of critical illness, offering more than one antibiotic simultaneously, even at different hours, can occur.
Cefotaxime antibiotic (Fig. 1a) is a third-generation cephalosporin antibiotic that can be used in a single dose or in combination with fluoroquinolone class antibiotics such as ciprofloxacin to treat infections in the urinary tract and respiratory tract. Cefotaxime is known to be effective in meningitis caused by Haemophilus influenzae, penicillin-sensitive Streptococcus pneumoniae, and Neisseria meningitides [1]. The peak concentration in the serum in the administration of cefotaxime 1 g infusion was reached after 30 minutes with a peak level of 41.1 μg/ml, and after 4 hours, the serum level was 1.5 μg/ml, the elimination half-life of cefotaxime was relatively fast so that the administration of cefotaxime intramuscularly or intravenously need to be repeated every 4–6 hours [2].
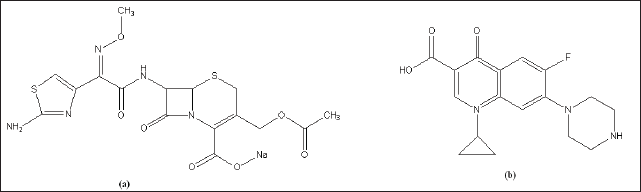 | Figure 1. Chemical structure of cefotaxime (a) and ciprofloxacin (b). [Click here to view] |
Ciprofloxacin (Fig. 1b) is a fluoroquinolone class of antibiotics that has been known to have activity for the treatment of bacterial infections of Staphylococcus aureus, Streptococcus pneumonia, Escherichia coli, and Haemophilus influenza dan Klebsiella pneumonia. Pharmacokinetic data of ciprofloxacin include binding to plasma proteins ranging from 20% to 40%, oral bioavailability of 75%, elimination half-life of 4 hours, and renal clearance of 300 ml/minute [3].
Various methods for determining cefotaxime concentration have been developed as a single analyte or simultaneously with other cephalosporin antibiotics. The instruments used also vary, namely spectrophotometry UV [4], HPTLC [5], HPLC-UV [6–12], UHPLC-MS/MS [13,14], LC-MS [15], and UPLC-MS/MS [16].
Several studies have successfully validated the analytical methods for determining ciprofloxacin levels in various matrices where the equipment used includes HPLC-UV [17–24] and HPTLC [25,26].
Based on several previous studies, it is known that simultaneous assay of several antibiotics is only for antibiotics from the same group as the cephalosporin group, either generation 1, 2, or 3, or for the fluoroquinolones group. However, until now, the development of analytical methods that determine different antibiotic classes simultaneously has never been carried out where it is known that the administration of combinations of different antibiotic classes for certain diseases can occur. The availability of a validated method for determining the levels of cefotaxime, which are third-generation cephalosporins and ciprofloxacin, which are fluoroquinolone antibiotics simultaneously, has never been developed.
The equipment available in various hospitals and clinical laboratories that can be used to help determine cefotaxime and ciprofloxacin drug levels to support TDM certainly requires a validated analytical method that is in accordance with the available equipment.
Developing an analytical method that can simultaneously determine the levels of cefotaxime and ciprofloxacin in human plasma requires special attention considering the presence of the two analytes to be measured in small quantities. The HPLC–UV method is one of the analytical methods for determining cefotaxime and ciprofloxacin levels in human plasma, considering that UV HPLC detectors can provide good selectivity and sensitivity for assaying levels in biological samples where drug concentrations are known to be measured in small quantities.
The bioanalytical method for determining cefotaxime and ciprofloxacin levels simultaneously with HPLC-UV that has been developed is expected to be able to assist TDM of the use of these two antibiotics in patients either on single antibiotics therapy or a combination of both in certain cases of infection.
MATERIALS AND METHODS
Chemicals and reagents
The materials used were cefotaxime sodium (Sigma-Aldrich, Singapore), ciprofloxacin (Abcam, UK), levofloxacin (Sigma-Aldrich, Singapore), methanol (MeOH) and acetonitrile (ACN) HPLC grade (Tedia, USA), potassium dihydrogen phosphate (E-Merck, Germany), distilled water (PT. Jayamas Medica Industri, Indonesia), orthophosphate acid 85% (E-Merck, Germany), phosphate-buffered saline (PBS) (Vivantis Technologies Sdn Bhd, Malaysia), and blank human plasma that was obtained from the Indonesian Red Cross in Yogyakarta-Indonesia.
Chromatographic conditions
The system used is high-performance liquid chromatography using L-2000 Hitachi with an L-2130 pump and equipped with a Hitachi L-2420 UV-Vis detector. The LC system used chromatographic column Luna Phenomenex® C18 (250 × 4.6 mm i.d; 5μm). The mobile phase consists of an isocratic mixture of phosphate buffer 0.02 M (pH 3.0), ACN, and MeOH (80:12:8, v/v). The mobile phase was filtered through with a 0.45 μm nylon membrane filter (Micro-Lab Scientific, Hongkong) and degassed ultrasonically before use. In total, 20 μl of samples were injected into the systems with a flow rate maintained at 1.0 ml/minute, with UV detection at 280 nm.
Preparation of standard solutions
The stock solution of 1,000 μg/ml of cefotaxime sodium was prepared in PBS. The stock solution of levofloxacin (as an internal standard) and ciprofloxacin was prepared by dissolving separately using distilled water until a final concentration of 1,000 μg/ml was reached. Furthermore, the stock solutions of cefotaxime were dissolved using PBS to obtain various concentration in the range of 2–100 μg/ml (as working solutions) and ciprofloxacin working solutions (1–15 μg/ml) was prepared by diluting ciprofloxacin stock solutions with distilled water. The concentration of levofloxacin working solutions was prepared at 50 μg/ml.
Sample preparation
An aliquot of 500 μl of blank plasma was spiked with an appropriate concentration of cefotaxime and ciprofloxacin working solutions to obtain calibration standard concentrations at range 0.2–10 μg/ml and 0.2–5 μg/ml respectively, then 50 μl of levofloxacin working solution (50 μg/ml) to the sample was pipetted. Homogenize the plasma sample for 30 seconds, followed by deproteinization using 100 μl of 10% acetic acid solution and 500 μl of ACN, vortex for 2 minutes, and centrifuge at 10,000 rpm for 10 minutes. The supernatant was taken and put into separate vials; the deproteinization procedure was repeated two times; all supernatants separated at each stage were then combined in one vial. Evaporate the supernatant with heat airflow until dry. The residue was added with 500 μl of mobile phase and filtered using a 0.22 μm membrane syringe filter before being injected into the HPLC systems.
Wavelength determination
In this study, the determination of the appropriate wavelengths for simultaneously determining cefotaxime and ciprofloxacin levels was carried out with HPLC-UV, which was initiated by scanning the wavelengths of cefotaxime, ciprofloxacin, and internal standard levofloxacin using a spectrophotometer-UV in the range of 200–400 nm.
System suitability test
System suitability tests were carried out to determine the best chromatographic systems for analysis. A total of six replicate samples containing cefotaxime and ciprofloxacin analytes were analyzed using the method developed. The chromatographic parameters observed were % relative standard deviation (%) of retention time, asymmetry factor, and the number of theoretical plates and resolution.
Validation parameters
Method validation refers to standard guidelines set, including selectivity, linearity, accuracy, precision, lower limit quantification (LLOQ), stability, and dilution integrity.
Selectivity
The selectivity test was carried out by comparing blank sample chromatograms with the spiked samples. Selectivity is proven by using six different blank samples to be analyzed and evaluated for the presence of interfering compounds. Observe the presence of chromatogram interference around the retention time of cefotaxime, ciprofloxacin, and levofloxacin. Evaluate the separation of the peaks for the three compounds studied against plasma blanks.
Calibration curve and linearity
A calibration curve is needed to find the relationship between the nominal analyte concentration to the response of the instrument. The calibration curve in this study uses the same biological matrix as the study sample, namely human plasma. The calibration curve range is made using a minimum of six concentration levels, including LLOQ, which is the lowest concentration of the analyte that is still quantified, and upper limit of quantification (ULOQ), which is the highest concentration of the calibration curve.
A calibration curve should be prepared by preparing blank plasma, zero samples (blank plasma added to an internal standard without analyte), and at least six different concentration levels to find linearity values (including ULOQ and LLOQ). The concentration series used to evaluate linearity in determining cefotaxime levels ranged from 0.2 to 10 μg/ml, while the concentration of ciprofloxacin calibration curve levels from 0.2 to 5.0 μg/ml. An aliquot of 50 μl of levofloxacin solutions (50 μg/ml) was added to each sample concentration on the calibration curve. The ratio of the peak area of the analyte to the internal standard was plotted against the correlated measured concentration. Linearity is determined based on the calculation of the correlation coefficient. The percentage of error recovery at each concentration must be ± 20% (LLOQ) and ±15% at the other concentration on the calibration curve [27].
Accuracy and precision
Accuracy and precision are determined at four different concentration levels: LLOQ, low QC, medium QC, and high QC by five replicates at each concentration level. LLOQ is the lowest concentration of analyte that can still be measured and meets the criteria for accuracy and precision with an acceptable %CV ≤ 20%. The concentration for low QC is 3 × LLOQ, medium QC (concentration levels are in the range of 30%–50% of the calibration curve), and high QC is the concentration set at 75% of the highest concentration on the calibration curve [27].
The concentration of cefotaxime for determining accuracy and precision in a row LLOQ, low QC, medium QC, and high QC is 0.2, 0.6, 5.0, and 7.5 μg/ml, whereas, for ciprofloxacin, it was respectively 0.2; 0.6; 2.5 and 3.75 μg/ml. Replicate five times at each concentration level. Accuracy is determined by comparing the measured concentration to the actual concentration of the analyte and is expressed in percent deviation or percent error.
The acceptable criteria for accuracy, expressed as a percent error from the average measured concentration to the actual concentration, is in the range of ±15%, except for the LLOQ, which is ≤20%. The precision of the method is evaluated under terms of repeatability where the acceptable % CV does not exceed 15% at each concentration level, except at LLOQ, which does not exceed 20%.
Stability
Short term stability
A stability test should be carried out to ensure that each step in sample preparation and sample analysis, including subsequent storage conditions, does not affect the concentration of the analyte. Stability testing was carried out using low and high QC samples, where each concentration level was replicated three times.
Freeze and thaw stability
Low and high QC samples were stored in the freezer, then allowed to thaw at room temperature. After complete thawing, store the sample back in the freezer. The number of repetitions of the freezing and thawing process is adjusted to the sample analysis procedure.
The mean concentration at each level should be within ±15% of the nominal concentration.
Stability testing was carried out on human plasma samples after they were stored for 24 hours in the freezer.
Carryover
Carryover is carried out by injecting a blank plasma sample after injection on the highest concentration sample on the calibration curve. The peak area of the analyte after injection of the highest concentration of sample does not exceed 20% of the peak area of the LLOQ and 15% of the peak area of the internal standard.
Dilution integrity
Dilution integrity is a procedure to ensure that the procedure does not affect the measured concentration of the analyte and still meets the parameters of accuracy and precision. This procedure is carried out by preparing a concentration analyte in plasma that is higher than the highest concentration on the calibration curve, then adding a plasma blank until the measured concentration is within the range of the calibration curve. The mean accuracy of the dilution sample QC must be within ±15% of the nominal concentration and the precision (%CV) <15%.
RESULT AND DISCUSSION
Determination of drug concentration in a biological matrix that is present in small quantities requires a series of steps to separate the analyte from the other component, which in this case is the plasma protein. For this reason, optimization has been carried out regarding the deproteinization method, which is able to separate analytes from the interfering components. Some solvents that can help the deproteinization process include acids or bases and organics solvents such as ACN and MeOH.
In this study the optimization of the deproteinization procedure was carried out by varying the amount of solvent used, the composition of the solvent for extraction, and the number of repetitions of extraction which was able to provide the best measurement results.
The extraction method that has been successfully developed related to the deproteinization technique uses 10% acetic acid solution and ACN which is relatively easily available and proven to be able to separate cefotaxime and ciprofloxacin from the other components in plasma. Samples that have been dried can be stored in the refrigerator until it is time to measure levels of HPLC–UV.
Wavelength determination
Based on the overlay of UV spectrum patterns obtained for three compounds, it appears that at a wavelength of 280 nm, the UV spectral of cefotaxime and levofloxacin overlap at the same point (280 nm), and at that wavelength, ciprofloxacin gives an absorption around the maximum (Fig. 2). Therefore, a wavelength of 280 nm was chosen to simultaneously determine cefotaxime and ciprofloxacin levels with internal standard levofloxacin.
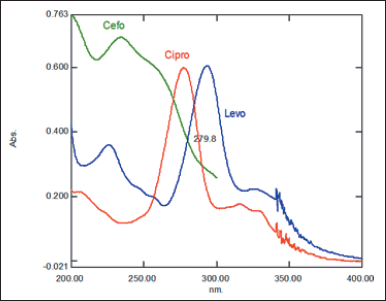 | Figure 2. UV Spectrum of cefotaxime (Cefo), ciprofloxacin (Cipro) and levofloxacin (Levo). [Click here to view] |
System suitability
The appropriate mobile phase is selected by optimizing a series of solvent compositions, pH of the mobile phase, and flow rate, which can provide good separation in a short time. The chromatographic system parameters evaluated were retention time, asymmetric factor, number of theoretical plates (N), and resolutions.
When optimizing the composition of the mobile phase, it was found that the use of a mixture of two solvents as the mobile phase, namely, phosphate buffer (0.02 M) pH 3.0 and ACN, was not able to separate ciprofloxacin analyte and levofloxacin internal standard. Unlike the case when using three solvents with the composition of phosphate buffer pH 3.0 (0.02 M), ACN and MeOH can give a good result.
The selected chromatographic system used a mixture of mobile phases with successive composition, namely, buffer phosphate pH 3.0 (0.02 M), ACN dan MeOH (80:12:8 v/v), flow rate 1.0 ml/minute at a wavelength of 280 nm. The separation of cefotaxime and ciprofloxacin in human plasma has been successfully carried out with the result that meets the standards required for the bioanalytical method, namely % CV retention time and peak area ≤ 2%, asymmetry factor ≤2, resolution >2.00 and the number of theoretical plates (N) > 2,000. The result of the system suitability test is shown in Table 1.
Selectivity
The selectivity test was carried out to determine whether the method development was selective for separating cefotaxime and ciprofloxacin analytes as well as levofloxacin internal standard from other components in human plasma. The method developed in the study succeeded in separating endogenous compounds present in plasma with cefotaxime, ciprofloxacin, and internal standard levofloxacin properly. The other plasma components eluted in less than 6 minutes, while the peak chromatograms of the analyte were separated with excellent resolution within 8 to 13 minutes, as evidenced by the resolution values of 6.23, and 3.55 for each analyte. The difference between the plasma blank chromatogram and the plasma that has been spiked with cefotaxime, ciprofloxacin, and levofloxacin is shown in Figure 3.
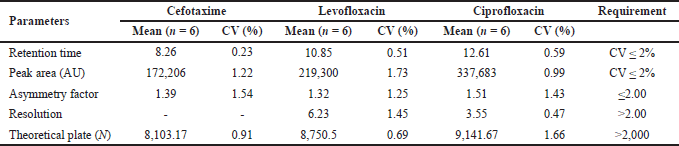 | Table 1. System suitability test. [Click here to view] |
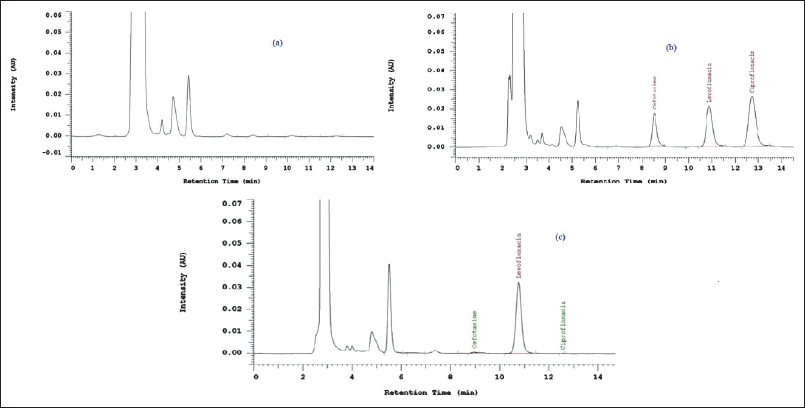 | Figure 3. Chromatogram of blank plasma (a), Blank plasma spiked with cefotaxime, ciprofloxacin, and internal standard levofloxacin (b), Blank plasma spiked with internal standard levofloxacin (c) in mobile phase phosphate buffer pH 3.0 (0.02 M):ACN:MeOH (80:12:8 v/v). [Click here to view] |
Linearity
Linearity is determined by performing a linear regression analysis of the calibration curve to obtain the intercept, slope, and correlation coefficient. The linear regression equation and the calculated parameters are shown in Table 2, with the recovery values for each concentration within the range of the calibration curve according to the requirements, namely ≤ 20% at LLOQ and ≤15% at other concentrations on the calibration curve. The linearity of the method, which is expressed as the coefficient of correlation (r) value for cefotaxime, is 0.9986 in the concentration range 0.2–10 μg/ml, while ciprofloxacin is 0.9981 (0.2–5.0 μg/ml). LLOQ is the lowest concentration of analyte that can be measured reliably and the result meets the accuracy and precision requirements. The graph of the linear regression equation of cefotaxime and ciprofloxacin is shown in Figure 4.
Accuracy and precision
Assessment of the accuracy of the developed analytical method is based on the average percent error of the measured concentrations to the nominal concentrations of the analyte. The within-run accuracy of the cefotaxime and ciprofloxacin for QC samples, as expressed in percent error, were in the range 8.3–12.05 and 1.96–5.27, while the percent error for LLOQ was 2.04 and 17.93. The between-run accuracy for the cefotaxime samples was in the range of 0.76–8.26, while for ciprofloxacin, it was 0.66–12.96.
The range of the coefficient of variation (%) for assessing precision within-run and between-run cefotaxime was 0.87–5.09 and 1.16–9.42, respectively. Meanwhile, within-run and between-run precision of ciprofloxacin was obtained in the range of 0.01–2.3 and 0.02–2.55. Figure 5 shows the chromatograms of LLOQ (a), low QC (b), medium QC (c), and high QC (d).
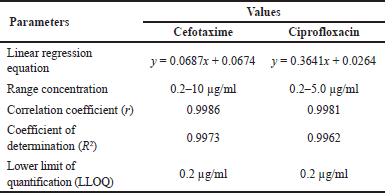 | Table 2. Linear regression parameters of the calibration curve. [Click here to view] |
After a series of optimizing the development of analytical methods to simultaneously determine cefotaxime and ciprofloxacin with HPLC-UV in human plasma, the method can be declared valid with within-run and between-run accuracy and precision values shown in Table 3. This result meets the requirements in accordance with the guidelines for the validation of the bioanalytical method issued by the European Medicines Agency.
Stability
Short-term stability testing was carried out to assure that the method developed relating to sample storage during preparation and storage before measurement still meets the requirements, considering that the determination of drug levels in patient blood samples is not carried out directly but through the storage stage prior to measurement. In this study, short-term stability was tested at two different storage conditions, namely after the sample was stored at temperature for 4 hours according to the length of sample preparation time and after storage at 2°C–8°C for 24 hours according to the storage conditions prior to sample analysis. The result of stability testing for both short-term stability and freeze and thaw stability are shown in Table 4.
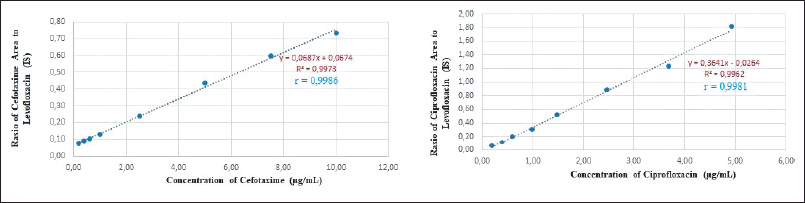 | Figure 4. Calibration curve of cefotaxime and ciprofloxacin. [Click here to view] |
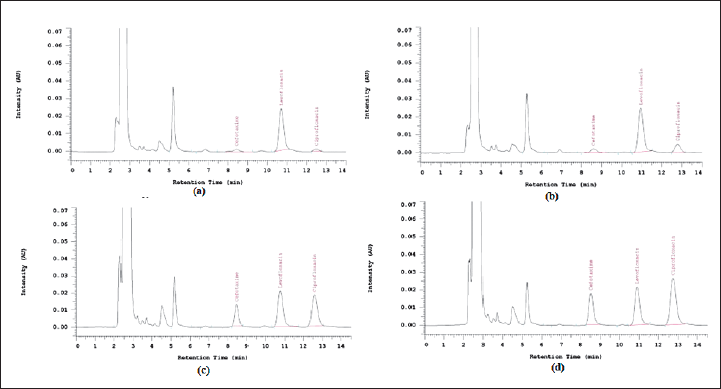 | Figure 5. Accuracy test chromatogram using LLOQ (a), Low QC (b), Medium QC (c), and High QC (d) Samples. [Click here to view] |
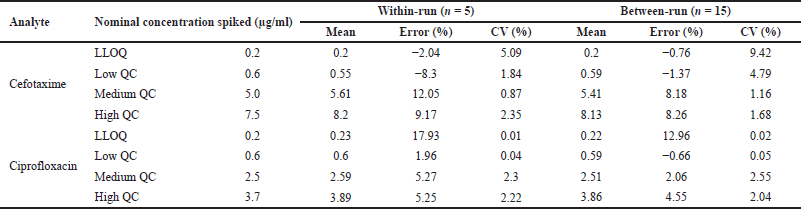 | Table 3. Accuracy and precision for determination cefotaxime and ciprofloxacin in spiked human plasma. [Click here to view] |
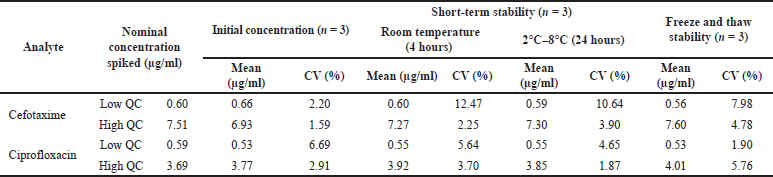 | Table 4. Stability test results (short-term stability and freeze and thaw stability). [Click here to view] |
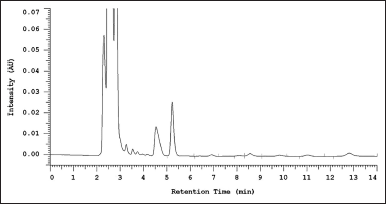 | Figure 6. Chromatogram of carry-over blank plasma after injection of the highest concentration of standard curve. [Click here to view] |
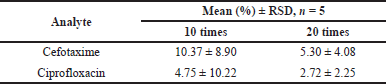 | Table 5. Dilution integrity results. [Click here to view] |
Carryover
In the validation of the analytical method, the carryover of blank plasma was determined by injection of the blank plasma after injection of the highest concentration sample on the calibration curve. This aims to ensure that the results of subsequent measurements do not affect accuracy and precision. Figure 6 shows the blank plasma chromatogram after measuring the highest sample concentration of the calibration curve.
Dilution integrity
In the dilution integrity test, plasma samples were prepared with cefotaxime and ciprofloxacin concentrations above the highest concentrations on the calibration curve, then diluted 10 and 20 times using a plasma blank. The addition of levofloxacin as an internal standard was carried out on a diluted sample, then the measurable drug level was determined based on the analytical method developed. The results of the dilution integrity test are shown in Table 5.
CONCLUSION
This research has succeeded in developing a validation analytical method for simultaneous determination of cefotaxime and ciprofloxacin in human plasma by HPLC-UV in a short time, simple and accurate, which is supported by good results, namely linearity, selectivity, accuracy, precision, and stability. The method was declared valid for the simultaneous determination of cefotaxime and ciprofloxacin concentrations in the range of cefotaxime 0.2–10 μg/ml and ciprofloxacin 0.2–5 μg/ml. The percent error for the QC samples of cefotaxime ranged from 0.76 to 12.05, while for ciprofloxacin analytes, it was in the range of 0.66–12.96.
ACKNOWLEDGMENTS
The author would like to express his gratitude to the Ministry of Education, Research, and Technology, Republic of Indonesia, for the domestic postgraduate scholarship.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The ethics committee approval was issued by the Medical and Health Research Ethics Committee, Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada --Dr. Sardjito General Hospital with Ref No: KE/FK/0324/EC/2022, March 23, 2022.
CONFLICTS OF INTEREST
All authors declared no potential conflicts of interest.
ETHICAL APPROVALS
The ethics committee approval was issued by the Medical and Health Research Ethics Committee, Faculty of Medicine, Public Health and Nursing Universitas Gadjah Mada—Dr. Sardjito General Hospital with Ref No: KE/FK/0324/EC/2022.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Birken SLK. Chapter 117: Septic and septic shock. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy?: a pathophysiologic approach. 6th ed. Chapter 117. New York, NY: McGraw Hill Professional; 2005. pp 2175–87.
2. Fu KP, Aswapokee P, Ho I, Matthijssen C, Neu HC. Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother [Internet]. 1979 Nov [cited 2020 Dec 24];16(5):592–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC352911/
3. Wishart D, Feunang Y, Guo A, Lo E, Marcu A, Grant J, et al. Ciprofloxacin—DrugBank [Internet]. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017 Nov 8 [cited 2018 Jan 17]. doi: https://doi.org/10.1093/nar/gkx1037. Available from: https://www.drugbank.ca/drugs/DB00537
4. Talib Humeidy I. Spectrophotometric determination of cefotaxime sodium in pharmaceutical formulations. Mater Today Proc [Internet]. 2021 Jan 1 [cited 2022 Jul 19];47:6043–9. Available from: https://www.sciencedirect.com/science/article/pii/S2214785321035513
5. Eric-Jovanovic S, Agbaba D, Zivanov-Stakic D, Vladimirov S. HPTLC determination of ceftriaxone, cefixime and cefotaxime in dosage forms. J Pharm Biomed Anal [Internet]. 1998 Dec [cited 2019 Jan 20];18(4–5):893–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S073170859800274X
6. Badgujar RS, Banerjee SK, Venkateshwarlu G. Development and validation of RP-HPLC method for simultaneous determination of cefotaxime sodium and sulbactum sodium in bulk and injection. Int J Chem Sci. 2010;8(3):1502–10.
7. Chaudhari B, Sridhar P, Moorkoth S, Lewis L, Mallayasamy S. Validation of an HPLC method for estimation of cefotaxime from dried blood spot: alternative to plasma-based PK evaluation in neonates. Bioanalysis. 2021 Aug 1;13:1245–58.
8. Hakim L, Bourne DWA, Triggs EJ. High-performance liquid chromatographic assay of cefotaxime, desacetylcefotaxime and ceftriaxone in rat plasma. J Chromatogr B Biomed Sci App [Internet]. 1988 Jan [cited 2019 Jul 3];424:111–7. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378434700810816
9. Kumar CHA, Gurupadayya BM, Sloka N. Determination and validation of cefadroxil, ceftriaxone and cefotaxime by using N-bromosuccinamide in human plasma and pharmaceutical dosage form. Int J Res Pharm Sci. 2011;2(2):206.
10. Ling SSN, Yuen KH, Barker SA. Simple liquid chromatographic method for the determination of cefotaxime in human and rat plasma. J Chromatogr B [Internet]. 2003 Jan 5 [cited 2022 Jul 19];783(1):297–301. Available from: https://www.sciencedirect.com/science/article/pii/S1570023202006578
11. Lalitha N, Sanjay Pai PN. Development and validation of RP-HPLC method for estimation of cefotaxime sodium in marketed formulations. J Basic Clin Pharm [Internet]. 2009 Dec [cited 2023 Aug 11];1(1):26–8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4158899/
12. Sinha S, Bhavsar SK, Thaker AM. Development and validation of HPLC method for quantification of cefotaxime in plasma of Patanwadi sheep. Explor Anim Med Res. 2015;2:6.
13. Guerra Valero YC, Dorofaeff T, Roberts JA, Lipman J, Coulthard MG, Sparkes L, et al. Development and validation of a UHPLC-MS/MS method to measure cefotaxime and metabolite desacetylcefotaxime in blood plasma: a pilot study suitable for capillary microsampling in critically ill children. Anal Bioanal Chem [Internet]. 2021 Jul 1 [cited 2021 Oct 17];413(17):4483–91. Available from: https://doi.org/10.1007/s00216-021-03411-7
14. Lefeuvre S, Bois-Maublanc J, Hocqueloux L, Bret L, Francia T, Eleout-Da violante C, et al. A simple ultra-high-performance liquid chromatography-high resolution mass spectrometry assay for the simultaneous quantification of 15 antibiotics in plasma. J Chromatogr B [Internet]. 2017 Oct [cited 2023 Aug 11];1065–6:50–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1570023216315252
15. Saranya CL, Thejaswini JC, Gurupadayya BM, Sruthi BYK. Simultaneous determination of cefotaxime sodium and paracetamol by LC-MS. IOSR J Pharm IOSRPHR [Internet]. 2014 [cited 2021 Oct 17];04(02):12–8. Available from: http://www.iosrphr.org/papers/v4i02/Version-1/C0421012018.pdf
16. Abdulla A, Bahmany S, Wijma RA, van der Nagel BCH, Koch BCP. Simultaneous determination of nine β-lactam antibiotics in human plasma by an ultrafast hydrophilic-interaction chromatography–tandem mass spectrometry. J Chromatogr B [Internet]. 2017 Aug 15 [cited 2022 Jul 19];1060:138–43. Available from: https://www.sciencedirect.com/science/article/pii/S1570023217303197
17. Elsadig H, Ahmed E. Stability study of ciprofloxacin hydrochloride under stress conditions using reverse phase -high performance liquid chromatography method. Der Pharm Sin. 2012;7 :217–23.
18. Aksoy B, Küçükgüzel ?, Rollas S. Development and validation of a stability-indicating HPLC method for determination of ciprofloxacin hydrochloride and its related compounds in film-coated tablets. Chromatographia [Internet]. 2007 Sep [cited 2022 Jul 19];66(S1):57–63. Available from: http://link.springer.com/10.1365/s10337-007-0287-6
19. Al-Ghazawi M, AbuRuz S. Determination of ciprofloxacin in dried blood spots for therapeutic drug monitoring. Chromatographia [Internet]. 2010 Jun 1 [cited 2022 Jul 19];71(11):999–1005. Available from: https://doi.org/10.1365/s10337-010-1568-z
20. Amini M, Khanavi M, Shafiee A. Simple high-performance liquid chromatographic method for determination of ciprofloxacin in human plasma. Iran J Pharm Res. 2004;3(2):99–101.
21. Imre S, Dogaru MT, Vari CE, Muntean T, Kelemen L. Validation of an HPLC method for the determination of ciprofloxacin in human plasma. J Pharm Biomed Anal [Internet]. 2003 Sep [cited 2018 Jan 10];33(1):125–30. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0731708503001511
22. Mukti AA, Jannah F, Nurrochmad A, Lukitaningsih E. Development and validation method for quantitative determination of ciprofloxacin in human plasma and its application in bioequivalence test. Asian J Pharm Clin Res. 2016;9(3):89–95.
23. Neckel U, Joukhadar C, Frossard M, Jäger W, Müller M, Mayer BX. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal Chim Acta [Internet]. 2002 Jul [cited 2019 Jun 18];463(2):199–206. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0003267002004294
24. Scheer F. Pharmacokinetics of piperacillin and ciprofloxacin in critically ill patients undergoing continuous venovenous haemodialysis or haemodiafiltration. J Pharm Care Health Syst [Internet]. 2014 [cited 2020 Feb 6];01(04). Available from: http://omicsgroup.org/journals/pharmacokinetics-of-piperacillin-and-ciprofloxacin-in-critically-ill-patients-2376-0419-1-118.php?aid=31590
25. Novakovic J, Nesmerak K, Nova H, Filka K. An HPTLC method for the determination and the purity control of ciprofloxacin HCl in coated tablets. J Pharm Biomed Anal [Internet]. 2001 Jul [cited 2019 Jan 27];25(5–6):957–64. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0731708501003879
26. Rao JR, Mulla TS, Yadav SS, Rajput MP, Bharekar VV. Validated HPTLC method for simultaneous estimation of ciprofloxacin hydrochloride and dexamethasone in bulk drug and formulation. Int J Chem Tech Res. 2012;4(4):1589–94.
27. European Medicines Agency. Guideline on bioanalytical method validation. Eur Med Agency. 2015. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf.