INTRODUCTION
A major section of the market is engaged by oral dosage formulations since it is the most preferred route of drug administration (Arora et al., 2006). Drug formulations after administration that are intended for producing uninterrupted therapeutic activity should maintain the drug concentrations in the region of therapeutic window region for a prolonged time period without frequent fluctuations which are possible by controlled along with sustained drug release formulations (Jain et al., 2011). Conventional delivery formulations release the medicament immediately and while considering the nature of the preparation either the release profiles are instantaneous or extended. In certain conditions above patterns are not appropriate, based on circadian rhythms and diseases which follow these rhythms in their pathophysiology it demands a special release pattern which involves the delivery of the drug at the site of activity after lag time which is beneficial for many therapies and the above stated condition is fulfilled by “pulsatile drug delivery system” (PDDS) (Patel and Patel, 2010). They are helpful for drugs with extreme first-pass effects, drugs administered for diseases that follow chronopharmacological behavior, and drugs with specific absorption windows inside Gastrointestinal tract (GIT), targeting to the colon (Sirisha et al., 2012).
PDDS rapidly delivers drug after an initial pre-determined time termed as lag time (Youan, 2004). In these formulations immediately after the administration of dosage form, there is no initial drug release during lag time later the drug will be released in the form of a pulse after an off-release period (Patel and Patel, 2010). In pulsatile formulations, the delivery of the drug is synchronized with the biological rhythms involved in the disease to generate the utmost therapeutic effect. They provide exclusive drug delivery at the site of activity according to the time point, therefore offer special, temporal delivery with high patient compliance (Reddy et al., 2009). Certain disease conditions do not require the release of the drug for the entire duration constantly rather they require the drug existence at the required site at the right point of time in the right concentrations to produce expected therapeutic activity following the circadian rhythm of disease and these conditions are demanding the production of “PDDS” (Patel and Patel, 2015). The drug release design is represented in Figure 1. Examples include Rheumatoid arthritis which is a chronic inflammatory autoimmune disorder, the symptoms include stiffness, swelling, and pain of more than one joint in the body which is observed most severely during the morning, myocardial infarction, bronchial asthma, rheumatoid arthritis, angina pectoris, peptic and duodenal ulcer, hypertension, etc. (Amidon and Leesman, 1993) when a persistent drug release is not desired pulsed release have to be formulated in a way such that total and quick drug release (Venkataswamy and Nallaguntla, 2021) is attained after a lag time establishing a fundamental rationale for formulating PDDS (Krogel and Bodmeier, 1999), classification of various medications based on their use is represented in Figure 2.
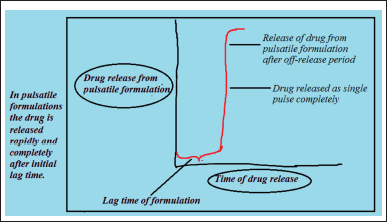 | Figure 1. Drug release profiles of pulsatile systems. [Click here to view] |
Circadian rhythms
Circadian rhythms are 24-hour cycle changes that are considered as normal course of action that happen in relation to the light and dark effects (Hema et al., 2019), which is seen in most of the living beings including animals, plants, and microbes, usually circadian rhythms are synchronized according to internal biologic clocks which are associated with the sleep-wake cycle, many functions in the human body like gastric acid production, metabolism, physiology, sleep patterns, hormone production are regulated through them (Ayyar and Sukumaran, 2021), the structure of circadian rhythm of body is depicted in Figure 3.
Chronotherapeutics
Chronotherapeutics deals with the drug delivery matching to inherent events of disease over a definite time period (Yesudanam et al., 2018).
Chronotherapy
Harmonization of the biological rhythms and medicinal treatment is entitled as chronotherapy.
Chronobiology
Chronobiology deals with the biological mechanism of diseases according to a time structure (Bisht, 2011). Chrono indicates time while biology relates to the study, otherwise science, of life.
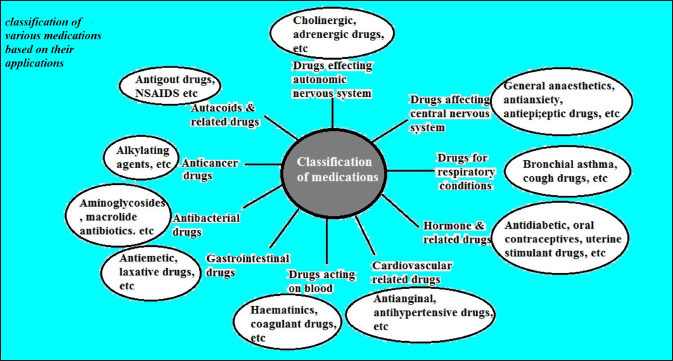 | Figure 2. Classification of various medications. [Click here to view] |
Chronopharmacokinetics
Chronopharmacokinetics includes analysis of time-based variations in drug pharmacokinetic parameters like absorption, distribution, metabolism, and excretion. Diurnal alterations in phenomena like secretion of the gastric acid, motility of the gastrointestinal tract, blood stream to the gastrointestinal tract, the drug protein binding reactions, the activity of liver enzymes (Bruguerolle and Lemmer, 1993), blood movement to kidneys and pH of urine could play a role in time-dependent variation of drug levels in the plasma.
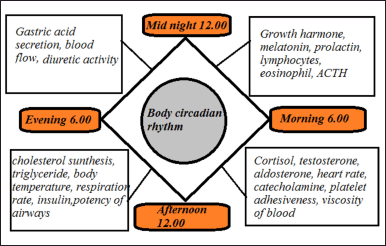 | Figure 3. Structure of circadian rhythm of body. [Click here to view] |
SITUATIONS ENCOURAGING FORMULATION OF PDDS
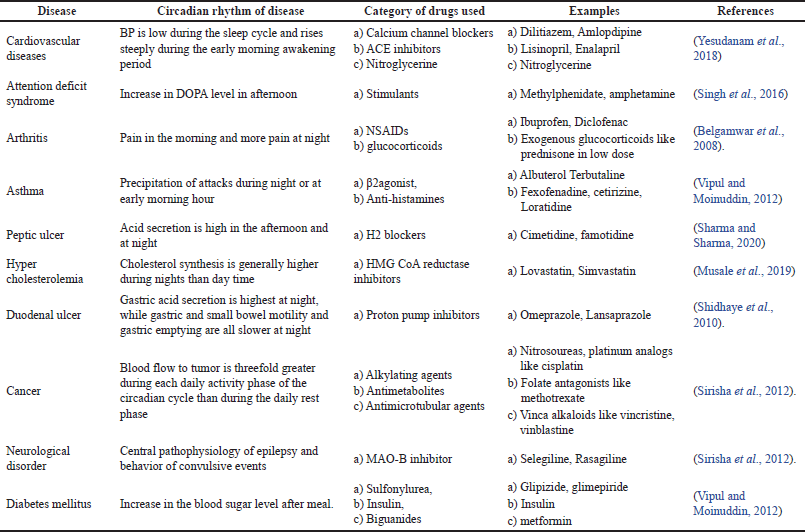
The following situations which are indicated in Table 1, necessitate pulsatile formulations which are valuable for patients with improved therapeutic efficacy. The circadian rhythm of several diseases when symptoms are exaggerated is demonstrated in Figure 4.
Bronchial asthma
Asthma is considered as frequent disease which follows the circadian rhythms appearing in its pathophysiology is asthma, the airway resistance increases progressively during night time in asthmatic patients which indicates the part of circadian rhythms in pathogenesis along with the management of asthma. Histamine is a bronchoconstictor that will be discharged during early mornings (Brahma and Gali, 2012), including cortisol an anti-inflammatory substance discharged in the morning time causes more asthma attacks in morning intervals which requires the release of a bronchodilator at a time point when symptoms are high, producing therapeutic activity (Youan, 2004).
 | Table 1. Conditions benefitted by chronotherapy. [Click here to view] |
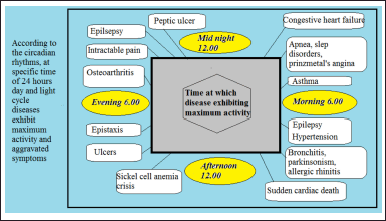 | Figure 4. Circadian rhythm of various diseases when symptoms are exaggerated. [Click here to view] |
Peptic ulcers
Based on observations and studies it is evident that the gastrointestinal tract is highly subjectedto circadian rhythms, which could be explained by during nights high levels of gastric acid is secreted while gastric motility including gastric emptying is slow, such that owed to slow emptying and motility drug disintegration, dissolution, and absorption may be reduced (Dhurke, 2020). Treatment of patients with peptic along with duodenal ulcers includes suppression of nocturnal acid secretion which is believed to be high during the nights, for an effective treatment instead of administering the H2 antagonist over the complete day, administration of these histamine blockers during bed time is preferred because maximum acid secretion, pain caused due to ulcers, and perforation of gastric ulcers along with duodenal ulcers are more common at night (Farhud and Aryan, 2018). It requires the administration of these drugs as PDDS at bedtime which would deliver the drug at night after predestined lag time producing the expected therapeutic efficiency (Humphries et al., 1991).
Cardiovascular disease
The pattern of circadian rhythms is observed in many cardiovascular disorders which include Blood pressures (BPs), heart rate, stroke volume, cardiac output, and blood stream of the cardiovascular system. For illustration, in the morning capillary resistance, and vascular reactivity will be high which decreases later during the day time (Brahma and Gali, 2012). It is observed that during morning’s platelet aggregation increases while fibrinolytic activity decreases which leads to hypercoagulability within the blood. Heart rate and BP are increased in the morning hours. During midafternoon the BP decreases which is minimum at midnight, an apparent rise in BP is noticed upon awakening which is termed as the morning surge (Pandit et al., 2017; Pasic et al., 1998). Myocardial infarction is observed to be further recurrent during the morning with most of the events happening between 6 A.M. and noon. The reasons reported include the release of catecholamines, cortisol, a raise in platelet aggregation, and vascular tone. Chronotherapy is advisable in such conditions to deliver the drug in the right concentration during a time of extreme requirement. Therapies include calcium channel blockers, Angiotensin-converting enzyme (ACE) inhibitors, nitroglycerine.
Cardiovascular disease is one of the causes of death worldwide (Ma et al., 2021). Experimental fluid dynamics plays an essential role in investigating pulsatile blood flow characters in the in vitro conditions using imitation arterial vessels for providing insight into disease conditions, the progression of disease, and design optimization for disease intervention (Stanley et al., 2023). Numerous in vitro works are done to predict cardiovascular disease conditions. Drug delivery formulations are developed utilizing magnetized nanoparticles as the carriers for delivering therapeutic agents (Hewlin and Tindall, 2023) in cardiovascular flow for the treatment of cardiovascular diseases (Hewlin et al., 2019).
Arthritis
Patients suffering from rheumatoid arthritis present symptoms like high pain that commonly mounts in the morning time and reduces as the day headway, while patients suffering from osteoarthritis have a tendency of more pain at night while low during the morning. Chronotherapeutics and chronobiology of pain explain the occurrence of the circadian rhythm in plasma level concentration of C-reactive protein along with interleukin-6 in patients suffering from rheumatoid arthritis (Vipul and Moinuddin, 2012). Chronotherapy in these conditions provides programmed discharge of drugs which includes Non-steroidal anti-inflammatory drugs (NSAIDS) like Ibuprofen, ketoprofen and glucocorticoids for all conditions of arthritis such that ensuring high blood levels of the drug is available at the location of activity when the pain is high (Lemmer, 1991).
Hypercholesterolemia
Circadian rhythms play a great role in hepatic cholesterol synthesis and it is described that synthesis of cholesterol is usually elevated during night than a day and maximum production of cholesterol occurs early morning which is 12 hours after the last meal (Lemmer, 1991). Studies conducted in patients suggest that drugs like Hydroxymethylglutaryl-CoA reductase inhibitors-atorvastatin, and lovastatin used for inhibiting cholesterol synthesis and for treating hypercholesterolemia are more effective as evening dose when compared to morning dosing. Previously these drugs were administered during the morning but after understanding the circadian rhythms involved in cholesterol synthesis the dosage regimen was changed to produce the required activity according to the pathogenesis of the disease (Prasanth et al., 2012). From the research conducted it is now recommended to administer the medication during the duration between the evening meal and bed time.
Diabetes
The purpose of insulin therapy is to simulate the routine physiologic pattern of endogenous insulin secretion in healthy individuals, with continuous basal secretion along with meal-stimulated secretion. The influence of circadian rhythms in variations of glucose levels and insulin in diabetes patients was widely studied and their clinical significance in insulin replacement in type I diabetes has been discussed earlier (Rada and Kandukuri, 2022). During the administration of proteins and peptides like insulin, their stability in the gastrointestinal tract should be taken into consideration, since they might be unstable because of the presence of high proteolytic enzyme activity in the upper GIT, in such situations colon is considered as preferred absorption site as presence of proteolytic enzymes in the colon is relatively low (Parcel et al., 2003). The above condition regarding the drug release specifically in the proximity of the colon could be possible by PDDS formulations indicating the drug will be initially not delivered in region of stomach region and small intestine, but the delivery of the drug is observed following lag time equal to 5 hours upon reaching the colon producing a time-dependent delivery. Time-dependent dosage forms deliver the drug subsequently aftera pre-programmed time delay and to attain the required conditions, the initial lag time should be equal to the time taken for the formulation to reach the colon.
Cerebrovascular accidents
The occurrence of cerebrovascular accidents surges in the morning (Rada and Kandukuri, 2022) from 10.00 am to 12.00 pm and decreases with afternoon and in the evening.
Pain
The degree of pain a patient is subjected to and the threshold of pain will not follow the same systematic pattern in all tissues (Rada and Kandukuri, 2022). Circadian rhythms are observed to play a role in the pattern of pain with respect to acute pain. It is recorded in cases such as dental surgery, where a high morning peak pain is observed during the first day after the surgery (Jones and Francis, 2000).
Sleep disorders
The time and duration of sleep necessary for each person will be mostly constant, but a variation is observed among individuals (Ritika et al., 2012). Sleep involves a circadian rhythm combination of changes in various processes including but not only limited to physiological, biochemical, and psychological processes.
Cancer
Research studies on humans and animals suggest that chemotherapy could be further effective with less toxicity if cancer drugs are administered to patients cautiously at selected times by gaining the benefit of tumor cell cycles, producing fewer side effects and toxicity to the normal healthy tissue (Patil et al., 2013). Circadian rhythms are observed in blood flow to the tumors which is generally threefold greater during every day activity phase than the day-to-day rest phase.
ADVANTAGES OF THE PULSATILE DRUG RELEASE FORMULATIONS
• Improved bioavailability, tolerability, reproducibility, and efficacy
• Patient compliance is improved (Sharma and Sharma, 2020)
• Used for drugs exhibiting chronopharmacological behavior
• Maintains constant medication levels at the site of activity so prevent peak-valley changes
• Reduces adverse effects and improves tolerability
• Dose dumping is reduced (Sharma and Sharma, 2020) with a limited risk of local irritation.
• The dosage of the medication can be reduced without decreasing the therapeutic effect.
• Improve stability and improve patient comfort
• Drug administration and medication adjusts to suit the human circadian rhythms
• Nearly persistent drug levels will be observed at the position of activity
• It permits the transport of poorly bioavailable medications which are unstable in GIT climate, for example, (peptide and protein) (Bhatt and Pethe, 2010; Musale et al., 2019)
• Decreases drug interaction because of the lower cytochrome P450isoenzymes.
• Pulse delivery allows various dosing in a solitary dosage form.
DISADVANTAGES AND LIMITATIONS OF PDDS
• Reproducibility of assembling and viability is not observed
• Multiple formulation steps are involved
• Large numbers of variables are involved during processing
• High production cost
• The capacity of loading of the drug is less and fragmented arrival of the drug (Musale et al., 2019).
• Advanced technology is required
• Instantaneous withdrawal of medicament is not possible.
• Drug dose manipulation in age groups like pediatrics and geriatrics might not be possible.
• In single unit pulsatile drug release system, in vivo variations are observed
• Numerous manufacturing steps in formulating a multiparticulate drug delivery system.
• The capacity of drug loading is low along with incomplete drug release
• Process insufficiency of manufacturing reproducibility and efficacy
• In vitro-in vivo correlation is unpredictable
NECESSITY OF FORMULATING PULSATILE DRUG DELIVERANCE SYSTEMS
• In disease conditions like bronchial asthma, angina pectoris, rheumatic infection, ulcer, arthritis, and hypertension which exhibit a biological rhythm (Rewar et al., 2014) in the disease condition.
• A predetermined lag time is necessary for those medications that experience degradation in the gastric medium due to the acidic pH.
• In situations when the drug should be dispensed to the distal part of GIT like colon delivery (Gazzaniga et al., 1994).
• Drugs that are exposed to extensive first-pass metabolism
• When the body rhythm is exhibiting circadian rhythms.
• If a rhythm variation is established in secretion of the gastric acid in the stomach, gastric purging, and gastrointestinal blood transfusion those situations also demand a pulsatile drug deliverance (Madhavi et al., 2020).
PROCESS OF DRUG DISCHARGE FROM PULSATILE DRUG DELIVERY FORMULATIONS
Any of the following mechanisms could be involved in the release of the drug from pulsatile formulations.
Osmosis
Within the interior of the formulation in contact with the drug particles, an osmotic pressure can be built up (Jones and Francis, 2000) when water is allowed to arrive into the system under the right circumstances which tend to force the drug from the system into the exterior environment. Osmotic agents resembling sodium chloride would also be used to create the osmotic pressure in the system.
Diffusion
When water and gastrointestinal fluids diffuse into the formulation and when drug particle interact with these aqueous fluids it leads to the formation of a drug solution (Venkataswamy and Nallaguntla, 2021), which diffuses across the release barrier coating of formulation to the exterior.
Erosion
As time proceeds depending on the nature and solubility of the polymer utilized some coatings erode gradually, resulting in drug release contained within the formulation (Valte et al., 2015).
Considering the route of administration pulsatile drug delivery formulations have been classified as oral, transdermal, and implants which are furthest classified into various categories depending on the mechanism with which the drug is released as represented in Figure 5. The pulsatile formulations administered orally are further categorized as denoted in the Table 2.
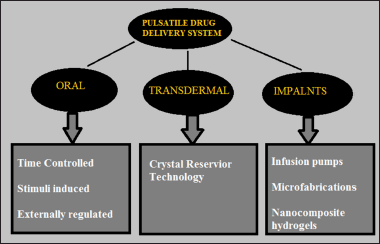 | Figure 5. Classification of PDDS based on route of administration. [Click here to view] |
PDDS AND METHODOLOGIES
Oral PDDS
Time controlled pulsatile drug delivery
Single unit pulsatile system
Capsule based systems
Pulsincap system. The formulation is mostly produced as a capsule, for fabricating pulsatile delivery required lag time is obtained by utilizing a polymeric hydrogel-plug which upon exposure to dissolution fluids gets propelled away either due to swelling or eroding, assisting in the drug release (Krögel and Bodmeier, 1998). It comprises of a capsule with a water-soluble cap and water impassable body of capsule which is covered with coating materials like insoluble materials like ethyl cellulose, encircling the drug reservoir and sealed with a hydrogel plug. Upon administration, when the soluble cap interacts with fluids it dissolves such that the plug swells and expands, the plug will be pushed out of the capsule following the lag time such that the drug is liberated rapidly, as displayed in the Figure 6. Depending on the position of the hydrogel plug and the dimensions of the plug the lag time will be controlled (Sirisha et al., 2012). Polymers like insoluble however permeable and swellable polymers e.g., polymethacrylates, Congealed melted polymers like, e.g., saturated polyglycolated glycerides, glyceryl monooleate, compressed Erodible polymers like e.g., hydroxypropylmethyl cellulose, Polyethylene oxide (PEO), polyvinyl alcohol can be employed in the approach of pulsincap systems.
Capsular system depending on osmosis
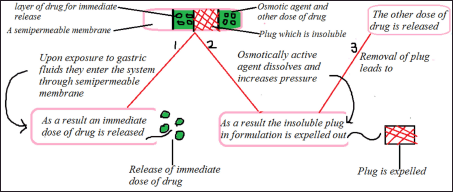
Port system. The system consists of the capsule that is coated with the membrane of semi-permeable material. The capsule encloses a plug that is insoluble and contains a vehicle which is osmotically active along with drug formulation (Reddy et al., 2009). Upon interaction with the dissolution medium, fluid enters into the system through semi-permeable layer which dissolves the osmotic agent and creates osmotic pressure so that leading to the expulsion of the insoluble plug after a particular lag time (Makhija and Vavia, 2003; Patel and Patel, 2015). These types of formulations are utilized in delivering methylphenidate in treating attention deficit hyperactivity disorder (Conley et al., 2006). The delivery of medication from these formulations is shown in Figure 7.
Formulations based on the principle of an expandable orifice. The formulations existing in liquid form could be delivered through this method, which produces the benefit of prolonged release along with improved bioavailability. The drug to be delivered will be absorbed into extremely porous particles and the release of drug will be through the orifice of semi-permeable capsule assisted by swelling the osmotic membrane after the interference layer has been dissolved (Balaban et al., 1993). The formulation consists of a capsule wall that consists of an elastic material that further holds an orifice, with the progress of osmotic pressure inside the capsule elevates initiated by stretching the wall of the capsule (Neeharika and Jyothi, 2015). The opening is small and when the elastic partition relaxes the drug flow will stop, when the wall is expanded the orifice enlarges and helps in the drug release. Depending on the barrier layer thickness along with the semi-permeable film thickness can produce a lag time of up to 10 hours (Dash et al., 2020). It is appropriate for polypeptides, polysaccharides, insoluble drugs, etc.
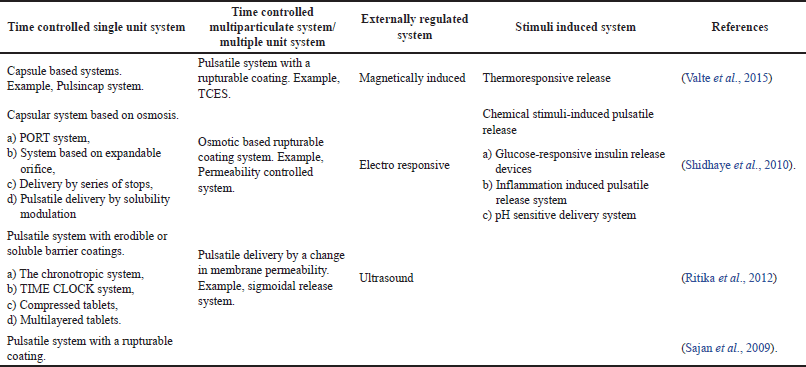 | Table 2. Orally administered PDDS and methodologies of pulsatile drug delivery. [Click here to view] |
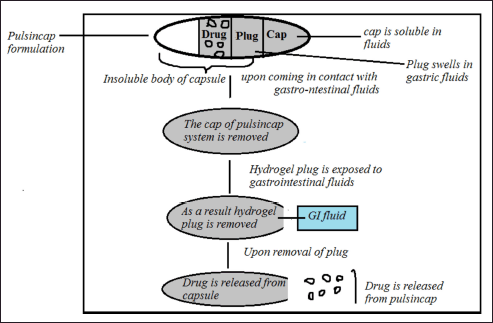 | Figure 6. Drug release from pulsincap system. [Click here to view] |
 | Figure 7. Schematic representation of PORT system. [Click here to view] |
Delivery using series of stops. It is appropriate for capsules administered as implants. The capsule encompasses two compartments partitioned by a movable slider, drug along with water-absorbable osmotic agent is placed in the first and second compartment respectively (Shidhaye et al., 2010). This arrangement provides pulsatile delivery of drugs. The sequence of stops barricades the passage of the drug by providing a necessary off-release period (lag time) which could be overwhelmed when osmotic pressure increases beyond the threshold level (Vani and Reddy, 2021). The total number of the stops and location of stops alongside the dimension of the capsule determine the total number and incidence of pulses, in addition, the arrangement of partition governs the intensity of pulse.
Pulsatile release by modulation of solubility. The solubility modulation of formulation design offers pulsatile deliverance of drugs which necessitates chronotherapeutic system. These categories of formulations were particularly established for the delivery of drugs like salbutamol sulphate which comprises of a substance similar to sodium chloride (NaCl) as a modulating vehicle (Ramakrishna and Rani, 2016). The NaCl quantity required was lower than the quantity desired to provide saturation within the fluid which enters the device. The deliverance of a drug in a pulsatile fashion depends on the overall drug solubility. Salbutamol exhibits solubility in a range 275 mg/ml in the presence of water furthermore 16 mg/ml in the existence of the NaCl saturated solution, whereas NaCl exhibits solubility in a range 321 mg/ml with water as a solvent and its solubility in saturated solution would be 320 mg/ml. The above values indicate that drug solubility would be the principle function that influences the concentration of the modulator that should be utilized in the formulation, however, the solubility of the modulator is not dependent on the concentration of the drug (Ozeki et al., 2003). The solubility modulating agent employed in formulation could include an organic salt, inorganic salt, and organic acid (solid). The fraction of drug incorporated along with the modulator might be fluctuated to produce pulsatile deliverance of drugs.
Pulsatile formulations with erodible or soluble barrier coatings
Maximum PDDS which are formulated include reservoir-based devices which will be coated with a layer of polymer that acts as a barrier (Belgamwar et al., 2008). The discharge of medication from the device is regulated by erosion, dissolution of barrier coating after a definite lag period, the thickness of the coating layer could be altered which, helps in controlling the required lag time.
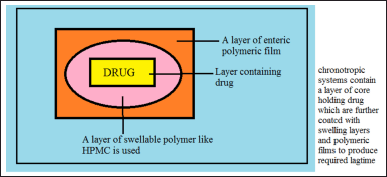
The chronotropic formulation system. In these formulations drug containing core acts as a reservoir onto which the layer of swellable hydrophilic hydroxypropylmethyl cellulose (HPMC) polymer is coated, as represented in Figure 8. It produces the required lag time, by altering the viscosity gradients of HPMC and polymer layer thickness (Gazzaniga et al., 2008). Colon targeting is achievable by coating the device with enteric polymeric film and gastrointestinal tract emptying time variations can be reduced (Chandani et al., 2012).
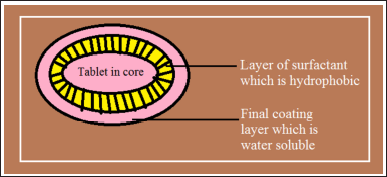
TIME CLOCK system. It is a pulsatile drug deliverance device that is established on the principle that, coating an aqueous dispersion on solid dosage formulation (Venkataswamy and Nallaguntla, 2021). The solid dosage formulation or the core material will be coated by aqueous dispersion of a hydrophobic surfactant layer at 75°C with materials like beeswax, carnubawax, etc. An outer water-soluble coating is utilized to increase adhesion toward the core coating layer, which is shown in Figure 9. When the device interacts with gastrointestinal fluids or dissolution fluid, rehydration of the dispersion is observed which produces the redispersion of the formulation (Youan, 2010). The essential lag time might be regulated by changing film thickness. After attaining lag time, the core instantly discharges the drug dose from the device (Sirisha et al., 2012).
Compressed tablets. The procedure of compression coating includes the direct compression technique. The core materials along with coating layers are compressed, avoiding the usage of the coating solutions. The outward tablet of compression-coated tablet delivers the initial dose, instantly disintegrating in the stomach and the interior layer is composed of constituents that will be insoluble in the pH of gastric media though they will be discharged into the intestinal region (Rane et al., 2009). Initially, the coating polymer layer is filled in the die cavity, then core tablet which is previously compressed will be placed in the center, then a top layer of coating polymer is placed followed by compression at the required hardness (Ramteke et al., 2013; Yan et al., 2002). Press-coated formulations of tablets provide the necessary lag time essential for the pulsatile system.
 | Figure 8. Illustration of chronotropic system. [Click here to view] |
 | Figure 9. TIME CLOCK system formulation. [Click here to view] |
The foremost shortcoming of above-stated technique is a large quantity of coating materials is needed along with difficulty to place the cores appropriately. Benefits of the press-coated pulsatile drug deliverance system include protection from moisture, light, acid-sensitive drugs, etc.
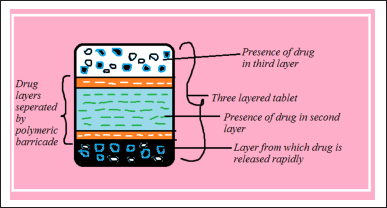
Multilayered tablets. This three-layered tablet formulation includes two drug comprising layers parted by a polymeric barricade layer which is free from the drug. The coating will be applied on the tablet on all sides except on the top portion with a polymer like ethyl cellulose (Kumar, 2018). The preliminary drug dose included in the upper layer will be released quickly from the uncoated portion of the tablet upon interaction with fluids like a dissolution medium. From the extreme bottom of the tablet after the erosion and dissolution of the HPMC layer the second pulse will be obtained (Bussemer et al., 2003). The arrival of the second pulse will be governed by gelling along with the dissolution of the barricade layer as indicated in Figure 10. The polymers utilized for gelling comprises of cellulose derivatives such as hydroxyl propyl methyl cellulose, methyl cellulose, polyvinyl alcohols with different molecular weight, and also coating materials incorporate ethyl cellulose, cellulose-acetate propionate, methacrylic co-polymers. Methacrylic polymers, polyalcohols acrylic (Arora et al., 2006).
Pulsatile formulations with principle of rupturable coating
These formulations eject the drug after the disintegration of the coating layer. By utilizing effervescent excipients and swelling agents pressure is generated in the formulation that helps to rupture the layer of coating (Amritha et al., 2022). For example, an effervescent blend containing citric acid along with sodium bicarbonate is incorporated in the tablet core and it will be coated with a layer of ethyl cellulose. Upon interaction with dissolution fluid when the fluid penetrates the system it produces carbon dioxide due to the chemical reactions involved, producing pulsatile drug delivery when the lag time which will be equal to the time taken by coating to rupture (Bhatia et al., 2014). The discharge of the drug will be affected by coating properties along with coating thickness also effects the drug deliverance. The greater the coating film thickness greater will be lag time and the tablet hardness (Bussemer et al., 2003), extremely swelling agents includes cellulose products like hydroxypropyl cellulose with low substitution, sodium starch glycollate, cross carmellose sodium, might be incorporated to acquire capsular system comprising drug and swelling agent along with a rupturable layer of polymer. Swelling of all these substances helps in rupturing the coating layer leading to drug release.
 | Figure 10. Design of multilayered tablets. [Click here to view] |
Multiparticulate or multiple unit pulsatile release systems
Pulsatile approach using rupturable coating
Time-controlled explosion system (TCES). In multiparticulate formulations, the drug will be coated on base material like non-pareil sugar seeds subsequently followed by a layer of swelling layer and a top coating layer with insoluble material. The swelling layer is formed using materials similar to cellulose derivatives like sodium carboxymethyl cellulose, L-hydroxypropyl cellulose, sodium starch glycollate, along with polymers similar to polyvinyl acetate, etc. The effervescent system consists an admixture of tartaric acid with sodium bicarbonate and citric acid (Sajan et al., 2009). On interaction with water, the swelling layer enlarges which leads to rupturing of the film visualizing successive instant drug release (Verma and Garg, 2001). This method is typically independent of drug solubility and pH. The formulations predetermined lag time might be differed by changing the coating layer thickness or incorporating an excessive amount of lipophilic non-polar plasticizer in the outermost layer of formulation (Sajan et al., 2009).
Osmotic system based on principle of rupturable coating
These formulations contain a core that encloses drug, along with low-density solids with an lipid material which is a liquid-like mineral oil with disintegrants. Cellulose acetate coating is applied to the core formulation (Pathak, 2014; Shetty and Selvam, 2016). When immersed into the aqueous medium as water or fluid penetrates into the core formulation it displaces lipid material (Chen, 1996). Once the lipid material is exhausted an interior pressure increases till a critical pressure is attained, resulting in the separation of coat.
Pulsatile release by alteration in the membrane permeability
Existence of various counter-ions in a medium controls the permeability along with water acceptance of the acrylic polymers holding quaternary groups. Eudragit is one of the best examples and polymer of preference for this function, within the polymer side chain it usually consists of quaternary ammonium units which are positively polarized and they are constantly supplemented with hydrochloride counterions which are negative in nature (Chen, 1996). The ammonium units present within the Eudragit being hydrophilic enables the interaction of polymer and water, hence, changes the permeability then permits water to invade the active core in a regulated manner. This is an essential principle to accomplish an accurately definite lag time. The existence of relation among film thickness along with lag time will be commonly observed (Ramteke et al., 2013). Subsequently after programmed lag time permeability of coating increases as a consequence of the interaction between acetate and the polymer, hence the complete dose will be released within minutes.
Stimuli induced pulsatile systems
In these formulations, the drug will be released when stimulated by a biological factor such as temperature or chemical stimuli, etc. These systems include the following.
Temperature induced pulsatile formulations
Pulsatile drug delivery devices were developed utilizing the principle of temperature-induced drug release by developing thermoresponsive hydrogel formulations (Rada and Kandukuri, 2022). In these formulations the polymer experiences swelling or de-swelling period in response toward changes in the temperature which regulate the delivery of drug in the swollen condition of thepolymer (Valte et al., 2015).
Chemical stimuli induced pulsatile formulations
Glucose-responsive insulin release devices
Numerous approaches have been established which are capable of responding to variations in the glucose level concentration. The best example is pH susceptible hydrogel comprising glucose oxidase enzyme immobilized in the hydrogel (Kamalpuria et al., 2017). When the concentration of glucose increases in the blood glucose oxidase transforms glucose into a gluconic acid that will modify the pH of the formulation. This alteration in pH encourages the swelling of polymer which will initiate the release of insulin. As the glucose levels decrease in the blood the gluconic acid concentration will decrease and the system decreases discharge of insulin (Thakur et al., 2021).
Inflammation induced pulsatile formulations
Upon encountering with an injury, fracture, physical or chemical stress inflammation is observed at the above stated sites. From the inflammation responsive cells, hydroxyl radicals will be produced. Studies conducted by Yui and the co-workers concentrated on inflammation-induced free hydroxyl radicals and then formulated drug deliverance systems depending on that approach. They have utilized hyaluronic acid that is exclusively degraded by hyaluronidase or in the existence of free radicals (Ali et al., 2010). The degradation of hyaluronic acid in the occurrence of hyaluronidase is extremely little in the normal health condition of individual; however, degradation is commonly high moreover quick when hyaluronic acid will be introduced at sites of inflammation, such that it is feasible to provide treatment for the patients suffering with inflammatory diseases utilizing anti-inflammatory medicament encompassed hyaluronic acid gels like one of novel implantable drug deliverance systems.
pH sensitive pulsatile drug delivery formulations
The formulation consists of two components while one of which includes immediate release chamber and the other compartment is a pulsed chamber that delivers drug in response to variations in pH (Ali et al., 2010). Considering the point that existence of various pH environments in different parts through the entire gastrointestinal tract, choosing pH-dependent polymeric materials will help to design formulations in such a way that drug release will be observed at the targeted site or at the specified site of activity, examples of these polymers contain sodium carboxy methyl cellulose, cellulose acetate phthalate, eudragits, polyacrylates, etc. (Kuila et al., 2018). The utilization of the above polymers releases the drug in the small intestine.
Externally regulated pulsatile drug release formulations
These devices need external means to induce the drug deliverance in a pulsatile fashion and they include the following.
Magnetically stimulated systems
These formulations contain a polymeric matrix holding drug and magnetic steel beads. Once exposed to a magnetic field the swinging of beads in the matrix generates compressive and tensile forces, which is the principle function and acts as a pump for forcing the drug out of the matrix in elevated concentrations (Kuila et al., 2018). Materials like nickel, iron, cobalt magnetite, and steel can be utilized to create necessary magnetic field in the device.
Electro responsive systems
The release of drug from these devices involves the application of an electric field on the rate controlling layer or directly on the solute or any of above-stated method can be employed so that the passage of active pharmaceutical ingredients through membrane can be controlled (Kuila et al., 2018). The polymer occurs in two redox states however only one is appropriate for ion binding. In this redox state ions of drug are in bound condition, in this environment it causes the membrane to swell, the pore size is reformed and the permeability of hydrogel membranes is also changed which controls drug transport (Patil et al., 2013).
Ultrasonically stimulated pulsatile drug delivery formulations
Degradation of polymer is noticed upon exposure to ultrasound waves which facilitates the delivery of drug molecules present in the device (Thakur et al., 2021). This technique is specifically used for intensifying the drug movement through biological barriers like skin, lungs, and blood vessels, etc. These Ultrasonic waves initiate erosion of polymeric matrix, and therefore, regulate the release of the drug from the device.
PDDS for transdermal route
Crystal reservoir technology
It comprises of small patches and exhibits controlled release of the drug, which is dependent on fractional crystallization of drug depending on supersaturation of adhesive, helping in drug release. The existence of solute in molecular form along with solid crystals improves drug concentration with an uninterrupted drug supply (Shidhaye et al., 2012). Once drug is utilized in its molecular solute form it will be absorbed into the skin the crystals dissolve to conserve the greatest thermodynamic action at the interaction site. By adjusting the concentration of solute in molecular form release of the medicament could be controlled.
PDDS for implants
Infusion pumps
These devices are surgically positioned in the subcutaneous region of the abdomen, helping in insulin therapy holding a reservoir of insulin (Ozeki et al., 2003). A catheter connects the device to the region of peritoneal cavity through muscle layers such that it floats easily and helps in delivery of the insulin through intra peritoneal route (Shidhaye et al., 2012). The dose of insulin can be adjusted by the patient utilizing an electronic device connected to the pump.
Microfabrication
These formulations consist of reservoirs of drug enclosed in membrane which is encompassed of active silicon-based microchip membrane through a thin coating of gold. As the voltage is applied across the device the release of drug is observed as anode membranes encircling the drug reservoirs are dissolved due to an electrochemical gradient (Valte et al., 2015). Studies conducted utilizing gold microchip with saline solution indicated a perfect pulsatile drug deliverance system by controlling the delivery of drug.
Several formulations of PDDS are existing in the market manufactured with distinctive technologies to modulate the drug release were designed for patient benefits, as represented in the Table 3. The course of drug release and alteration of required lag time will be possible by utilizing various strategies in originating dosage form, various chronotherapeutic advanced technologies which are existing in the market are demonstrated in Table 4.
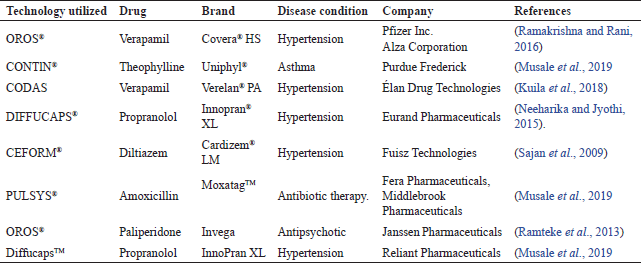 | Table 3. Marketed products of pulsatile drug delivery formulations. [Click here to view] |
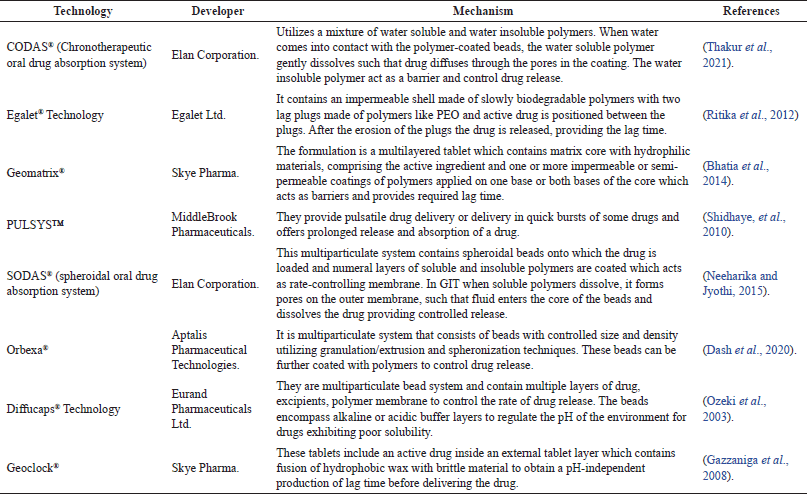 | Table 4. Chronotherapeutic advanced technologies available in the market. [Click here to view] |
CONCLUSION
It is necessary and obligatory to formulate innovative drug delivery systems which improve the therapeutic activity of the drug at the site of action by producing a clinical response. These formulations release drug in accordance with the biological and circadian rhythm involved in disease, so which offers special benefits for patients suffering from diseases that follow a rhythm in their pathophysiology. Successful development of chronotherapeutic drug delivery system requires a thorough understanding of the time structure involved in circadian rhythms, including the rhythm of disease along with its pathophysiology and 24 hours day and light pattern in the symptom exaggeration. Extensive advancement has been observed in formulating these systems and numerous preparations are exists in the market, however further research and clinical investigation is mandatory to attain complete advantages from these formulations. It might be indicated that pulsatile systems offer assuring benefits to patients through their inventive technologies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ali J, Baboota S, Ahuja A, Saigal N. Distinctive features of “chronotherapeutic” and “pulsatile” drug delivery systems negating the practice of their interchangeable terminology. J Drug Tar, 2010; 18(6):413–9.
Amidon GL, Leesman GD, inventors. University of Michigan, assignee. Pulsatile drug delivery system. US Patent 5,229,131. 1993-06-20.
Amritha R, Sivakumar R, Haribabu Y. A review on pulsatile drug delivery system: drug scheduling based on biological rhythm. Res J Pharm Tech, 2022; 15(3):1359–64.
Arora S, Ali J, Ahuja A, Baboota S, Qureshi J. Pulsatile drug delivery systems: an approach for controlled drug delivery. Indian J Pharm Sci, 2006; 68(3):295–300.
Ayyar VS, Sukumaran S. Circadian rhythms: influence on physiology, pharmacology, and therapeutic interventions. J Pharmacokinet Pharmacodyn, 2021; 48:321–38.
Balaban SM, Pike JB, Smith JP, Baile CA. ALZA Corporation, assignee. Osmotically Driven Delivery Devices with Pulsatile Effect. US Patent 5209746. 1993-05-11.
Belgamwar VS, Gaikwad M, Patil GB, Surana S. Pulsatile drug delivery system. Asian J Pharm, 2008; 2(3).
Bhatia S, Kumar B, Mittal S. Oral chronotherapeutics: future of drug delivery systems. Int J Sci Study, 2014; 2(4):55–8.
Bhatt DA, Pethe AM. Nanotechnology: a promising drug delivery for poorly water soluble drugs. J Pharm Res, 2010; 3(8):1748–175.
Bisht R. Chronomodulated drug delivery system: a comprehensive review on the recent advances in a new sub-discipline of chronopharmaceutics. Asian J Pharm, 2011; 5(1):1–8.
Brahma CK, Gali GVS. Chronotherapeutics drug delivery systems challenges to pharmaceutical field. J Glob Trends Pharm Sci, 2012; 3(3):779–91.
Bruguerolle B, Lemmer B. Recent advances in chronopharmacokinetics: a review. Methodological problems. Life Sci, 1993; 52(23):1809–24.
Bussemer T, Dashevsky A, Bodmeier R. A pulsatile drug delivery system based on rupturable coated hard gelatine capsules. J Control Release, 2003; 93(3):331–9.
Chandani G, Ganesh B, Preeti K. A comprehensive review of pulsatile drug delivery system. Pharm Innov, 2012; 1(7):99–104.
Chen CM, inventor. Andrx Pharmaceuticals, Inc, assignee. Multiparticulate Pulsatile drug delivery system. US Patent 5508040. 1996-04-16.
Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin, 2006; 22(10):1879–92.
Dash SK, Khan AS, Padhan A, Karna N. Formulation and evaluation of pulsatile drug delivery system of atenolol by using natural gum. Int J Pharm Sci Res, 2020; 11(7):3229–42.
Dhurke R. A review on chronomodulated drug delivery systems. Int J Recent Sci Res, 2020; 11(8):39578–87.
Farhud D, Aryan Z. Circadian rhythm, lifestyle and health: a narrative review. Iran J Public Health, 2018; 47(8):1068–76.
Gazzaniga A, Iamartino P, Maffione G, Sangalli ME. Oral delayed-release system for colonic specific delivery. Int J Pharm, 1994; 108(1):77–83.
Gazzaniga A, Palugan L, Foppoli A, Sangalli ME. Oral pulsatile delivery systems based on swellable hydrophilic polymers. Eur J Pharm Biopharm, 2008; 68(1):11–8.
Hema J, Vaseem AA, Pandit JN, Farogh A. Pulsatile drug delivery system: an overview with special emphasis on losartan and captopril. Res J Pharm Tech, 2019; 12(7):3175–88.
Hewlin Jr RL, Ciero A, Kizito JP. Development of a two-way coupled Eulerian-Lagrangian computational magnetic nanoparticle targeting model for pulsatile flow in a patient-specific diseased left carotid bifurcation artery. Cardiovasc Eng Technol, 2019; 10(2):299–313.
Hewlin Jr RL, Tindall JM. Computational assessment of magnetic nanoparticle targeting efficiency in a simplified circle of Willis arterial model. Int J Mol Sci, 2023; 24(3):2545.
Humphries TJ, Root JK, Hufnagel K. Successful drugspecific chronotherapy with the H2 blocker famotidine in the symptomatic relief of gastroesophageal reflux disease. Ann N Y Acad Sci, 1991; 618:517–8.
Jain D, Raturi R, Jain V, Bansal P, Singh S. Recent technologies in pulsatile drug delivery. Biomatter, 2011; 1(1):57–65.
Jones JW, Francis J. Softgels: consumer perceptions and market impact relative to other oral dosage forms. Adv Ther, 2000; 17(5):15–20.
Kamalpuria N, Dhir S, Jain S. The latest methods and technologies of pulsatile drug delivery system: a review. Int J Pharm Life Sci, 2017; 8:5446–58.
Krögel I, Bodmeier R. Pulsatile drug release from an insoluble capsule body controlled by an erodible plug. Pharm Res, 1998; 15(3):474–81.
Krogel I, Bodmeier R. Floating or pulsatile drug delivery systems based on coated effervescent cores. Int J Pharm, 1999; 187(2):175–84.
Kuila A, Dhandapani NV, Bhowmik H, Soni A, Kumar KH. Chronotherapeutic drug delivery system: an emerging approach to treat circadian rhythmic related disease. Asian J Pharm Clin Res, 2018; 11(8):15–20.
Lemmer B. Circadian rhythms and drug delivery. J Control Release, 1991; 16(1–2):63–74.
Ma T, Zhang Z, Chen Y, Su H, Deng X, Liu X, Fan Y. Delivery of nitric oxide in the cardiovascular system: implications for clinical diagnosis and therapy. Int J Mol Sci, 2021; 22(22):12166.
Madhavi AV, Reddy DRB, Venugopal M, Srihari N, Chenniah P, Koteswarao P. Formulation and evaluation of pulsatile drug delivery system of zafirlukast. J Drug Deliv Ther, 2020; 10(2):122–8.
Makhija SN, Vavia PR. Controlled porosity osmotic pump based controlled release system of pseudoephedrine. J Control Release, 2003; 89(1):5–18.
Musale RJ, Jawanjal P, Kulkarni V. Review on pulsatile drug delivery system and their marketed product. World J Pharm Pharm Sci, 2019; 8(4):1448–65.
Neeharika MS, Jyothi JB. Chronotherapeutics: an optimizing approach to synchronize drug delivery with circadian rhythm. J Crit Rev, 2015; 2(4):31–40.
Ozeki Y, Ando M, Watanabe Y, Danjo K. Evaluation of novel one step dry coated tablets as platform for delayed—release tablets. J Control Release, 2003; 95(1):51–60.
Pandit V, Kumar A, Ashawat MS, Verma CP, Kumar P. Recent advancement and technological aspects of pulsatile drug delivery system—a laconic review. Curr Drug Targets, 2017; 18(10):1191–203.
Parcel P, Vishnupad KS, Venkatesh GM. Timed pulsatile drug delivery systems. 2003; US Patent No. 6627223.
Pasic J, Shapiro D, Motivala S, Hui KK. Blood pressure morning surge and hostility. Am J Hypertens, 1998; 11(2):245–50.
Patel R, Patel J. Novel technologies of oral controlled release drug delivery system. Sys Rev Pharm, 2010; 1(2):128–32.
Patel VR, Patel VP. Pulsatile drug delivery system—a review. Int J Pharm Sci Res, 2015; 6(9):3676–88.
Pathak D. Novel concept of drug delivery based on chronotherapy: a review. Int J Pharm Res Tech, 2014; 4(2):23–7.
Patil ND, Bari MM, Barhate SD. A review on novel approach pulsatile drug delivery system. Int J Pharm Sci Rev Res, 2013; 21(1): 209–22.
Prasanth VV, Modi MP, Mathew ST. Pulsatile: a tool for circardian rhythm—a review. J Drug Del Ther, 2012; 2(1):58–65.
Rada SK, Kandukuri S. Chronotherapeutic systems: novel systems of drug delivery. Int J Pharm Sci Res, 2022; 13(1):86–94.
Ramakrishna M, Rani PA. Review on recent trends and approach for pulsatile drug delivery systems. Eur J Pharm Med Res, 2016; 3(5):268–75.
Ramteke KH, Dhole SN, Chavanke MS. Recent technologies for pulsatile drug delivery system. Int J Pharm Chem Sci, 2013; 2(3):1369–76.
Rane AB, Gattani SG, Kadam VD, Tekade AR. Formulation and evaluation of press coated tablets for pulsatile drug delivery using hydrophilic and hydrophobic polymers. Chem Pharm, 2009; 57(11):1213–7.
Reddy JRK, Jyothsna MV, Saleem TSM, Chetty CMS. Review on: pulsatile drug delivery systems. J Pharm Sci Res, 2009; 1(4):109–15.
Rewar S, Bansal BK, Singh CJSV, Sharma M. New approaches in pulsatile drug delivery system: a review. Int J Pharm Biol Arch, 2014; 5(2):27–43.
Ritika S, Arjun S, Sunil K, Faraz J. Pulsatile drug delivery system: a review. Int Res J Pharm, 2012; 3(7):103–7.
Sajan J, Cinu TA, Chacko AJ, Litty J, Jaseeda T. Chronotherapeutics and chronotherapeutic drug delivery systems. Trop J Pharm Res, 2009; 8 (5):467–75.
Sharma K, Sharma D. A review on pulsatile drug delivery system and various approaches used to design. J Emerg Tech Inn Res, 2020; 7(6):1096–103.
Shetty A, Selvam TP. Review on chronotherapy: a novel drug delivery system. J Pharm Sci Bioscientific Res, 2016; 6(5):646–53.
Shidhaye S, Dhone A, Budhkar T, Surve C. Technologies in pulsatile drug delivery system. Int J Adv Pharm Bio Chem, 2012; 1(4):438–45.
Shidhaye SS, Lotlikar VM, Ghule AM, Phutane PK, Kadam VJ. Pulsatile delivery systems: an approach for chronotherapeutic diseases. Sys Rev Pharm, 2010; 1(1):56–61.
Singh NP, Ganarajan G, Kothiyal P. Pulsatile drug delivery system: a review. World J Pharm Pharm Sci, 2016; 5(5):479–91.
Sirisha VNL, Namrata M, Sruthi B, Harika I, Kumar KP, Rao YKK, Pranavi K. Pulsatile drug delivery system-a review. Int J Pharm Res Allied Sci, 2012; 1(3):13–23.
Stanley N, Ciero A, Timms W, Hewlin Jr RL. A 3-D printed optically clear rigid diseased carotid bifurcation arterial mock vessel model for particle image velocimetry analysis in pulsatile flow. ASME Open J Eng, 2023; 2:021010.
Thakur G, Wani SUD, Gautam SP. A review on recent advancement in pulsatile drug delivery systems. Int J Curr Pharm Res, 2021; 13(2):6–10.
Valte YB, Mahajan NA, Jadhav VD, Mogal R, Talele S. Review on pulsatile drug delivery system. Int J Pharm Res Sch, 2015; 4(2):120–9.
Vani C, Reddy KS. Pulsatile drug delivery system-a technique of delivering drug in accordance with biological clock—a review. Int J Adv Res, 2021; 9(05):101–24.
Venkataswamy R, Nallaguntla L. Review article on pulsatile drug delivery system. Asian J Pharm Clin Res, 2021; 14(6):48–59.
Verma RK, Garg S. Current status of drug delivery technologies and future directions. Pharm Technol Eur, 2001; 25(2):1–14.
Vipul P, Moinuddin M. Pulsatile drug delivery system for treatment of various inflammatory disorders: a review. Int J Drug Dev Res, 2012; 4(3):67–87.
Yan L, Chu JS, Fix JA. Colonic-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm, 2002; 235(1–2):1–15.
Yesudanam S, Soumya SP, Anusree S, Dr. William Arputha Sundar AS, Sam Jeeva Kumar E. Pulsatile drug delivery system—a review. World J Pharma Med Res, 2018; 4(12):130–42.
Youan BB. Chronopharmaceutics: gimmick or clinically relevant approach to drug delivery? J Control Release, 2004; 98(3):337–53.
Youan BB. Chronopharmaceutical drug delivery systems: hurdles, hype or hope? Adv Drug Deliv Rev, 2010; 62(9–10):898–903.