INTRODUCTION
Herbalism is a prevalent long-established medicinal practice worldwide based on the utilization of plant extracts (Acharya and Shrivastava, 2008). In different medication systems like allopathy, homeopathy, and Ayurveda, plants have been the principal source of drugs (Chaudhary et al., 2015). Most of the researchers were inclined to allopathic drugs at the beginning of the 20th century; however, in the last couple of decades, there has been an upsurge in the usage of herbal or plant-derived drugs and cosmetics. Around 25% of the drugs have been derived from plant parts only (Khaliq and Chaudhary, 2016). An elaborated description of several herbs with medicinal uses has been given in Ayurveda, Sushruta, and Charaka Samhita (K?rtikara and Basu, 1918). The genus Ficus (fig genus) contains around 40 genera consisting of 1,400 species of vines, herbs, shrubs, and woody trees (collectively known as figs) forming the largest genus of the family Moraceae (mulberry family) (Zerega et al., 2005). Some species are ornamental, e.g., F. lyrata (fiddle-leaf fig), while some are used as edible plants. A few prominent species of the genus Ficus are F. benghalensis, F. auriculata (roxburgh fig), F. religiosa (sacred fig), F. carica (edible fig), F. elastic (Rubber fig), and F. benjamina (weeping fig). They are found to occur worldwide in tropical and subtropical regions. Ficus species are the highest oxygen generator plants with the highest rate of photosynthesis. They typically contain a latex-like gummy substance in their vasculatures that has self-healing properties and play a role in the defense upon physical assaults to the plants.
The common name of F. benghalensis came from the Britishers who noticed a specific community called “Baniya” that used to have rest in its shade. The banyan tree is the national tree of India and is considered sacred and a symbol of spiritual knowledge (Gopukumar and Praseetha, 2015). It is native to many parts of the Asian continent, i.e., India, Myanmar, Thailand, Malaysia, Southeast Asia, and China. It is usually cultivated in parks, botanical gardens, and tropical areas throughout the world. In developing countries, about 80% of the population relies on alternative medicines as a source of primary care (Rupani and Chavez, 2018). On the contrary, in developed/industrially rich countries, the use of alternative medication is famous as a complementary way of care. In India, Afghanistan, Pakistan, and other Asian developing countries, the use of complementary medication like Sow-Rigpa and Ayurveda along with allopathy is a common practice. Such medicine practice uses different therapies that employ a complex of plant extracts and herbs (Rupani and Chavez, 2018; Verma et al., 2019). The potential effects of these extracts include body detoxification, free radical scavenging, cell cycle alteration, DNA repair, immune surveillance, autophagy, apoptosis mitigation, anti-angiogenic effect, anti-inflammatory effect, anti-metastatic activity, and modulation of the signaling pathway (Arumugam et al., 2014; Omóbòwálé et al., 2016; Patel et al., 2016).
This review presents the economic importance and recent developments pertaining to the beneficial aspects of F. benghalensis to human health and well-being. Scientific reports and patent literature on the medicinal and economic importance of various extracts of F. benghalensis have been studied and analyzed to bring out its significance as an antimicrobial and antioxidant agent and its potential role in various extenuating inflammations and diseases like cancer, diabetes, diarrhea, rheumatism, etc. The authors have also analyzed the patent literature to learn about the other commercial applications of its phytochemicals. This review highlights the potential of F. benghalensis in medicine and as a promising component for a variety of cosmetic products.
METHODOLOGY
Literature search
A variety of search engines and databases including Google Scholar, Semantic Scholar, Science Direct, Research Gate, Scopus, and PUBMED were explored for obtaining various scientific findings on medicinal and commercial applications, and patent filings of F. benghalensis. The keywords used for potential publications search included terms and Boolean operators: (“F. benghalensis”) AND (“Chemical compounds” OR “Bioactive compounds”) AND/OR (“Traditional uses” OR “Conventional applications”), and (“Ficus benghalensis” AND “Antioxidant”) AND/OR (“Anticancer” AND/OR “Antimicrobial” AND/OR “Anti-inflammatory”). For nanotechnological applications, (“Ficus benghalensis”) AND (“Nanoparticle” AND/OR “Nanotechnology”) were used. The comprehensive search yielded around 350 publications consisting mainly of research papers, review articles, short communications, conference proceeding papers, and book chapters. However, only research and review articles were considered for the present updated and comprehensive review.
Patent landscape
After going through the literature which was focused on F. benghalensis, the relevant keywords were shortlisted under two broad categories. The first category was related to medicinal importance and the second category was commercial usage of the plant. After shortlisting the keywords, search strings were made which were uploaded onto the search interface of a paid database named Thomson innovation. Different Boolean operators were used to narrow down the search results. Searches made on the databases were restricted to titles, abstracts, and claims. All the patent documents from different jurisdictions were analyzed and no international classification system code was used for carrying out the searches.
Preliminary screening of patents
After downloading the results, a preliminary analysis was carried out to shortlist the most relevant patent documents, and the shortlisting was based on analysis made on the titles, abstracts, and claims of the patent document. Preliminary analysis was important to remove nonrelevant patents focusing on the other aspects of the plant.
Detailed analysis of patents and report preparation
All patents describing any medicinal or commercial usage of the plant were shortlisted as relevant and analyzed in detail to find out various commercial applications of the plant that were listed in Table 4. The bibliographic data of the shortlisted patents was used to study the patenting trends, and the key players commercializing the preparations made from this plant.
CONVENTIONAL MEDICINAL/ETHNOMEDICINAL APPLICATIONS
Typically, different parts (Fig. 1) of F. benghalensis, i.e., leaf, stem bark, root, fruit, latex, and bud are used in medical folklore and preparation of Ayurvedic remedies to treat various kinds of diseases like liver and spleen enlargement, diabetes, dysentery, leprosy, diarrhea, leucorrhoea, lung infections, asthma, cough, prills, heart diseases, rheumatism, gonorrhea, seminal weakness, and skin disorders like burns, wounds, pimples, sores, and ulcers (Patel and Gautam, 2014). Table 1 lists the detailed ethnomedicinal uses of various parts of F. benghalensis.
 | Figure 1. Ficus benghalensis leaves, fruits, aerial roots, and stem bark. [Click here to view] |
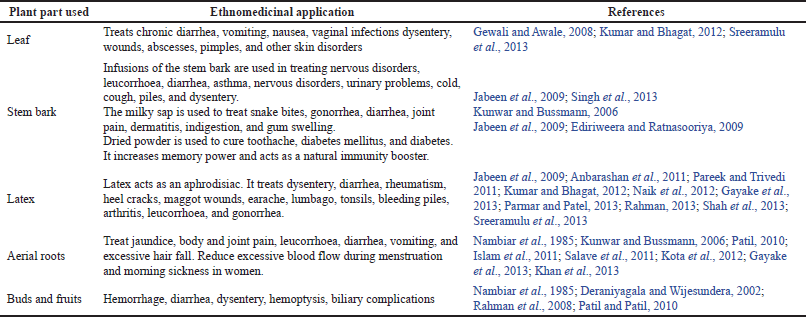 | Table 1. Ethnomedicinal applications of different parts of F. benghalensis. [Click here to view] |
BIOACTIVE COMPOUNDS/PHYTOCHEMISTRY
Plants are the source of various phytochemicals in the form of secondary metabolites, which function as a remedy for many human body ailments (Gopukumar and Praseetha, 2015; Rahman and Khanom, 2013). By preliminary phytochemical investigation, many phytochemical constituents have been reported in various parts of F. benghalensis which are responsible for its diverse medicinal and pharmacognostic properties. They contain terpenes, flavonols, flavonoids, triterpenoids, methyl esters, sterols, coumarins, carbohydrates, amino acids, saponins, and tannins. Table 2 lists the phytoconstituents of F. benghalensis.
PHARMACOLOGICAL ACTIONS
Antioxidant (free radical scavenging capacity) potential
Typically, antioxidant activity is the compound’s ability to inhibit or delay the oxidation process by free radical scavenging and oxidative stress reduction. Oxidative stress generates free radicals or reactive oxygen species that are the primary cause of inflammation and are found to be associated with the pathology of various diseases such as diabetes, cancer, aging, cardiovascular disease, and many inflammatory conditions. They react upon several bio-molecules and exert damage by removing electrons to enter into a stable state, rendering the cell in a condition of oxidative stress (Alzohairy, 2016; Kiranmai et al., 2011). Consequently, it becomes necessary to neutralize or stabilize these radicals for blocking or preventing oxidative stress exacerbation by providing an adequate supply of antioxidant compounds such as catalase, glutathione, superoxide dismutase, nitric oxide dioxygenase, and glutathione peroxidase which complement the job of body’s natural antioxidant defenses (Basir and Shailey, 2012; Gautam et al., 2015). These antioxidant compounds prevent multiple diseases by resisting oxidative stress through free radical scavenging, lipid peroxidation inhibition, and several other mechanisms. Supplementing in everyday diets in the form of oils, teas, and infusions is one of the simplest ways to supply such compounds to the body, e.g., supplementing natural extracts such as those derived from medicinal plants like F. benghalensis. Ficus species are a very rich source of natural antioxidants and various studies elucidated the outstanding antioxidant potential of F. benghalensis. In a study on ethanolic extract of F. benghalensis fruit, the maximum scavenging activity against 2, 2-diphenyl-1-picrylhydrazyl (DPPH) was reported to be 75.74% with a concentration at which 50% inhibition (IC50) occurs 32.20 μg/ml at 60 μg/ml concentration. Against 2, 2-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS), the maximum scavenging activity was reported to be 79.57% with IC50 13.69 μg/ml at a concentration of 30 μg/ml. Radical scavenging activity against nitric oxide and hydroxyl ions was found to be 51.96% with IC50 57.74 μg/ml and 57.02% with IC50 34.37 μg/ml, respectively, at 60 μg/ml concentration. The maximum phosphomolybdenum and Fe3+ reduction were reported to be 80.48% with a concentration at which 50% recovery (RC50) occurs 13.78 μg/ml, and 69.19% with RC50 18.71 μg/ml, respectively, at 60 μg/ml concentration (Tharini et al., 2018). In another study by Bhanwase and Alagawadi (2016), five-leaf extracts (hydroalcohol, n-butanol, n-hexane, water, and chloroform) were studied using DPPH and ABTS assay from concentrations 7.8–1,000 μg/ml. Vitamin C and quercetin were taken as reference standards, and scavenging activity against DPPH was observed in order Vitamin C > quercetin > n-hexane > hydroalcohol > water > chloroform > n-butanol with IC50 values 11.5, 15.4, 32.3, 28.2, >1,000, >1,000, and 125.0 for Vitamin C, quercetin, n-hexane, hydroalcohol, water, chloroform, and n-butanol, respectively. Scavenging activity against ABTS was observed in the order of Vitamin C > quercetin > hydroalcohol > n-hexane > water > n-butanol > chloroform, and IC50 was observed to be 6.40, 7.05, 52, 58.2, 491, >1,000, and 20.3, respectively. In both cases, hydroalcoholic and n-hexane extracts exhibited the strongest activity, whereas water, chloroform, and n-butanol extracts exhibited weak activity. Another investigation reported maximum scavenging (96.07% and 69.23%) against DPPH and hydrogen peroxide (H2O2), respectively, at a concentration of 250 μg/ml by aqueous extract of root (Gupta and Sharma, 2010). In yet another study, methanolic and ethanolic extracts of F. benghalensis root were reported to possess significant antioxidant activity with IC50 80.14, 982.93 and 38.66, 261.24, respectively, against DPPH and ferric reducing antioxidant power (FRAP) assay (Verma et al., 2015). Meanwhile, the stem bark aqueous extract was reported to have a significant inhibition of lipid peroxidation with IC50 80.24 μg/ml compared to tetraethoxypropane (Satish et al., 2013). Besides that, methanolic latex extract showed potential scavenging activity against DPPH, FRAP, and phosphomolybdenum with IC50 28.63, 49.82, and 31.84 μg/ml, respectively, compared to ascorbic acid and trolox (Yadav et al., 2011a, 2011b). Shukla et al. (2004) reported increased levels of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase in hypercholesterolemic rabbits treated with stem bark aqueous extract. Similarly, Manian et al. (2008) evaluated the antioxidant activity of fruit and stem bark extracts through various assays and obtained significant results. Apart from these, the seed ethanolic extract was reported to have the potential antioxidant capacity with plentiful amounts of secondary metabolites like polyphenols (Govindan and Francis, 2015). The abundant presence of phenolics, flavonoids, tannins, saponins, alkaloids, carbohydrates, fats, and oils reported in F. benghalensis is held responsible for its antioxidant capacity (Rao et al., 2014).
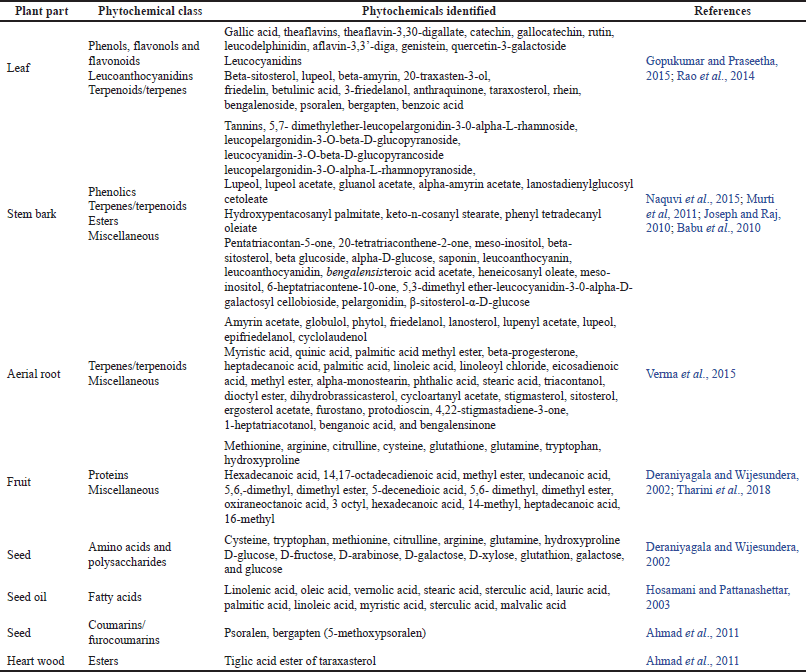 | Table 2. Phytochemicals present in different parts of F. benghalensis. [Click here to view] |
Antimicrobial activity
Many microorganisms like bacteria, fungi, and viruses cause various kinds of infections and deadly diseases when get entered any biological system. Antimicrobial agents are those natural or synthetic substances that kill or inhibit the microorganism’s growth. The search for novel antimicrobial compounds from plants and various other natural sources has increased in recent times since microorganisms, especially human pathogens are continuously developing drug resistance. Currently, plant-based medicines serve as the first line of defense in sustaining human health and fighting against many serious diseases for about 80% of the world’s population. A diverse set of studies published over time have well estimated the antimicrobial potential of F. benghalensis. Its fruit latex was found to possess more inhibitory effects than F. elastic fruit latex against Gram-positive (Streptococcus pyogenes and Staphylococcus aureus) and Gram-negative (Escherichia coli PBR322, E. coli each from stool and patients suffering urinary tract infection, E. coli resistant for ampicillin and tetracycline, Proteus mirabilis, Klebsilla pneumonia, Pseudomonas aeruginosa, Seratia spp., and Salmonella spp.) bacteria and fungus species like Candida (C. albicans, C. cruzii, C. tropicalis, C. sojae, and C. kefyr) (Faisal, 2017). A study by Afzal et al. (2020) evaluated the antimicrobial activity of leaf, root, and fruit against different bacteria and reported potent results. The zone of inhibition (ZOI) exhibited against S. aureus was reported to be 7.5, 5.4, 6.9, and 8.6 mm; against E. coli: 8.5, 9.6, 6.4, and 8.6 mm, against Pseudomonas protobacteria: 0.6, 0.4, 1.2, and 0.73 mm, and against Bacillus cereus: 0.5 0.6, 0.9, and 0.6 mm by ethanolic, methanolic, and aqueous extracts, respectively, in agar disc diffusion method. In another study, ethanolic extract from leaves has been reported to possess the strongest activity against a fish pathogen Aeromonas hydrophila with ZOI 10–12 mm (Tkachenko et al., 2016). In yet another study, aqueous and ethanolic extracts of roots were found to possess promising antimicrobial activity. The ZOI was reported to be 20, 15, and 12 mm for 25 mg/ml; 25, 20, and 18 mm for 50 mg/ml and 30, 24, and 22 mm for 75 mg/ml by ethanolic extract and 0, 16, and 0 mm for 25 mg/ml; 18, 0, and 0 mm for 50 mg/ml and 14, 12, and 14 mm for 75 mg/ml by aqueous extract, respectively, against S. aureus, E. coli, and K. pneumonia. Thus, maximum activity was reported against S. aureus by both extracts (Murti and Kumar, 2011). Meanwhile in yet another study, methanolic, aqueous, and petroleum ether extracts of fruit were compared and ZOI was reported to be 8, 6, and 5 mm for aqueous; 6, 0, and 0 mm for metanolic; and 5, 5, and 7 mm for petroleum ether extracts against E. coli, Salmonella typhi, and Lactobacillus acidophilus. Aqueous and ether extract of fruit was found to possess positive activity against all three bacteria. Aqueous extract possessed the highest inhibition for E. coli and ether extract for L. acidophilus. The methanolic extract was most effective against E. coli and no response was shown against L. acidophilus and S. typhi (Gaherwal, 2013). On the other hand, the antimicrobial activity of two compounds, genistein and catechine, isolated from methanolic leaf extract was tested using disc diffusion assay against different bacteria and fungi at doses level of 100 μg/ml. For reference standards, nystatin and streptomycin were used at dose levels of 50 and 25 μg/disc, respectively. Antibacterial activity was shown by both the compounds in comparison to nystatin and streptomycin against P. aeruginosa and B. cereus. No anti-fungal activity was reported for Candida lipolytica, Aspergillus ochraceus, Acchromyces lipolytica, and Sacchromyces cereviseae (Almahy and Alhassan, 2011). Similarly, stem bark exhibited remarkable antibacterial activity against S. aureus, K. pneumonia, and P. aeruginosa exhibiting 12, 13, and 10 mm ZOI, respectively, at 1 mg/ml concentration; and 0.1, 0.08, and 0.04 mg/ml minimum inhibitory concentration (MIC), respectively, against each tested organisms in comparison to standard drugs ampicillin, tetracycline, and chloramphenicol which exhibited MIC 0.03, 0.026, and 0.013 mg/ml, respectively, (Gayathri and Kannabiran, 2009). The bark extracts were found to possess more promising activity against enterotoxigenic E. coli isolated from diarrheal patients in a comparative study consisting of F. religiosa and F. benghalensis. At a concentration of 200 mg/ml, methanolic extracts possessed remarkable activity in comparison to aqueous and chloroform extracts, whereas hexane and petroleum ether extracts possessed zero activity (Uma et al., 2009). Yet another study by Aswar et al. (2008) exposed the significant antimicrobial potential of methanolic, aqueous, and chloroform extracts of underground roots against B. subtilis, P. aeruginosa, S. aureus (Gram +ve bacterial strains), K. pneumonia, E. coli, E. coli mutant (Gram −ve bacterial strains), and Aspergillus niger, a fungal species by cup plate method. Inhibitory activity of aqueous and methanolic extracts was observed at a concentration of 0.4 mg/well against all bacterial and fungal strains. The aqueous extract inhibited no bacterial or fungal strain at 0.04 and 0.004 mg/well concentration, whereas methanolic extract inhibited all bacterial and fungal strains at 0.4 mg/well concentration. At 0.04 mg/well concentration, inhibitory activity was reported against all bacterial strains; however, no activity was observed against fungal strains. At 0.004 mg/well concentration, activity was observed against all except E. coli, Aspergillus species, and B. subtilis. At concentrations 0.4 and 0.04 mg/well, chloroform extract inhibited all fungal and bacterial strains; however, no bacterial and fungal strains except for E. coli mutants and S. aureus were inhibited at concentrations 0.004 mg/well. In further studies, aqueous and hexane extracts of aerial roots exhibited sustained activity against different bacterial strains at 25, 50, and 75 mg/ml concentration, and the strongest activity against S. aureus (Singh and Geeta, 2010). Recently, aqueous leaf extract showed to exhibit significant activity against isolates of human immunodeficiency virus (HIV), HIV-1VB59 and HIV-1UG070 in PM1 and TZM-bl cell lines (Palshetkar et al., 2020). Thus, it can be concluded that F. benghalensis extracts could be potential sources of future antimicrobial drugs. However, further investigations are required to properly identify the bioactive compounds for comprehensive pharmacological studies and developing clinical applications.
Anticancerous/cytotoxic/antimitotic/antitumor/antiproliferative effect
Cancer is one of the most fatal diseases which badly affect the human population causing thousands of deaths annually throughout the world (De Flora et al., 2001). Death rate has increased many folds since 2000 due to cancer and in the year 2020, around 10 million deaths have been reported by World Health Organization. Biochemically, any abnormality or change in the cell division process that leads to uncontrolled cell division resulting in malignancy is cancer (Watson et al., 2004). Therefore, suppression of such uncontrolled cell division becomes crucial in patients with cancer (Lamy et al., 2006). Drugs used in chemotherapy for cancer treatment essentially work by targeting fast-dividing cells (antiproliferative/antitumor) and by inhibiting cell division (antimitotic), hence referred to as cytotoxic drugs. Hence, researchers nowadays are searching for substances that can be used in preventing cells from dividing. Owing to their great medicinal potential, medicinal plants are being studied extensively and explored for isolating anticancerous drugs to develop more efficient cancer treatment (Raheel et al., 2017). Aphale et al. (2018) evaluated the anticancer potential of Panchvalkala (a conventional Ayurvedic formulation consisting of equal proportions of bark materials from five different plants, namely, F. benghalensis, F. glomerata, F. religiosa, Ficus virens, and Thespesia populnea) in cervical cancer cell lines using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) dye assay. The cells were cultured with different concentrations of Panchvalkala and a significant reduction was reported in the viability of SiHa (75.2%) and HeLa (75.03%) cells after 24 hours at 80 μg/ml concentration of Panchvalkala. Further investigation by Aphale et al. (2021) reported the anticancer mechanism of Panchvalkala in cervical cancer cell lines as well as in mouse papilloma models. This study showed Panchvalkala induced programmed cell death/apoptosis and increased generic caspases expression in cervical cancer cells. The membrane potential of mitochondrion was reported to be reduced by Panchvalkala in both SiHa and HeLa cells in a dose-dependent manner which led to membrane depolarization resulting in apoptosis. Further, an increased expression of p53 and pRb, tumor suppressor proteins have also been reported at all doses in both SiHa and HeLa. At 40 μg/ml concentration of Panchvalkala, pRb, and p53 expression was increased by 1.63 and 2.38 folds, respectively,O in SiHa as compared to the control. On the contrary, phospho Rb expression was decreased by 2.40 folds. Furthermore, Panchvalkala affected the expression of human papillomavirus oncoproteins, E6 and E7. At 80 μg/ml, expression of E6 and E7 was decreased by 3.19 and 2.32 folds, respectively, in SiHa when compared to the control. On the other hand, at 80 μg/ml dose in HeLa, expression of E6 and E7 was decreased by 5.81 and 2.62 folds, respectively, compared to the control. Thus, mice treated with Panchvalkala showed a significant reduction in tumor growth as compared to the untreated group. Meanwhile, in yet another study, hydroalcoholic extract of F. benghalensis bark was reported to possess higher cytotoxicity against adenocarcinomic human alveolar basal epithelial cells (A549) with IC50 193.78 mg/ml and significantly lesser cytotoxicity to Chinese hamster ovary with IC50 193.78 mg/ml (Khanal and Patil, 2020). Ethanolic extract of aerial roots exhibited anticancer activity on breast cancer (MDA-MB-231), lung cancer (A549), and cervical cancer (Hela) cell lines with IC50 97.89, 17.81, and 49.27 μg/ml, respectively (Saloni and Sakthivel, 2019). Ethanol and ethyl acetate extracts of latex were found to exhibit promising antiproliferative activity against cell lines including peripheral blood lymphocytes, neuroblastoma, colorectal, and human breast cells. Ethanol extract has shown potent dose-dependent cytotoxic activity against human neuroblastoma cells (IMR 32) and human colorectal carcinoma (HCT 116) with IC50 123.27 and 99.82 μg/ml, respectively, whereas ethyl acetate extract has shown potent activity against human breast adenocarcinoma (MDA MB 231) with IC50 75.66 μg/ml colorectal (Tulasi et al., 2018a, 2018b). Another similar study reported the potent cytotoxic activity of ethanolic extract of latex against human breast cancer cell lines MCF-7 with IC50 101.55 μg/ml (Tulasi et al., 2018a, 2018b). An investigation conducted on combined leaf extract of F. benghalensis and F. religiosa showed that with an increase in the concentration of combined leaf extract of both the plants, cell viability decreased i.e., only 50% of the cells remained viable at 100 μg/ml concentration (Kumaresan et al., 2018). F. glomerata has also been investigated for its antimitotic and antiproliferative activity. The strongest antimitotic activity with a mitotic index of 28% and antiproliferative activity with only 8% viable cells were reported with an n-butanol fraction of stem bark methanolic extract at 4 mg/ml concentration using Allium cepa root tip and yeast cell models for antimitotic and antiproliferative activity, respectively (Raheel et al., 2017).
Antimutagenic activity
Apart from causing carcinogenesis and genotoxicity, mutagens are also involved in chronic inflammation, aging and inception and pathogenesis of numerous diseases including cardiovascular disorders, arthritis, diabetes, and hepatic disorders. By consuming natural antimutagens, the detrimental effects of such mutagens can be minimized. Natural antimutagens which include plant metabolites such as phenolics, flavonoids, carotenoids, coumarins, anthraquinones, tannins, and saponins are found in medicinal plants and are of meticulous importance in disease prevention. F. benghalensis exhibits a potential antimutagenic effect that has been elucidated by different studies. Aqueous heat-treated stem bark extract possessed significantly higher antimutagenic activity (as evaluated through Ame’s test) with IC50 70.24 μg/ml on Salmonella typhimurium TA100 strains in comparison to heat-treated root extract of Mongifera aleifera (IC50 value 99.20 μg/ml) against sodium azide (Satish et al., 2013). Meanwhile, methanolic stem bark extract exhibited excellent antimutagenic effect inhibiting micronucleus formation and chromosomal aberrations against cyclophosphamide-induced genotoxicity in Swiss-albino rat models through micronucleus assay and chromosomal aberration assay (Sharma et al., 2012).
Antihyperglycemic/antidiabetic activity
Diabetes mellitus is a kind of chronic endocrine disorder that is associated with high blood glucose level (BGL) (hyperglycemia), occurring either from insufficiency of insulin secretion, or its action, or both. It is a heterogeneous group of diseases that are characterized by defective metabolism of glucose, proteins, and fats affecting millions of people worldwide. The commonly occurring symptoms are polyurea (increased urine production), polyphagia (excessive eating), polydipsia (excessive thirst), and fatigue, weight loss (Otunola and Afolayan, 2019). It continues to be the most prevalent healthcare problem throughout the world and is estimated to increase from the present 382 to 471 million by the year 2035 (Bi et al., 2017; Ogurtsova et al., 2017). Various treatments such as insulin, diet, and pharmacological therapies which work via a variety of mechanisms in managing diabetes are currently being employed (Otunola and Afolayan, 2019). Despite the substantial progress in diabetes management through the use of various drugs and management strategies, diabetes continues to be a major medical challenge. Almost all the synthetic oral hypoglycemic drugs used for treating the disease have disadvantages including serious side effects, drug resistance, toxicity, and cost (Haque et al., 2010; Kumari et al., 2013). The use of herbal plants has an extensive folkloric history for treating blood sugar abnormalities. Ethanolic extract of fruit, bark, and aerial roots of F. benghalensis were investigated in alloxan-induced diabetic rat models. Varying intensities of hyperglycemia were produced in rat models by giving different doses of alloxan and a significant reduction of 31.73%, 18.33%, and 28.84% in BGLs was reported, respectively, through administration of fruit, aerial root, and bark ethanolic extracts compared to standard drug glibenclamide that reduced BGL by 34.4%. Thus, it was clearly revealed that fruit extract is a more potent antidiabetic agent than aerial root or bark (Sharma et al., 2010). Meanwhile, bark extract was reported to reduce the BGLs in streptozotocin (STZ)-induced diabetic rats by stimulating insulin secretions from beta cells of Islets of Langerhans. Reduction in BGLs was 48.61%, 61.66%, and 68.24% at dose levels of 150, 300, and 500 mg/kg, respectively, in STZ-induced rats as compared to glibenclamide-induced rats at a dose level of 0.5 mg/kg on 15th day (Kasireddy et al., 2021). Similarly, hydroalcoholic bark extract normalized lipid, renal, and hepatic profiles and reduced BGL by 29.26%, after 21 days of treatment in Alloxan-induced diabetic rats at dose levels of 120 mg/kg (Saifi et al., 2014). Meanwhile, anti-diabetic activity against alloxan-induced rat models was investigated by Ahmed et al. (2011) and a significant reduction in BGLs was reported with inhibited alpha-glucosidase and sucrose exhibiting IC50 77.0 μg/ml and IC50 141.0 μg/ml, respectively. Another study on stem bark by Gayathri and Kannabiran (2009) under the same experimental conditions reported similar results. Leucopelargonin, a compound reported in stem bark extract of F. benghalensis, held responsible for insulin production from beta cells and thereby regulating BGLs and hence regulating diabetes within 5 hours of oral administration compared to standard drug tolbutamide (Fig. 2A and B). Leucopelargonin-stimulated beta cells of Islets of Langerhans from STZ-induced diabetic rats to increase insulin production (Fig. 2A). Glucose enters the beta cell through glucose transporters and generates adenosine triphosphate (ATP) through a chain of processes. Augmented ATP/adenosine diphosphate ratio leads to the shutting down of the ATP-sensitive K+ channels in the cell membrane which results in extracellular Ca2+ influx and activates exocytosis of insulin granules (Fig. 2B).
Similarly, Pelargonidin 3-O-alpha-L rhamnoside, a flavonoid isolated from stem bark was reported to exhibit positive activity and reduced BGLs by 19% while improved glucose tolerance by 29% as compared to 25% and 66% respectively by glibenclamide. Moreover, insulin secretion by beta-cells was reported to be higher in pelargonidin derivative presence (Cherian et al., 1992). Meanwhile, Achrekar et al. (1991) and Khanal and Patil (2021) investigated bark activity against STZ-induced rats and results revealed enhanced glycolysis, glucose uptake, insulin secretions, and decreased gluconeogenesis. Another study by Singh et al. (2009) investigated aerial roots in normal and diabetic rat models and compared it with the standard drug Glipizide at 300 mg/kg dose level using glucose tolerance tests and fasting blood glucose studies. Results showed 40.7%, 54.8%, and 51.7% improvement in glucose tolerance of normal, semi, and mild-diabetic animals 3 hours post-exposure during the glucose tolerance test. Furthermore, fall reported in BGL at the most effective dose, i.e., 300 mg/kg, was found to be higher compared to Glipizide (2.5 mg/kg) in sub-diabetic, and almost similar in mild-diabetic rats. Glycemic elements Ca2+ and Mg have been reported in higher amounts (0.85% and 1.02%) in the most effective dosage by laser-induced breakdown spectroscopy technique. Ca2+ initiates insulin gene expression via calcium-responsive element-binding protein which ultimately results in insulin exocytosis. Mg has also been reported to be associated with diabetes management in past studies by Feng et al. (2020). BGLs have been found reported to fall in other groups of animals when the same doses of elemental Ca2+ and Mg were given, and further biochemical and pharmacological studies are in progress to explicate the action mechanism of the extracts in detail at a molecular level. Meanwhile, an alpha-glucosidase inhibitor that inhibits the aldose reductase enzyme was reported in F. benghalensis by an in-silico study. This enzyme is reported to be involved in glucose metabolism pathways that control diabetes. Three flavonoids, namely kaempferol, 3,4’,5,7-tetrahydroxy-3’-methoxy flavones, and apigenin found to exhibit high affinity toward aldose reductase and were predicted to be involved in the modulation of proteins via p53 signaling pathway (Khanal and Patil, 2019). Apart from these, ethanolic leaf extract reportedly reduced the cholesterol, triglycerides, and glucose levels in alloxan-induced diabetic albino rats (Saraswathi et al., 2013). Similarly, decreased levels of low-density and very low-density lipoprotein cholesterol by 60%, serum cholesterol by 59%, and triacylglycerol by 54% were reported in hypercholesterolemic rabbits upon treatment with bark aqueous extract. It further decreased lipid peroxidation as indicated by decreased TBARs with a consequent improvement in glutathione content in the blood (Shukla et al., 2004).
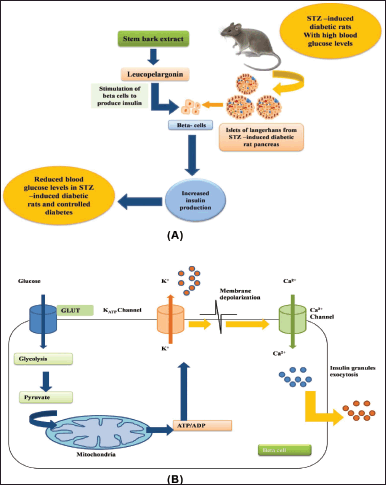 | Figure 2. (A) Antidiabetic effect of leucopelargonin in STZ-induced diabetic rats. (B) Key mechanisms involved in glucose-induced insulin secretion. [Click here to view] |
Antihyperlipidemic and hypocholesterolemic activity
Stem bark aqueous extract at a dose level of 50 mg/kg p.o. decreased the levels of low-density and very low-density cholesterol, serum cholesterol, and triacylglycerol by 60%, 59%, and 54% and increased the levels of glutathione reductase, catalase, superoxide dismutase, and glutathione peroxidase by 22%, 30%, 36%, and 90%, respectively in hypercholesterolemic rabbits (Shukla et al., 2004). A similar study on bark aqueous extract in hypercholesterolemic rabbits under similar experimental conditions reported increased high-density lipoprotein cholesterol to 30.6 mg% and decreased triacylglycerol to 89 mg% (25.6 and 188 mg%, respectively, in control), and 48% decrease in total cholesterol levels after 4 weeks of treatment (Shukla et al., 1995a, 1995b). Meanwhile, alloxan-induced sub-diabetic and diabetic rabbits were studied again under similar experimental conditions and decreased low-density lipoprotein, total cholesterol, and triglyceride levels from 34 to 16, 82 to 42.7, and 121 to 45 mg%, respectively, in sub diabetic and from 95 to 29, 118 to 51.7, and 416 to 81 mg%, respectively, in diabetic rabbits were reported (Shukla et al., 1995a, 1995b).
Anti-inflammatory activity
Inflammation plays a role as a defense mechanism against any harmful stimuli such as injury, irradiation, toxic compounds, and pathogens, thus initiating the healing process (Chen et al., 2018; Dai and Medzhitov, 2017). Fatty acid glucoside isolated from ethanolic leaf extract was found to exhibit significant anti-inflammatory activity against lipopolysaccharide-stimulated RAW 264.7 macrophages. It inhibited the activity of cyclooxygenases (enzymes which contribute to fever, pain, and inflammation), whereas increased the release and activity of nitric oxide and inducible nitric oxide synthase (enzymes that give anti-inflammatory responses). Furthermore, it directly binds to epidermal growth factor receptor with a high affinity as proposed by molecular docking studies suggesting it to be an inhibitor for the same that further inhibits the downstream phosphoinositide-3 kinase/Akt signaling cascade to reduce inflammation further (Alaaeldin et al., 2022). Meanwhile, methanolic leaf extract was reported to reduce paw edema by 65.21% at 200 mg/kg p.o. dose level as compared to diclofenac sodium reduced it by 62.31% in rat models with formalin-induced paw edema (Kothapalli et al., 2014). Similar studies on methanolic leaf extract against carrageenan-induced hind paw edema reported a significant reduction in paw edema at 10–100 mg/kg p.o. as compared to aspirin (Mahajan et al., 2012). On the other hand, aqueous extract of aerial root showed significant activity in a dose-dependent manner against carrageenan-induced paw edema, and cotton-pellet-induced granuloma in rats at 100–200 mg/kg p.o. dose levels (Deore et al., 2012). Meanwhile, ethanolic bark extract inhibited carrageenan-induced hind paw edema by 31.50%, 56.16%, and 69.86% at dose levels 50, 100, and 200 mg/kg, respectively, as compared to diclofenac sodium which inhibited by 72.60% at 5 mg/kg in rat models (Wanjari et al., 2011). Further, aqueous and methanolic bark extracts possessed effective activity against paw edema, cotton-pellet-induced granuloma, and acetic acid-induced vascular permeability by significantly preventing marker enzymes such as aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) in serum forming malondialdehyde in edematous tissue. They also reduced myeloperoxidase levels considerably (Thakare et al., 2010). In the meantime, another study compared stem barks of young and matured plants of F. benghalensis using cotton pellet-induced granuloma for chronic inflammation and carrageenan-induced hind paw edema for acute inflammation in rats. Ethanolic, chloroform, and petroleum ether extracts were studied and results were compared to indomethacin. At 300 and 600 mg/kg/day p.o. dose levels, extract of ethanolic bark of younger plants reduced paw edema by 37.64% and 69.04%, whereas mature plant extract reduced it by 55.03% and 65.54%, respectively, in the carrageenan-induced paw edema model. Younger plant ethanolic extract caused 19.27% and 39.03% reduction, whereas mature plant bark extract caused 14.12% and 34.25% reduction, respectively, in the cotton pellet granuloma model. Thus, the younger plant was concluded to possess relatively more activity than the mature plant. No activity was exhibited by chloroform and petroleum ether extracts (Patil and Patil 2010). Similarly, ethanolic extract reduced paw edema volume by 55.03% and 65.54%, whereas petroleum ether extract reduced it by 0.4.73% and 7.43% at 300 and 600 mg/kg, respectively, after 3 hours of treatment (Patil et al., 2009). Meanwhile, another study reported significant activity at 100–500 mg/kg p.o. dose levels against carrageenan-induced paw edema in rats (Navanath et al., 2009).
Immunomodulatory activity
Medicinal plants contain a rich amount of substances responsible for immunity induction. Any substance of biological or synthetic origin that helps stimulate, suppress, or modulate any aspect of the adaptive or innate immune system is known as an immunomodulator, and the mechanism is called immunomodulatory activity (Kumar et al., 2012). Naturally derived immunomodulators are generally categorized as high- and low-molecular-weight compounds. More specifically, classified literature shows that compounds responsible for immunomodulation are phenolics, carbohydrates, steroids, coumarins, terpenes, proteins, amino acids, alkaloids, glycoproteins, and other nitrogen-containing compounds. Different studies indicated the enormous immunomodulatory potential of F. benghalensis. Aqueous extract of panchvalkala at 100, 200 mg/kg b.w. dose levels increased thymus and spleen indices and induced splenocyte proliferation. Its in-vitro upregulated T-helper1 (Th1) (InterLeukin-2) cytokine levels are responsible for the activation of cytotoxic lymphocytes and promotion of activated T cells’ differentiation and proliferation and thereby enhanced killing effect of T-cells that further play an important role against tumors and viruses. The mouse papilloma model modulated the immune response by retarding tumor growth by shifting Th2 toward Th1 response (Aphale et al., 2021). The potential immunomodulatory activity was also reported from leaf hydroethanolic extract and its fractions using the Nitroblue tetrazolium test, candidacidal assay, and phagocytosis of killed C. albicans, which significantly stimulated phagocytic action of neutrophils. n-butanol and n-hexane fractions showed the highest activity with 89.66% and 80.33% stimulation in the phagocytic activity, respectively. Both of these fractions also showed the highest percentage (35.33% and 36.33%, respectively) of killed C. albicans at 1,000 mg/ml (Bhanwase and Alagawadi, 2016). Meanwhile, ethanol leaf extract was reported to enhance the growth of human peripheral blood mononuclear cells in a study conducted by Crossia et al. (2016). Tabassum et al. (2014) studied an aqueous extract of aerial root using hypersensitivity and hemagglutination reactions and hypersensitivity assays and reported increased hypersensitivity reactions and antibody titers producing a maximum response at 50 mg/kg b.w. Similarly, the immunostimulatory potential of growing aerial roots was studied for the first time by Sridevi et al. (2009). Remarkably increased percentage phagocytosis and phagocytic index by water and ethanolic extracts indicated higher phagocytic efficacy of polymorph nuclear cells due to more engulfment of Candida cells in comparison to control. In-vivo studies showed increased hypersensitivity reactions to antigen-sheep red blood cell antigen (SRBC) at 200 and 300 mg/kg dose levels. Other in-vitro and in-vivo studies on methanolic extract showed a significant increase in phagocytic index percentage as reported by Gabhe et al. (2006). Methanolic extract showed significant phagocytic activity (53%, 49%, and 46% at dose levels of 0.5, 1.0, and 2.0 mg/ml, respectively) as compared to control (31%), whereas aqueous extract showed 55% activity at 1.0 mg/ml compared to control (32%). This signifies that the phagocytic efficacy of the cells is enhanced by extracts as they cause more engulfment of the Candida cells versus control, and thereby stimulate a non-specific immune response. In-vivo studies indicated increased hypersensitivity reaction and antibody titer value to the SRBC antigen at dose levels of 100 and 200 mg/kg. Meanwhile, another study on methanolic root extract showed a significant increase in plaque-forming cells, increased circulating antibody titer, increased levels of lymphocytes and rosettes formation, and hematological parameters at dose levels of 100–300 mg/kg b.w. (Anarthe et al., 2016).
Wound healing potential
Wound healing is a process that occurs naturally after any kind of skin damage for proper repair and restoration of tissue’s normal function (Murti et al., 2011). An inflammatory response occurs following an injury, and the cells underneath the dermis start increased collagen production further regenerating epithelial tissue. Therefore, wound healing is a complex process typically consisting of three stages: inflammation, proliferation, and remodeling. Since ancient times, F. benghalensis has been used in wound healing (Gonzalez et al., 2016). The petroleum ether extract formulation of F. benghalensis leaves was found to be more potent and showed 100% wound healing in 21 days of the epithelization period in comparison to ethanol and aqueous extracts which took 25 and 28 days respectively in complete healing compared to standard nitrofurazone (marketed) which took 20 days (close to petroleum ether extract) for complete wound healing in albino rat models inflicted with excision wounds (Imran et al., 2021). Meanwhile, ethanolic and hydroalcoholic bark extracts showed complete wound healing activity in 17.19 and 18.37 days in excision wound models compared to the standard drug Soframycin (Raisagar et al., 2019). Similarly, various stages of wound healing through different wound models in rats were assessed using an aqueous extract of leaf and increased breaking strength due to increased collagen protein content, wound contraction, and hexosamine content were reported in rats’ incision, excision, and dead space wound models, respectively. The breaking strength was reported to be 439.55 and 526.72 g in oral and topical doses respectively as compared to 628.38 g of povidone-iodine, whereas total protein content was reported to be 9,397.97, 14,014.14, and 16,861.61 mg in oral, topical and povidone–iodine in incision model. The wound area on day 12 was reported to be 252.83, 196, and 60.5 mm² in oral, topical, and povidone-iodine excision models. A further wet and dry weight of granulation tissue was reported to be 178.5, 47.43, 104.1, 35, and 85, 16.16 in oral, topical, and povidone-iodine in dead space models (Chaudhary et al., 2014). A similar study reported the wound healing capacity of aqueous and ethanolic extracts of roots by the same experimental models in rats. The breaking strength of wounds was increased from 305.20 in normal control to 502.30 in aqueous and 455.80 in ethanol extract as compared to standard povidone iodine 429.70 in the incision model. In the excision model, aqueous and ethanolic extracts showed 100% and 94.83% wound contraction in 13.33 and 14.17 days, which was better than povidone-iodine which showed 92. 80% wound contraction in 16.00 days. The aqueous and ethanolic extracts, and standard povidone iodine showed 515.80, 97.33 mg/g, and 56.49 mg/ml; 479.00, 86.83 mg/g, and 45.08 mg/ml; and 351.80, 80.67 mg/g, and 40.80 mg/ml of granulation breaking strength, dry granulation weight, and hydroxyproline content respectively in dead space model in Wistar albino rats (Murti et al., 2011). Meanwhile, a similar study on bark aqueous and ethanolic extracts showed a significant reduction in the wound area and period of epithelialization in the excision wound model where ethanolic and aqueous extracts showed complete healing in 17.16 and 18.33 days respectively compared with control where complete healing occurred in 21.50 days. In the incision model, ethanolic and aqueous extracts showed significantly increased-breaking strength compared with a standard drug placebo (Garg and Paliwal, 2011).
Hepatoprotective
The liver is a crucial organ that regulates metabolic activities, storage, secretions, and body detoxification (Kumar et al., 2019). So, to ensure the proper functioning of the body, it is very important to protect it from different toxic substances acquired via contaminated food, water, synthetic medicines/drugs, and other factors. The hepatoprotective activity of ethanolic fruit extract was evaluated against acetaminophen, carbon tetrachloride (CCl4), and erythromycin-induced hepatotoxicity in goat liver, and effective results were obtained which could be attributed to its ability of free radical scavenging or restoring catalase (antioxidant enzyme) of the liver, compared to the standard drug, Silymarin (Karmakar et al., 2020). A similar in-vivo study on ethanolic fruit extract at a dose of 500 mg/kg p.o. against perchloromethane-induced hepatotoxicity in New Zealand albino rat models reported a significant reduction in otherwise elevated levels of liver biomarkers such as AST, ALT, and ALP, total serum bilirubin, and malondialdehyde upon fruit extract administration. This lipid-protective action can be attributed to the potential reduction of lipid peroxides by antioxidants like coumarins present in fruit extract (Ahad et al., 2021). Similarly, bark has also demonstrated promising activity against paracetamol and CCl4-induced hepatotoxicity models by significantly reducing levels of serum ALP, serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), and bilirubin (Baheti and Goyal, 2013). Meanwhile, Shinde et al. (2012) reported elevated levels of AST, ALT, and ALP, and decreased levels of total proteins and albumins against ethanol and CCl4-induced hepatotoxicity in rats by ethanolic leaf extract. Similarly, methanolic extract of aerial roots was reported to prevent the isoniazid-rifampicin-induced increase in the levels of serum marker enzymes and thiobarbituric acid reactive substances (TBARS) (a marker for oxidative stress) in the liver in experimental rats when orally administered. Furthermore, significantly increased total protein content and reduced glutathione levels were reported in treatment groups (Parameswari et al., 2012). Orally administered latex to albino rat models having paracetamol and CCI4-induced hepatotoxicity showed improved liver functions with significantly improved total protein levels, reduced SGPT, SGOT, ALP, and bilirubin levels (Chandrasekaran et al., 2012). Leucopelargonin derivative isolated from stem bark also showed promising results against carbon tetrachloride-induced hepatotoxicity in rats. Decreased levels of total cholesterol, low and high-density lipoproteins, triglycerides, biomarker enzymes like AST, ALT, and ALP in the liver and serum was reported. Hydroxymethylglutaryl-coenzyme-A reductase, glucose 6- phosphate dehydrogenase activities have also reduced, whereas the concentration of antioxidant enzymes in the liver was increased (Augusti et al., 2005).
Antihelminthic activity
Helminthes or parasitic worms affect a large portion of the population worldwide by causing different kinds of infections. For centuries, such parasites have been a big concern in the medical field, as they are prevalent infectious agents of multiple human diseases such as malnutrition, eosinophilia, pneumonia, and anemia in developing countries (Ambujakshi and Shyamnanda, 2009). Direct ingestion or penetration through the skin could be the probable reason for human infection. Hosts get deprived of food and consequently suffer blood loss, organ damage, and lymphatic obstruction which ultimately lead to death in many cases. Antihelminthic drugs kill or abolish the infesting helminth from the host body. During the past couple of decades, even though significant advancement has been made in understanding the transmission and treatment of these parasites, no efficient products could still be developed to gain complete control. Cases of resistance have also been reported to the indiscriminate use of certain drugs (Deore and Khadabadi, 2010). Modern synthetic medication system has proved to be effective in treating parasitic diseases but also leaves multiple side effects such as fever, nausea, dizziness, severe allergies, and headache. Crude drugs are comparatively less efficient than synthetic drugs in curing diseases but typically cause no side effects. In traditional medication systems, many plants are known to possess anthelmintic activity (Ghosh et al., 2011; Ravindra et al., 2008; Spiegler et al., 2017). Alcoholic and aqueous leaf extracts of F. benghalensis were investigated for their anthelmintic activity against the Indian earthworm Pheretima posthuma. Time taken for paralyzing and killing the earthworm was observed using different concentrations (25, 50, and 100 mg/ml) of each extract, 25 mg/ml concentration of Albendazole, and normal saline as control. Both extracts have been found to exhibit significant dose-dependent anthelmintic activity when compared to standard. The alcoholic extract exhibited the most potent activity by taking 2.12 minutes to paralyze and 7.34 minutes to kill earthworms at a concentration of 100 mg/ml compared to Albendazole, which took 2.36 minutes to paralyze and 6.42 minutes to kill earthworms at a concentration 25 mg/ml. Aqueous extract showed little lesser activity by taking 3.92 minutes for paralysis and 13.72 minutes for death at 100 mg/ml. On the contrary, no paralysis or death occurred in the control groups during the given period (Bhardwaj et al., 2012). Tuse et al. (2011), investigated methanolic extract of aerial roots using the above approach of paralyzing or killing Indian earthworms at 10, 20, and 50 mg/ml, and a dose-dependent activity was reported. Meanwhile, another study investigated the comparative anthelmintic activity of latex of F. religiosa, F. elastica, and F. benghalensis, and reported potent activity by F. benghalensis latex extract, which took 10.2 and 13.4 minutes for paralysis and 26.3 and 30.1 to completely kill the earthworm at 250 and 500 μl/ml dose levels, respectively. At the same time, the standard drug Metronidazole took 5 and 13.2 minutes, respectively, to paralyze and kill the earthworm at a dose level of 10 mg/ml (Hari et al., 2011). Yet another study compared aqueous extracts of F. benghalensis, F. religiosa, and F. carica fruits where F. benghalensis was found to be most potent killing all the test organisms within an hour of exposure (Sawarkar et al., 2010). Similarly, Aswar et al. (2008) investigated aqueous, methanolic, chloroform, and petroleum ether root extracts which took 3.44, 3.02, 3.71, and 4.03 minutes to paralyze and 4.34, 4.36, 4.91, and 6.18 minutes to kill the earthworm, respectively, at 20 mg/ml. On the other hand, Albendazole, the standard drug took 2.68 and 5.29 minutes to paralyze and kill respectively.
Antiarthritic activity
Herbal emulgel of F. benghalensis ethanolic aerial root extract showed antiarthritic activity compared to the standard drug diclofenac emulgel through the in-vitro release method (Sonali et al., 2021). In another study, maximum inhibition of protein denaturation and membrane stabilization of aqueous stem bark extract at a dose of 1,000 μg/ml was reported to be 45.31 and 62.50, respectively, while that of methotrexate was reported to be 87.50 and 81.25 at 100 μg/ml dose respectively in a study by Joshi et al. (2021). Meanwhile, aqueous and methanolic extract protein denaturation activity was found to be 83.80, whereas diclofenac sodium possessed 91.45 in the egg albumin method. On the contrary, the extract had 75.77, and diclofenac sodium possessed 90.60 in the bovine serum method at 400 μl/ml (Rajalakshmi and Tamilarasi 2019). On the other hand, ethanolic and aqueous extracts of aerial roots against complete Freund’s adjuvant-induced arthritis model in Wistar rats revealed a significant decrease in paw swelling with the highest percentage inhibition of 63.64% by ethanolic extract as compared to water extract which showed 31.82% inhibition at dose levels of 300 mg/kg b.w. p.o. Results were compared to the standard drug Indomethacin (Bhardwaj et al., 2016). The antiarthritic activity of stem bark methanolic extract was reported to be slightly more effective than that of diclofenac sodium and lesser than that of methotrexate and dexamethasone at 400 mg/kg/day p.o. against formalin and complete Freund’s adjuvant-induced arthritis in rats (Thite et al., 2014).
Antidiarrhoeal activity
Diarrhea ranges from mild to severe illness, a significant cause of malnutrition among children especially in developing countries. It causes about 4–5 million deaths annually across the world. Many plants including F. benghalensis possess the antidiarrheal activity and so act as a beneficial remedy for alleviating human suffering. Mahalakshmi et al. (2014) evaluated methanolic leaf extract against castor oil-induced diarrhea in Swiss albino mice and reported a significant reduction in the number of stools compared to control in a dose-dependent manner. On the other hand, a dose-dependent decrease in diarrhea was reported in Wistar albino rats in a study conducted on ethanolic bark extract in various experimental models, viz., gastrointestinal motility test, castor oil-induced diarrhea, Prostaglandin E2 (PGE2)-induced enteropooling. The extract showed a significant decrease in fecal output and dropping frequencies compared to the castor oil-treated rats, especially at 400 and 600 mg/kg doses. Considerable reduction in the charcoal meal propulsion and inhibition of PGE2-induced enteropooling was also reported (Patil et al., 2012). Similarly, the antidiarrheal activity of ethanolic aerial root extract at 400 mg/kg was evaluated against castor oil-induced diarrhea; PGE2-induced enter pooling, and gastrointestinal motility in charcoal meal test in albino rat models using standard anti-diarrhoeal agent diphenoxylate and atropine. Mean defecations per animal in 4 hours were reported to be 1.37 and 2.21. On the contrary, the mean number of wet feces per animal was 0.0 and 1.96 in rats treated with diphenoxylate and plant extract, respectively, in castor oil-induced diarrhea. The antimuscarinic drug atropine and plant extract also decreased propulsive movement of the intestine in the charcoal meal treated models, which was reported to be 34.2 and 50.2, respectively. Similarly, PGE2-induced exteropooling was also inhibited by plant extract, which was reported to be 2.97 and 1.25 ml in PGE2 alone and PGE2 plus plant extract, respectively (Mukherjee et al., 1998).
Antistress and antiallergic potential
“Stress is an emotional and physical tension that causes psychological and physiological disturbance when one’s emotions are challenged or when a certain demand in life is not fulfilled” (Murugesu et al., 2021). In the current era of a competing and demanding world, increased depression and even suicides are reported in individuals having stressful lives. Stress and intolerance have become very common, especially in youth. Physiological and psychological effects from natural sources like herbal medicines and yoga are much more helpful in treating such issues compared to modern synthetic drugs. Many scientific studies reveal the potential antistress and antiallergic activity of F. benghalensis (Jahagirdar et al., 2020; Khaliq, 2017; Vignesh et al., 2019). Ethanol, aqueous, and ethyl acetate extracts of stem bark showed potent antistress and antiallergic activity against milk-induced asthma in mice, which is mediated by decreasing eosinophils and leucocyte count. Conversely, no activity was reported from petroleum ether and chloroform extracts (Taur et al., 2007a, 2007b). Another study investigated methanolic extract of fruit at dose levels 125, 250, and 500 mg/kg b.w. using different tests, viz., swimming endurance, anoxia stress tolerance, and immobilization stress on Wistar albino rats and Swiss albino mice models. A remarkable dose-dependent antistress activity of fruit extract was reported; thus, it is considered as an adaptogenic agent in stress management (Jahagirdar et al., 2020). During stress, brain cells release a neurotransmitter, namely acetylcholine. Methanolic bark extract was studied for acetylcholinesterase inhibitory activity with an IC50 228.3 μg/ml against neuroblastoma cell lines, namely SHSY5Y (Vignesh et al., 2019). Flavonoids, rutin, and quercetin isolated from F. benghalensis leaf exhibited significant antistress activity, probably due to inhibition of monoamine oxidase-A and B, thereby increasing monoamines levels in tail suspension test, by preventing desensitization of central components of hypothalamic-pituitary-adrenal and non-specifically increased resistance in acetic acid-induced writhing test, by modulating receptors for gamma amino butyric acid and acting as an antagonist for N-methyl D-aspartate receptor in pentylenetetrazol-induced convulsions. The results were comparable to the standard drug diazepam (Lotankar et al., 2016).
Analgesic activity
An analgesic is any drug/substance used to achieve analgesia, i.e., relief from pain. Methanolic leaf extract showed potential analgesic activity in acetic acid-induced writhing and Eddy’s hot plate methods in rat models at dose levels of 100 and 200 mg/kg b.w. compared to potent drugs aspirin and diclofenac sodium (Mahajan et al., 2012). Meanwhile, a similar study on methanolic leaf extract reported significant activity at dose levels 200 mg/kg p.o., using Eddy’s hot plate method in rat models compared to Diclofenac sodium at 10 mg/kg p.o. (Kothapalli et al., 2014). Similarly, aqueous and ethanolic leaf extract at dose levels of 200 mg/kg, i.p. have shown effective analgesic activity compared to aspirin (Yadav et al., 2011a, 2011b). In yet another study, ethanolic bark extract showed 26.61%, 41.93%, and 61.28% whereas aqueous extract showed 40.72%, 52.02%, and 69.75% inhibition of writhing at dose levels of 100, 200, and 400 mg/kg p.o., respectively, compared to aspirin, which showed 75% inhibition (Garg and Paliwal, 2014). Methanol, ethanol, chloroform, and petroleum ether extracts of stem bark exhibited significant analgesic activity in acetic acid-induced writhing, hot plate method, and tail immersion tests compared to potent drugs like diclofenac sodium, aspirin, and pentazocine as reported by Thakare et al. (2010) and Chavan et al. (2015a, 2015b). Significant writhings were reported at 200 mg/kg b.w. against root extract by Panday and Rauniar (2016).
Anticoagulant activity
The process of obstructing blood clotting or thrombus formation, thereby preventing blood clotting is anticoagulant activity. Antithrombotic drugs are not satisfactory these days and often result in vascular relapses. Thus, there is a need to develop bioactive anticoagulant and antithrombotic drugs that are novel, more effective, and less toxic. Hence, research on developing anticoagulant drugs from herbal medicinal plants is encouraged. Investigation of methanolic leaf extract showed significant activity in human plasma with a prothrombin time of 21.7 seconds compared to the control (13.3 seconds). Activated partial thromboplastin time was reported to be 67.3 seconds compared to the control, which was 43.3 seconds. Therefore, delayed prothrombin and activated partial thromboplastin times were reported compared to the control (Ambreen et al., 2019).
Antinociceptive effect
The stem bark extract showed significant antinociceptive activity in Swiss albino mice at 100, 200, and 400 mg/kg p.o. in tail-flick and formalin tests. A remarkable increase in the time elapsed till the tail flicking from the thermal stimuli, i.e., extended duration of pain response, and a decrease in the licking response duration was reported with tested doses. Dose and duration-dependent results obtained were compared to morphine (Rajdev et al., 2018). Meanwhile, another study reported significant inhibition of clonidine-induced dose-dependent catalepsy in mice by one of the fractions of bark aqueous extract (Taur and Patil, 2009). Apart from this, ethanol and ethyl acetate extracts of stem bark at dose levels of 50 mg/kg p.o. were studied using tail-flick, hot-plate latency, allodynia, and acetic acid writhing tests in mice. Significantly increased pain threshold and decreased serum glucose levels, writher counts, and weight and thickness of the hind paw were reported (Varija et al., 2011).
Anticataleptic activity
Catalepsy is a medical condition characterized by unconsciousness or loss of sensation accompanied by body rigidity. Aqueous and ethyl acetate bark extracts showed potent anticataleptic activity in cataleptic mice injected with clonidine and haloperidol (1 mg/kg). Extracts were given at a dose of 50 mg/kg, i.p., each, and the results were compared to the standard drug pheniramine maleate. Aqueous extract showed potent inhibitory activity for clonidine-induced catalepsy, followed by ethyl acetate extract, which is mediated by histamine release from the mast cells of mice. Polar constituents in these solvents are held responsible for the antihistaminic activity. Haloperidol-induced catalepsy was inhibited by none of the extracts (Taur et al., 2007a, 2007b).
Larvicidal activity
The larvicidal activity of leaf methanolic extract was evaluated against early second, third, and fourth instar larvae of three vector mosquitoes, Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti. 50% lethal concentration (LC50) was reported to be 41.43, 60.44, and 56.54 ppm against early second; 58.21, 76.41, and 70.29 ppm against third; and 74.32, 89.55, and 80.85 ppm against fourth instar larvae of C. quinquefasciatus, A. stephensi, and A. aegypti, respectively, at dose levels between 25 and 200 ppm (Govindarajan, 2010). On the other hand, methanolic leaf extract exhibited the highest efficacy with LC50 and 90% lethal concentration of 100.88, 159.76 ppm, and 56.66, 85.84 ppm, respectively, against early third instar larvae of Culex tritaeniorhynchus and Anopheles subpictus (Govindarajan et al., 2011).
Antipyretic activity
The antipyretic activity of ethanol, water, and chloroform leaf extracts at dose levels of 200 mg/kg b.w. i.p. was evaluated against Brewer’s yeast-induced pyrexia in rats. Water and chloroform extracts significantly reduced elevated body temperatures, whereas ethanol extract showed no activity. The results were compared to aspirin at 100 mg/kg (Yadav et al., 2011a, 2011b). Another study against yeast-induced hyperthermia in rat models using ethanolic and aqueous extracts of bark showed significant activity at all doses compared to the standard drug paracetamol (Garg and Paliwal, 2014).
TOXICITY STUDIES
Toxicological screening is crucial in the drug development process, be it the development of a new drug or expanding the existing ones. According to the norms of the Food and Drug Administration, United States, screening any new molecule is crucial for its potential therapeutic action and toxicity potential by means of animal models. Plants have metabolites that may have antagonistic or synergistic behavior; a few may lead to anaphylactic shock, have serious intoxications, or some may result in hypersensitivity reactions. Consequently, assessing the toxic and adverse effects of plant extracts and phytoconstituents isolated which are to be used for humans becomes crucial (Renata-Maria, 2019). During the toxicity studies, the aerial root extract showed no toxicity up to 5,000 mg/kg and was considered safe. Another study reported extract dose levels of up to 3,000 mg/kg b.w. safe in Wister albino rats (Renata-Maria, 2019). Similarly, no mortality or any other abnormal functionality was caused in motor activities, muscular weakness, feeding behavior, and fecal output in albino mice models up to 2,000 mg/kg by 50% ethanolic leaf extract (Bhardwaj et al., 2016). A number of other studies further supported these results.
RECENT NANOTECHNOLOGICAL APPLICATIONS
The field of nanotechnology is enormous and expanding due to its potential applicability in a variety of areas such as medicine and drug development, engineering, chemistry, and cosmetics. Nanoparticles (NPs) typically display new or improved properties than their original counterparts depending upon their morphology, size, and distribution. Recently, researchers and scientists have been piqued by nanotechnology due to its crucial mechanisms of cellular-level penetration and targeted delivery of the compound of interest (Lee et al., 2019). The metals (e.g., Ag, Au, Cu, Zn, Se, Mg, and Pt) and metal oxides (e.g., Ag2O, TiO2, and ZnO) nanomaterials have immense therapeutic benefits in the medical fields and these have also been employed in drug delivery applications, biosensors, and diagnostic imaging (Katas et al., 2018; Yaqoob et al., 2020). However, green synthesis is preferred over chemical synthesis because of its cost-effectiveness, short production time, and environmentally safe nature. Therefore, plant extracts and microorganisms such as fungi and bacteria are extensively used as reducing and capping agents for synthesizing NPs. Primary and secondary metabolites such as lipids, proteins, amino acids, saponins, tannins, phenolics, and flavonoids present in plants themselves act as reducing agents. Moreover, NP synthesis using plant extracts is more straightforward and advantageous than microbial synthesis, which involves a tedious and time-consuming process of culturing and maintaining the cell. Thus, such methods are significantly more environmental friendly and produce waste that is more biodegradable than usual techniques exhibiting contrary effects. Extracts from different plants, including F. benghalensis, have been used in synthesizing metallic NPs such as silver, gold, copper, zinc, selenium, and zirconium, which can be used directly. These NPs possess more effective properties and are required in a scanty amount than their bulk counterparts for producing the same effect (Saxena et al., 2012). NPs from F. benghalensis have been used in applications such as dealing against oral and other pathogens, mouthwash preparations, cancer treatment, etc. A detailed study of NPs synthesized using F. benghalensis extracts to date is described in Table 3. It is noteworthy here that all the studies consisting of NP synthesis are Indian.
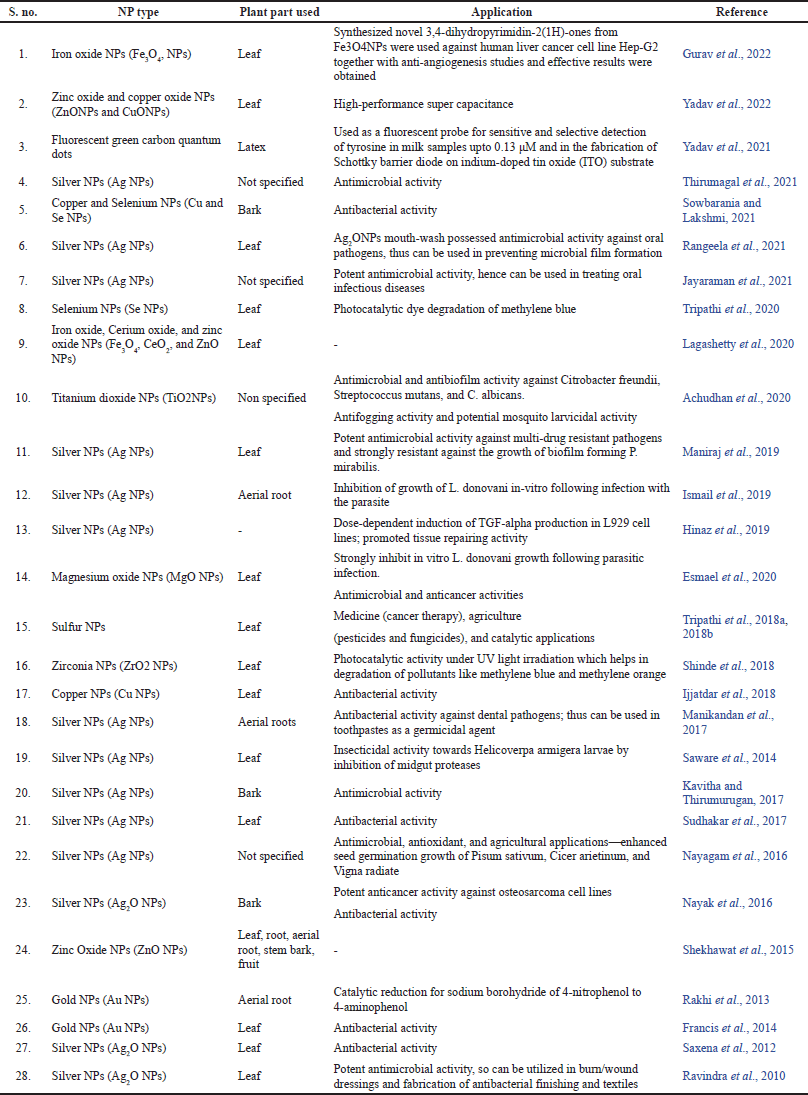 | Table 3. Various NPs synthesized using different parts of F. benghalensis and their applications. [Click here to view] |
PATENT LANDSCAPE ON F. BENGHALENSIS
Patents filed in different areas
A comprehensive study on patent analysis and a keyword-based search on the Thomson Innovation database gave 316 patent results. A title, abstract, and claim-based screening of the patent documents resulted in 38 relevant patent results where the utility of F. benghalensis plant parts or extracts for any commercial application was claimed in the applications like treating diabetes, denture stomatitis, neurological disorders, obesity, vaginal infections, asthma, cough, skin care, udder sore, male-infertility, hair fall, aging, animal skin disorders, bloating, baldness, hair growth and blackening, antioxidative stress, pesticidal, anti-inflammatory, anti-arthritic, anti-mastitis, vegan cheese, fuel supplement, insect repellent, plant growth promoter, antioxidant, aphrodisiac, and mono and bimetallic NPs synthesis. Though many medicinal properties have been reported in the literature, there are negligible commercial products available in the market that employs any part/extract of F. benghalensis; thus, very few companies have filed patents for any medicinal product from the same.
Skin care
One of the most distinguishing commercial applications of F. benghalensis is in improving the esthetic appearance of aging skin. Its phytochemicals are used in a topical cream that manifests itself by decreasing one or more of the facial lines, wrinkles, or sagging skin when applied externally. Different extracts have good antioxidant and anti-oxidizing properties which are used in external preparation for the skin (Millikin et al., 2008; Tani et al., 2009). Its phytochemicals increase collagen expression and fibroblasts and keratinocytes proliferation, and reduce collagenase expression (Ayusawa et al., 2008). F. benghalensis ethanolic extract (plant part not specified) provides a safe and inexpensive collagen production promoter that exhibits excellent skin aging-preventing and improving effects by promoting collagen biosynthesis in fibroblasts (Ayusawa et al., 2008). The extraction is carried out by a mixture of a solvent comprising a polar organic solvent and water, for 1–8 hours at a temperature of 30°C–90°C, in which the polar organic solvent ethanol is present in an amount of 40% by volume (Ptchelintsev, 2010). Furthermore, the phytochemicals ameliorate the damage caused to the skin by ultraviolet light, thereby maintaining the state of youthful skin (Ayusawa et al., 2007). They also have known for alleviating skin damage due to heavy metal or fine dust. Phytochemicals have an excellent effect of adsorbing heavy metal and fine dust, an excellent effect on the prevention of collagen biosynthesis inhibition due to the fine dust, and an effect of inhibiting hyaluronic acid decomposition (Kim et al., 2017), as listed in Table 4.
Hair care
Compounds from aerial root help in increasing hair growth and decreasing hair loss. F. benghalensis extract when exposed to hair follicles increases the viability of hair follicles (Nookaraju, 2014). The method involves fractionating aqueous extract of an aerial root tip, with n-hexane to obtain an n-hexane fraction and a first residue, which was subjected to a dichloromethane extraction to get the dichloromethane fraction and a second residue, later this residue was subjected to ethyl acetate extraction to get ethyl acetate fraction and a third residue, this was subjected to a methanol extraction to obtain a methanol fraction and a fourth residue, this was finally subjected to water extraction to obtain a water fraction (Bhavale, 2018). Phytochemicals from F. benghalensis are known to promote melanin production from melanocytes and to prevent gray hair (Hashimoto et al., 2010), as listed in Table 4.
Another contrasting application is in removing hairs to decrease hair growth or increase hair loss over the skin of the face, arms, chest, back, legs, etc. A sub-fraction from an aerial root portion is obtained by preparing aerial root tip crude extract followed by extracting the crude extract using n-Hexane, and later partitioning the first extract with chloroform using chromatography. From the sub-fraction, an unsaturated fatty acid, linoleic acid is isolated which is used to decrease hair growth and/or increase hair loss (Armani et al., 2011), as listed in Table 4.
Management of metabolic disorders and diseases
F. benghalensis methanolic extract showed the highest amylase inhibition activity, whereas aqueous extract inhibited glucose uptake which is good to control diabetes and obesity. Moreover, its potential for inhibiting lipase enzymes could make it a candidate drug molecule to cure obesity (Khan et al., 2019). F. benghalensis extract is used in a composition that not only effectively heals normal wounds but diabetes mellitus-associated wounds as well (Kushwah and Prasad, 2019; Majumder and Beveenahalli, 2020). It is also claimed for the management of denture stomatitis. Its extracts have an anticandidal effect on acrylic denture base resin (Philip et al., 2020). They are well known for the therapeutic and prophylactic management of neurological disorders including epilepsy and other seizure-related neurological disorders and associated conditions (Jayantibhai, 2016; Singh, 2017). Their effectiveness in the treatment of vulvovaginal candidiasis (Maurya, 2021), asthma (Patel, 2020), anti-inflammatory and anti-arthritic (Sarojini, 2017), cough, antitussive, throat soothing (Sharma, 2018), mastitis (Omvati et al., 2017), male infertility (Kumar et al., 2018a, 2018b), bloat (Vankar ,2014), oxidative stress (Masahiro et al., 2010), aphrodisiac (Pushpangadan et al., 2006) is also claimed in different patent applications as listed in Table 4.
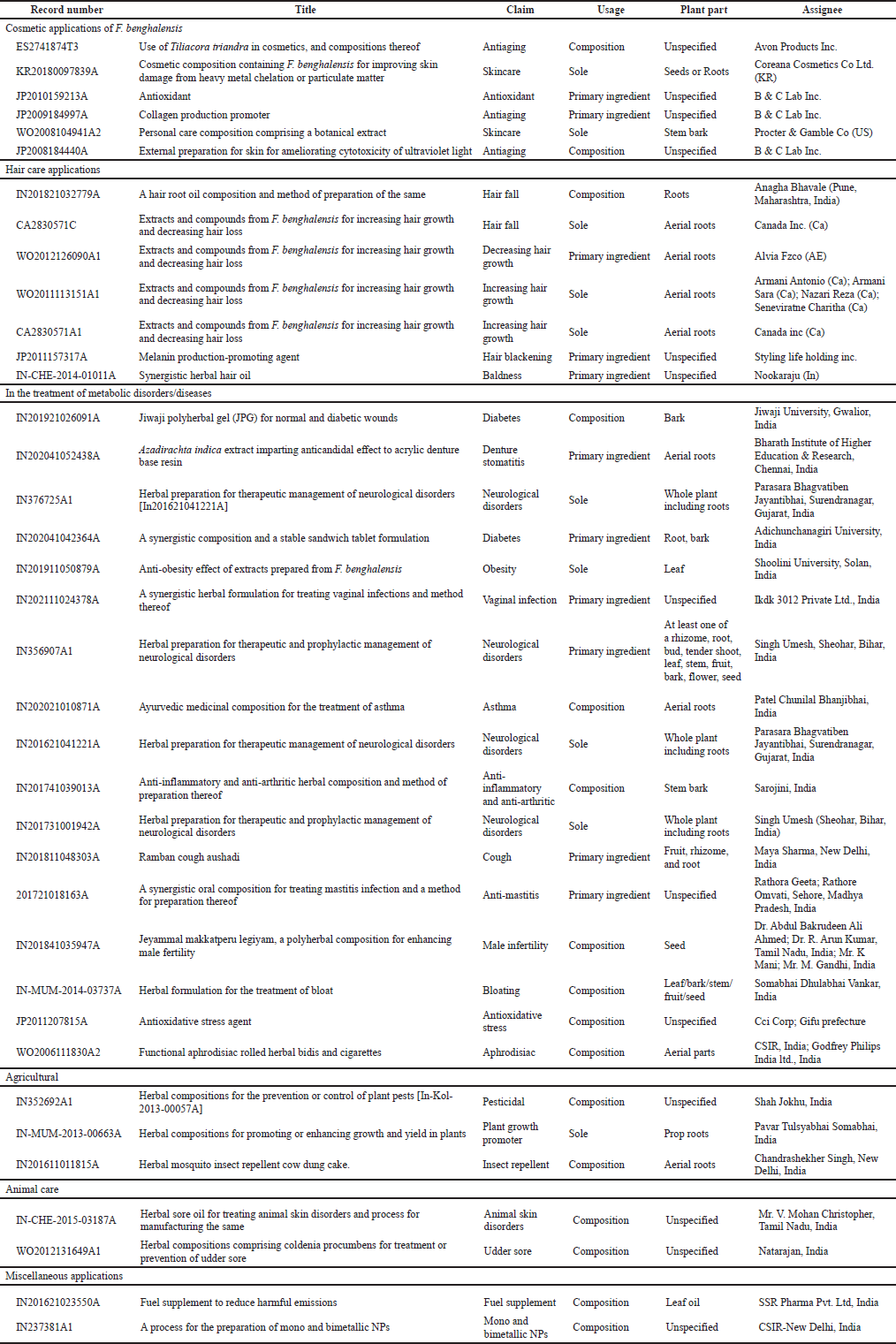 | Table 4. Commercial applications of F. benghalensis claimed in patent documents filed globally. [Click here to view] |
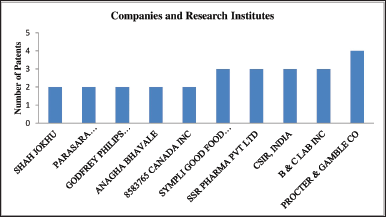 | Figure 3. Companies and research institutes active in patenting various applications of F. benghalensis. [Click here to view] |
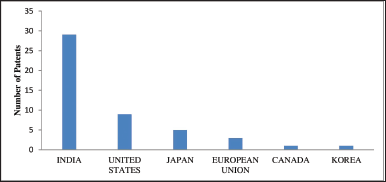 | Figure 4. Bar chart showing the number of patents filed by respective countries. [Click here to view] |
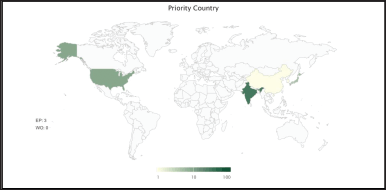 | Figure 5. Patenting activities heat map of F. benghalensis globally. [Click here to view] |
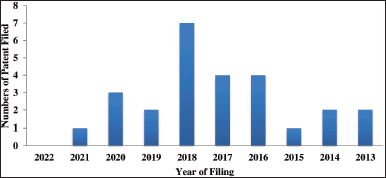 | Figure 6. Year wise filing trend of patents on F. benghalensis. [Click here to view] |
Animal care
In the case of animals, the F. benghalensis extracts have been claimed to be effective in treating animal skin disorders like warts, sore teats (Christopher, 2015), and udder sores (Natarajan, 2012). Studies also reported treatment of puerperal fever, caked bag and milk, chronic dermatitis, etc., by different extracts as listed in Table 4.
Agricultural application
F. benghalensis extracts in composition have been claimed to have an insect repellant (Singh, 2016) and pesticidal effect (Jokhu, 2013). Its different parts have also been claimed to be enhancing the growth and yield of other cultivated plants (Somabhai, 2013).
Miscellaneous applications
Other miscellaneous applications include the use of plant-derived oils when combined in a defined ratio to our conventional fuels viz. petrol and diesel resulting in the depletion of harmful gases during engine combustion. The addition of such oils can reduce the use of sulfur significantly in fuels, thereby protecting the environment from harmful pollutants (Sandeep and Surjan, 2016). Further, there are claims of the use of F. benghalensis extracts in metallic NPs synthesis (Murali et al., 2006), as listed in Table 4.
Key players in the cosmetics industry
B & C LAB Inc. is a company providing health-related services and is based in Osaka, Japan. This company has filed three patents claiming the use of F. benghalensis in cosmetic preparations has an antiaging effect. CCI Corporation is another Japan-based company mainly in engineering products. This company has filed a patent claiming the use of F. benghalensis extracts as an antioxidant stress agent in a composition. Styling Life Holding Inc. is a Tokyo, Japan-based company claiming a cosmetic preparation of the plant for hair blackening. Avon Products, Inc. or Avon, is an American British multinational cosmetics, skincare, fragrance, and personal care company, based in London that claims the F. benghalensis use in antiaging compositions. Same way, Coreana is a Korean cosmetic company that has claimed its application in skin care, as shown in Figure 3.
Country data
Data on countries show that only six countries have filed patents using F. benghalensis and except India, no other country is claiming its medicinal applications in the form of a patent. Further, the data shows India as the leading country filing patents on its use in medicinal, cosmetics, and other herbal preparations. The US followed by Japan and Europe are other countries filing patents on cosmetic applications only. Important to note here is that except India no other country is claiming the medicinal properties of F. benghalensis, as shown in Figure 4. Other global players are only focusing on cosmetic applications. Figure 5 is showing a heat map of countries involved in the patent filing.
Patent filing trend
Year-wise filing trend shows that patent filing on F. benghalensis started in 2013 and peaked by 2018, and not many patents are filed showing poor interest of researchers at a global level in exploring the plant for various medicinal and cosmetic applications (Fig. 6). Legal status analysis of the patents on F. benghalensis indicates that 15 patents have been granted and another 19 are under examination which shows the moderate interest of the commercial organization in this particular plant.
CONCLUSION AND FUTURE PROSPECTS
It is a well-known fact that phytochemicals are the non-nutritive, naturally occurring compounds present in plants that as ages have shown significant potential in controlling, preventing, and treating human diseases. In past times, F. benghalensis has gained a lot of attention for its enormous pharmacological potential contributed by an abundant amount of phyto-compounds present in it. Besides the presence of common phenolics, flavonoids, coumarins, terpenes, fatty acids, leucoanthocyanin, etc., distinctive compounds such as bengalenoside and benganoic acid have also been reported from F. benghalensis. The present study confirmed its enormous applications in terms of anti-inflammatory, antidiabetic, antimicrobial, antioxidant, anticancerous, anti-stress, antiproliferative, analgesic, antidiarrheal, larvicidal, etc. activities that form a scientific platform of its use in the traditional medication system. Moreover, tremendous potential in terms of nanotechnological applications against different pathogens has also been explored. A study on patent literature has shown patents that have been filed in different areas to claim the usage of this plant in skin care, hair care, management of metabolic disorders and diseases, animal care, and a few agricultural applications. However, the effectiveness of this plant in several other areas is yet to be scientifically explored.
Due to the side effects of chemically synthesized drugs/cosmetic ingredients, and the emerging antibiotic-resistant pathogenic bacteria, the world is looking towards natural sources of bioactive compounds that can replace chemically synthesized drugs. In such a scenario, plants play vital role due to their immense and diverse pharmacological benefits owing to their easily available phytochemicals with a good shelf life and few side effects. Phytochemicals can be a promising alternative to antibiotics and can help reduce the usage of antibiotics. There are conclusive evidence that phytomedicines are a potential source of antimicrobial agents for future use; therefore, the characterization of different phytochemicals is vital to hunt for novel compounds having antimicrobial potential. Thus, there is a need for more research in optimizing the processes for the isolation and characterization of these phytochemicals on a commercial scale. Besides this, further studies using animal models should be conducted to analyze the further potential of the compounds, thereby increasing the likelihood of the most optimal use of compounds of interest.
On the whole, the current compilation of phyto-constituents with their respective pharmacological potential will be beneficial in providing the perspective information on the existing data and pharmacological aspects that might be required for further experimental value addition to this plant and to fill the research gap. However, further extensive studies are required to be done in the future to completely explore its potential.
AUTHOR CONTRIBUTIONS
All authors agreed to submit the article to the current journal; gave final approval of the version to be published; made significant contributions to conception and design, data collection, analysis, and interpretation; participated in its writing or critically revised it for important intellectual content; and agreed to be accountable for every aspect of the work. According to the requirements/guidelines of the International Committee of Medical Journal Editors, all of the authors are qualified to be authors.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed is included within this article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Acharya D, Shrivastava A. Indigenous herbal medicines: tribal formulations and traditional herbal practices. Aavishkar Publishers and Distributior, Jaipur, India, 2008.
Achrekar S, Kaklij GS, Pote MS, Kelkar SM. Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: mechanism of action. In Vivo (Athens, Greece), 1991; 5(2):143–7.
Achudhan D, Vijayakumar S, Malaikozhundan B, Divya M, Jothirajan M, Subbian K, González-Sánchez ZI, Mahboob S, Al-Ghanim KA, Vaseeharan B. The antibacterial, antibiofilm, antifogging, and mosquitocidal activities of titanium dioxide (TiO2) nanoparticles green-synthesized using multiple plant extracts. J Environ Chem Eng, 2020; 8(6):104521. CrossRef
Afzal T, Ali Q, Malik A. Phenolic compounds proliferation by HPLC: to find out antibacterial activities in Ficus benghalensis plant extract. Int J Botany Stud, 2020; 5(2):140–4.
Ahad HA, Haranath C, Varam NJ, Ksheerasagare T, Krishna JV, Teja ST. Liver shielding activity of Ficus benghalensis fruit extracts contrary to perchloromethane prompted toxic hepatitis in New-Zealand albino rats. Res J Pharm Technol, 2021; 14(7):3739–43. CrossRef
Ahmad S, Rao H, Akhtar M, Ahmad I, Munawar M. Phytochemical composition and pharmacological prospectus of Ficus bengalensis Linn. (Moraceae)—a review. J Med plant Res, 2011; 5(28):6393–400. CrossRef
Ahmed F, Chavan S, Satish A, Punith KR. Inhibitory activities of Ficus benghalensis bark against carbohydrate hydrolyzing enzymes—an in vitro study. Pharmacogn J, 2011; 3(20):33–7.
Alaaeldin R, Hassan HA, Abdel-Rahman IM, Mohyeldin RH, Youssef N, Allam, AE, Abdelwahab, SF, Zhao QL, Fathy M. A new EGFR inhibitor from Ficus benghalensis exerted potential anti-inflammatory activity via Akt/PI3K pathway inhibition. Curr Issues Mol Biol, 2022; 44(7):2967–81. CrossRef
Almahy HA, Alhassan NI. Studies on the chemical constituents of the leaves of Ficus benghalensis and their antimicrobial activity. J Sci Technol, 2011; 13(3):118–24.
Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med, 2016; 2016:1–11. CrossRef
Ambreen S, Tariq M, Masoud MS, Ali I, Qasim M, Mushtaq A, Ahmed M, Asghar R. Anticoagulant potential and total phenolic content of six species of the genus Ficus from Azad Kashmir, Pakistan. Trop J Pharm Res, 2019; 18(6):1245–51. CrossRef
Ambujakshi HR, Shyamnanda TH. Anthelmintic activity of Gmelina arborea roxb. leaves extract. Int J Pharma Res Dev, 2009; 9(1):1–5.
Anarthe SJ, Pravalika A, Malavika E, Ganga Raju M. Assessment of immunomodulatory activity of Ficus benghalensis Linn. aerial roots. Int J PharmTech Res, 2016; 9(1):153–63.
Anbarashan M, Parthasarthy N, Padmavathy A. Ethno-floristic survey in sacred groves, Pudukottai district, Tamil Nadu-India. J Med Plant Res, 2011; 5(3):439–43.
Aphale S, Pandita S, Raina P, Mishra JN, Kaul-Ghanekar R. Phytochemical standardization of Panchavalkala: an Ayurvedic formulation and evaluation of its anticancer activity in cervical cancer cell lines. Pharmacogn Mag, 2018; 14(58):554–60. CrossRef
Aphale S, Shinde K, Pandita S, Mahajan M, Raina P, Mishra JN, Kaul GR. Panchvalkala, a traditional Ayurvedic formulation, exhibits antineoplastic and immunomodulatory activity in cervical cancer cells and C57BL/6 mouse papilloma model. J Ethnopharmacol, 2021; 15(280):114405. CrossRef
Armani A, Armani S, Seneviratne C, Nazari R, inventors. Armani Antonio (Ca); Armani Sara (Ca); Nazari Reza (Ca); Seneviratne Charitha (Ca), assignee. Extracts and compounds from Ficus benghalensis for increasing hair growth and decreasing hair loss. World International Patent Organisation Application. WO2011113151A1. 2011-03-18.
Arumugam A, Agullo P, Boopalan T, Nandy S, Lopez R, Gutierrez C, Narayan M, Rajkumar L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol Ther, 2014; 15:26–34. CrossRef
Aswar M, Aswar U, Watkar B, Vyas M, Wagh A, Gujar K. Antihelmintic activity of Ficus benghalensis. Int J Green Pharm, 2008; 2:170. CrossRef
Augusti K, Anuradha PS, Smitha K, Sudheesh M, George A, Joseph M. Nutraceutical effects of garlic oil, its nonpolar fraction and a Ficus flavonoid as compared to vitamin E in CCl4 induced liver damage in rats. Indian J Exp Biol, 2005; 4(3):437–44.
Ayusawa M, Mohammad NH, Yamashita R, inventors. B & C Lab Inc, assignee. Collagen production promoter. Japanese Patent. 2009184997A. 2008-02-08.
Ayusawa M, Mohammad NH, Yana Y, Yamashita R, inventors. B & C Lab Inc, assignee. External preparation for skin for ameliorating cytotoxicity of ultraviolet light. Japanese Patent. 2008184440A. 2007-01-30.
Babu K, Sabesan GS, Rai S. Comparative pharmacognostic studies on the bark of four Ficus species. Turk J Bot, 2010; 34(3):215–24. CrossRef
Baheti JR, Goyal RK. The methanolic extract of Ficus benghalensis and its fraction induces antihepatotoxic activity in vivo: possible involvement of antioxidant action. Planta Med, 2013;79:PB6. CrossRef
Basir S, Shailey S. Strengthening of antioxidant defense by Azadirachta indica in alloxan-diabetic rat tissues. J Ayurveda Integr Med, 2012; 3:130. CrossRef
Bhanwase AS, Alagawadi KR. Antioxidant and immunomodulatory activity of hydroalcoholic extract and its fractions of leaves of Ficus benghalensis Linn. Pharmacogn Res, 2016; 8(1):50–5. CrossRef
Bhardwaj LK, Anand L, Chandrul KK, Patil KS. In-vitro anthelmintic activity of Ficus benghalensis Linn. leaves extracts. Asian J Pharm Clin Res, 2012; 5(4):118–20.
Bhardwaj LK, Chandrul KK, Sharma U. Evaluation of anti-arthritic activity of Ficus benghalensis Linn. root extracts on Freund’s adjuvant induced arthritis in rats. J Phytopharm, 2016; 5(1):10–4. CrossRef
Bhavale A, inventor. Anagha Bhavale (Pune, Maharashtra, India), assignee. A hair root oil composition and method of preparation of the same. Indian Patent. 201821032779A. 2018-08-31.
Bi X, Lim J, Henry CJ. Spices in the management of diabetes mellitus. Food Chem, 2017; 15(217):281–93. CrossRef
Chandrasekaran C, Dethe S, Mundkinajeddu D, Pandre M, Balachandran J, Agarwal A, Hiraganahalli D. Hepatoprotective and antioxidant activity of standardized herbal extracts. Pharmacogn Mag, 2012; 8(30):116–23. CrossRef
Chaudhary S, Alok S, Jain SK, Chanchal D, Dongray A. Phytopharamacology and pharmacognostic properties of Ficus benghalensis—a review. Int J Pharmacogn Phytochem Res, 2015; 2:560–9.
Chaudhary AA, Khan M, Ansari S, Chauhan V. Phytochemical screening and antibacterial efficacy of Ficus benghalensis using in vitro models. Int J Pharm Sci Rev Res, 2014; 24:276–9.
Chavan S, Jadhav R, Kharat D, Mankar S, Godge R. Evaluation of analgesic activity and phytochemical screening of Clitoria ternatea Linn. Br J Pharm Res, 2015a; 6:255–60. CrossRef
Chavan SS, Jadhav RS, Kolhe SS, Bhambar RS Tambe VD. Evaluation of analgesic activity and phytochemical screening of Ficus bengalensis Linn bark. Der Pharm Lett, 2015b; 7(5):22–7.
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation associated diseases in organs. Oncotarget, 2018; 9:7204–18. CrossRef
Cherian S, Kumar RV, Augusti KT, Kidwai JR. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J Biochem Biophys, 1992; 29(4):380–2.
Christopher VM, inventor. Christopher VM (Karaikal, Tamil Nadu, India), assignee. Herbal sore oil for treating animal skin disorders and process for manufacturing the same. Indian Patent. IN-CHE-2015-03187A. 2015-06-24.
Crossia W, Dahms HU, Muthukumar K, Kaviarasan T, Thirunalasundari T, James RA. In-vitro study: immunomodulatory and cytotoxicity effects of ethanolic leave extracts of Aegle marmelos and Ficus benghalensis. J Coast Life Med, 2016; 4:217–24. CrossRef
Dai X, Medzhitov R. Memory beyond immunity. Nat Cell Biol, 2017; 550:460–1. CrossRef
De Flora S, Izzotti A, Agostini F D, Balansky RM. Multiple points of intervention in the prevention of cancer and other mutation related diseases. Mutat Res, 2001; 480:9–22. CrossRef
Deore AB, Mule SN, Sapakal VD. Evaluation of anti-inflammatory activity of Ficus benghalensis in rats. J Biol Act Prod Nat, 2012; 2(2):85–9. CrossRef
Deore SL, Khadabadi SS. Antiproliferative activity of saponin fractions of Chlorophytum borivilianum. Pharmacogn J, 2010; 2:33–7. CrossRef
Deraniyagala S, Wijesundera R. Ficus benghalensis. National Science Foundation, Colombo, Sri Lanka, 2002.
Ediriweera E, Ratnasooriya W. A review on herbs used in treatment of diabetesmellitus by Sri Lankan ayurvedic andtraditional physicians. AYU, 2009; 30(4):373.
Esmael HH, Saheb EJ, Hasoon SA. Eco-friendly synthesis of magnesium oxide nanoparticles using Ficus benghalensis leaf extract and its anti-leishmaniasis activity. Biochem Cell Arch, 2020; 20(1):1859–66.
Faisal ZG. Antimicrobial activity of Ficus benghalensis and Ficus elastica fruit latex against selected bacteria and fungi. Int J Sci Basic Appl Res, 2017; 31(3):21–6.
Feng J, Wang H, Jing Z. Role of magnesium in type 2 diabetes mellitus. Biol Trace Elem, 2020; 196:74–85. CrossRef
Francis G, Thombre R, Parekh F, Leksminarayan P. Bioinspired synthesis of gold nanoparticles using Ficus benghalensis (Indian Banyan) leaf extract. Chem Sci Trans, 2014; 3(1):470–4.
Gabhe S, Tatke P, Khan T. Evaluation of the immunomodulatory activity of the methanol extract of Ficus benghalensis roots in rats. Indian J Pharmacol, 2006; 38:271. CrossRef
Gaherwal, S. Anti-bacterial activity of Ficus benghalensis (banyan) fruit extract against different bacteria. Int J Microbiol Res, 2013; 4(2):177–9.
Garg VK, Paliwal SK. Analgesic and anti-pyretic activity of ethanolic and aqueous extracts of Ficus benghalensis. Int J Pharm Pharm Sci, 2014; 6(3):231–4.
Garg VK, Paliwal SK. Wound-healing activity of ethanolic and aqueous extracts of Ficus benghalensis. J Adv PharmTechnol Res, 2011; 2:110–4. CrossRef
Gautam M, Gangwar M, Singh S, Goel R. Effects of Azadirachta indica on vascular endothelial growth factor and cytokines in diabetic deep wound. Planta Med, 2015; 81:713–21. CrossRef
Gayake D, Awasarkar U, Sharma P. Indigenous traditional medicinal plant resources from Ahmednagar District, Maharastra, India. Asian J Biomed Pharm Sci, 2013; 3(22):1–5.
Gayathri M, Kannabiran K. Antimicrobial activity of Hemidesmus indicus, Ficus benghalensis and Pterocarpus marsupium roxb. Indian J Pharm Sci, 2009; 71:578–81. CrossRef
Gewali MB, Awale S. Aspects of traditional medicine in Nepal. Institute of Natural Medicine, University of Toyama, Toyama, Japan, 2008.
Ghosh AK, Das SS, Dey M. Determination of anthelmintic activity of the leaf and bark extract of Tamarindus indica linn. Indian J Pharm Sci, 2011; 73:104. CrossRef
Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing—a literature review. An Bras Dermatol, 2016; 91:614–20. CrossRef
Gopukumar ST, Praseetha PK. Ficus benghalensis Linn–the sacred Indian medicinal tree with potent pharmacological remedies. Int J Pharm Sci Rev Res, 2015; (32)1:223–7.
Govindan V, Francis GS. Qualitative and quantitative determination of secondary metabolites and antioxidant potential of Ficus benghalensis Linn seed. Int J Pharm Pharm Sci, 2015; 7:118–24.
Govindarajan M, Sivakumar R, Amsath A, Niraimathi S. Mosquito larvicidal properties of Ficus benghalensis L. (Family: Moraceae) against Culex tritaeniorhynchus Giles and Anopheles subpictus Grassi (Diptera: Culicidae). Asian Pac J Trop Med, 2011; 4:505–9. CrossRef
Govindarajan M. Larvicidal efficacy of Ficus benghalensis L. plant leaf extracts against Culex quinquefasciatus say, Aedes aegypti L. and Anopheles stephensi L. (Diptera: Culicidae). Eur Rev Med Pharmacol Sci, 2010; (2):107–11.
Gupta V, Sharma S. In vitro antioxidant activities of aqueous extract of Ficus bengalensis Linn. root. Int J Biol Chem, 2010; 4:134–40. CrossRef
Gurav R, Gurav A, Salunkhe-Gawali S, Jadhav S, Choudhari P, Sankpal S, Hangirgekar S. Ficus benghalensis leaf extract in biosynthesis of Fe3O4 for Fe3O4@ Ag-S-CH2-COOH: A novel catalyst for synthesis of new 3, 4-dihydropyrimidin-2 (1H)-ones and their anticancer evaluation. Appl Organomet Chem, 2022; 36(3):6547. CrossRef
Haque N, Salma U, Nurunnabi TR, Uddin MJ, Jahangir MFK, Islam SMZ, Kamruzzama M. Management of type 2 diabetes mellitus by lifestyle, diet and medicinal plants. Pak J Biol Sci, 2010; 14:13–24. CrossRef
Hari BV, Kumar PS, Devi DR. Comparative in-vitro anthelmintic activity of the latex of Ficus religiosa, Ficus elastica and Ficus bengalensis. J Phytol, 2011; 3(3):26–30.
Hashimoto H, Tani K, Yamashita R, Ayusawa M, inventors. Styling Life Holding Inc, assignee. Melanin production-promoting agent. Japanese Patent. 2011157317A. 2010-02-02.
Hinaz N, Priya VV, Gayathri R. In vitro tissue repairing properties of green synthesized silver nanoparticles from Ficus benghalensis. Drug Invt Today, 2019; 11(6):1432–4.
Hosamani KM, Pattanashettar RS. Occurrence of unusual fatty acids in Ficus benghalensis seed oil. Ind Crops Prod, 2003; 18:139–43. CrossRef
Ijjatdar S, Gaherwal S, Prakash MM. Synthesis of plants extract mediated copper nanoparticles and thir impact on pathogenic bacteria. Mater Sci, 2018; 5:779–86.
Imran M, Sharma JN, Kamal M, Asif M. Standardization and wound-healing activity of petroleum, ethanolic and aqueous extracts of Ficus benghalensis leaves. Pharm Chem J, 2021; 54:1057–62. CrossRef
Islam F, Jahan F, Seraj S, Malek I, Sadat A. Bhuiyan M. Variations in disease and medicinal plant selection among folk medicinal practitioners: a case study in Jessore district, Bangladesh. Am Eurasian J Sustain Agric, 2011; 5(2):282–91.
Ismail HH, Hasoon SA, Saheb EJ. The anti-leishmaniasis activity of green synthesis silver oxide nanoparticles. Ann Trop Med Public Health, 2019; 22:28–38. CrossRef
Jabeen A, Khan MA, Ahmad M, Zafar M, Ahmad F. Indigenous uses of economically important flora of Margallah Hills National Park, Islamabad, Pakistan. Afr J Biotechnol, 2009; 8(5):763–84.
Jahagirdar AQF, Hugar S, Patil V, Nanjappaiah AKH. Screening of antistress activity of Ficus benghalensis fruit extract. Res J Pharm Technol, 2020; 13:191. CrossRef
Jayantibhai PB, inventor. Parasara Bhagvatiben Jayantibhai (Surendranagar, Gujarat, India), assignee. Herbal preparation for therapeutic management of neurological disorders [In201621041221A]. Indian Patent. 376725A1. 2016-12-02.
Jayaraman S, Rajeshkumar S, Jeevitha M, Thirumagal K. Controlling oral pathogens using Ficus benghalensis mediated silver nanoparticles. J Pharm Res Int, 2021; 33:98–105. CrossRef
Jokhu S, inventor. Shah Jokhu (In), assignee. Herbal compositions for the prevention or control of plant pests [In-Kol-2013-00057A]. Indian Patent. 352692A1. 2013-01-16.
Joseph B, Raj SJ. Phytopharmacological and phytochemical properties of three Ficus species—an overview. Int J Pharm Bio Sci, 2010; 1:246–53.
Joshi DG, Jat RK, Patil SB. In vitro protein denaturation and membrane stabilising anti-arthritic activity of aqueous extracts of bark of Ficus benghalensis L. against methotrexate. Pharm Innov J, 2021; 10(4):689–92. CrossRef
Karmakar S, Paul S, Biswas NM, Khanam J, Kar SK, Mukherjee H, Poddar S. A pharmacological audit and advancement on the recent trend of research on Ficus benghalensis L. including its in vitro hepatoprotective activity. Clin Phytosci, 2020; 6(84):1–3. CrossRef
Kasireddy GBS, Nadithe L, Chinnam P. Experimental evaluation of hypoglycemic effect of bark extract of Ficus benghalensis in streptozotocin-induced diabetic rats. Natl J Physiol Pharm Pharmacol, 2021; 11:1. CrossRef
Katas H, Moden NZ, Lim CS, Celesistinus T, Chan JY, Ganasan P, Abdalla SSI. Biosynthesis and potential applications of silver and gold nanoparticles and their chitosan-based nanocomposites in nanomedicine. J Nanotechnol, 2018; 2018:1–13. CrossRef
Kavitha M, Thirumurugan V. Synthesis of nanoparticles from Ficus benghalensis bark and evaluation of its antimicrobal and antioxidant activity. Asian J Innov Res, 2017; 2(1):38–48.
Khaliq HA, Chaudhary BA. Pharmacognostic and phytochemical studies on Parthenium hysterophorus L. J Biomed Pharm Res, 2016; 5(1):65–75.
Khaliq HA. A review of pharmacognostic, physicochemical, phytochemical and pharmacological studies on Ficus bengalensis L. J Sci Innovative Res, 2017; 6(4):151–63. CrossRef
Khan A, Chandel K, Khan H, Kolish M, inventors. Shoolini Univ Biotechnology & Management Science (In), assignee. Anti-obesity effect of extracts prepared from Ficus benghalensis. Indian Patent. 201911050879A. 2019-12-10.
Khan SU, Khan RU, Mehmood S, Ullah I, Ullah Z, Zahoor M. Study of prominent indigenous medicinal plants of village, Ahmad Abad, District Karak, KPK, Pakistan. J Med Plant Stud, 2013; 1(4):121–7.
Khanal P, Patil B. Gene set enrichment analysis of alpha-glucosidase inhibitors from Ficus benghalensis. Asian Pac J Trop Biomed, 2019; 9:263. CrossRef
Khanal P, Patil BM. In vitro and in silico anti-oxidant, cytotoxicity and biological activities of Ficus benghalensis and Duranta repens. Chin Herb Med, 2020; 12:406–13. CrossRef
Khanal P, Patil BM. Consolidation of network and experimental pharmacology to divulge the antidiabetic action of Ficus benghalensis L. bark. 3 Biotech, 2021; 11(5):238. CrossRef
Kim T, Lee JY, Joo HB, Lee CM, Lee KK, inventors. Coreana Cosmetics Co Ltd (Kr), assignee. Cosmetic composition containing Ficus benghalensis for improving skin damage from heavy metal chelation or particulate matter. KR20180097839A. 2017-02-24.
Kiranmai M, Mahender Kumar CB, Ibrahim MD. Free radical scavenging activity of neem tree (Azadirachta indica A. Juss var., Meliaceae) root bark extract. Asian J Pharm Clin Res, 2011; 4(4):134–6.
K?rtikara KR, Basu BD. Indian medicinal plants. Sudhindra Nath Basu, M.B. Panini Office, Bhuwanéswari Asrama, Bahadurganj, India, 1918. CrossRef
Kota BP, Teoh AW, Roufogalis BD. Pharmacology of traditional herbal medicines and their active principles used in the treatment of peptic ulcer, diarrhoea and inflammatory bowel disease. In: Brzozowski PT (Ed.). InTech Open Access Publisher, Rijeka, Croatia, 2012.
Kothapalli PK, Sanganal SJ, Shridhar NB, Narayanaswamy HD, Narayanaswamy M. In-vivo anti-inflammatory and analgesic screening of Ficus benghalensis leaf extract in rats. Asian J Pharm Sci, 2014; 4(4):174–8.
Kumar D, Arya V, Kaur R, Bhat ZA, Gupta VK, Kumar V. A review of immunomodulators in the Indian traditional health care system. J Microbiol Immunol Infect, 2012; 45:165–84. CrossRef
Kumar R, Bhagat N. Ethnomedicinal plants of district Kathua (J&K). Int J Med Aromat Plants, 2012; 2(4):603–11.
Kumar RA, Ahmed ABA, Gandhi M, Mani K, inventors. Dr Abdul Bakrudeen Ali Ahmed; Dr R Arunkumar (Thanjavur, Tamil Nadu, India); Mr K Mani; Mr M Gandhi, assignee. Jeyammal Makkatperu Legiyam a polyherbal composition for enhancing male fertility. Indian Patent. 201841035947A. 2018-09-25.
Kumar Y, Gautam G, Mishra P. Evaluation of hepatoprotective activity of Carica papaya and Ficus benghalensis latex on thioacetamide induced hepatotoxicity in rats. Int J Adv Res, 2018; 6:294–9. CrossRef
Kumar Y, Gautam G, Mishra PK. Protective role of Carica papaya and Ficus bengalensis latex against CCl4 induced liver toxicity in experimental rats. J Drug Deliv Ther, 2019; 9(3):465–9.
Kumaresan S, Ramasamy R, Jayachandran PR. Antioxidant and cytotoxic activity of combined extracts prepared using Ficus religiosa and Ficus benghalensis leaves against cervical cancer cell line (hela). Asian J Pharm Clin Res, 2018; 11:407. CrossRef
Kumari KD, Suresh KP, Samarasinghe K, Handunnetti SM, Samaranayake, TSP. Evaluation of a traditional Sri Lankan herbal beverage (water extract of dried flowers of Aegle marmelos, Bael fruit) in type II diabetic patients. J Diabetes Metab, 2013; 4(6). CrossRef
Kunwar RM, Bussmann RW. Ficus (Figure) species in Nepal: a review of diversity and indigenous uses. Lyonia, 2006; 11(1):85–97.
Kushwah L, Prasad GBKS, inventors. Jiwaji Univ, assignee. Jiwaji polyherbal gel for normal and diabetic wounds. Indian Patent. 201921026091A. 2019-06-30.
Lagashetty A, Ganiger SK, Preeti RK, Reddy S, Pari M. Microwave-assisted green synthesis, characterization and adsorption studies on metal oxide nanoparticles synthesized using Ficus benghalensis plant leaf extracts. New J Chem, 2020; 44:14095–102. CrossRef
Lamy S, Blanchette M, Michael-Levesque J. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptors phospharylation. J Carcinogenesis, 2006; 27:989–96. CrossRef
Lee NY, Ko WC, Hsueh PR. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol, 2019; 10:1153–3. CrossRef
Lotankar AR, Wankhede S, Sharma JB, Momin AJ. Anti-stress activity of flavonoids rutin and quercetin isolated from the leaves of Ficus benghalensis. Int J Pharm Pharm Res, 2016; 5(4):5–19. CrossRef
Mahajan MS, Gulecha VS, Khandare RA, Upaganlawar AB, Gangurde HH, Upasani CD. Anti-edematogenic and analgesic activities of Ficus benghalensis. Int J Nutr Pharmacol Neurol Dis, 2012; 2(2):100. CrossRef
Mahalakshmi M, Parimala M, Shoba FG. Evaluation of anti-diarrhoeal potential of methanol extract of Ficus bengalensis Linn. leaf and Mangifera indica Linn. stem bark and root bark. Int J Pharmacogn Phytochem Res, 2014; 6(3):454–8.
Majumder P, Beveenahalli R, inventors. Adichunchanagiri University (In), assignee. A synergistic composition and a stable sandwich tablet formulation. Indian Patent. 202041042364A. 2020-09-29.
Manian R, Anusuya N, Siddhuraju P, Manian S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus benghalensis L. and Ficus racemosa L. Food Chem, 2008; 107(3):1000–7. CrossRef
Manikandan V, Velmurugan P, Park JH, Chang WS, Park YJ, Jayanthi P, Cho M, Oh BT. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. Biotechnology, 2017; 7:72. CrossRef
Maniraj A, Kannan M, Rajarathinam K, Vivekanandhan S, Muthuramkumar S. Green synthesis of silver nanoparticles and their effective utilization in fabricating functional surface for antibacterial activity against multi-drug resistant Proteus mirabilis. J Cluster Sci, 2019; 30:1403–14. CrossRef
Masahiro I, Kazutaka M, Hironori M, Masafumi I, inventors. CCI Corp; Gifu Prefecture, assignee. Antioxidative stress agent. Japanese Patent. 2011207815A. 2010-03-30.
Maurya AJ, inventor. Ikdk3012 Private Ltd (In), assignee. A synergistic herbal formulation for treating vaginal infections and method thereof. Indian Patent. 202111024378A. 2021-06-01.
Millikin CL, Goodman LJ, Bissett DL, Robinson LR, Osborne R, inventors. Procter & Gamble Co (Us), assignee. Personal care composition comprising botanical extract. World International Patent Organisation Application. WO2008104941A2. 2008-02-27.
Mukherjee PK, Saha K, Murugesan T, Mandal S, Pal M, Saha B. Screening of anti-diarrhoeal profile of some plant extracts of a specific region of West Bengal, India. J Ethnopharm, 1998; 60:85–9. CrossRef
Murali S (National Chemical Laboratory, Pune, India), Absar A (National Chemical Laboratory, Pune, India), Shankar SS (National Chemical, Pune, India), inventors. CSIR (IN), assignee. A process for preparation of mono and bimetallic nanoparticles. Indian Patent. 237381A1. 2006-05-24.
Murti K, Kumar U, Panchal M. Healing promoting potentials of roots of Ficus benghalensis L. in albino rats. Asian Pac J Trop Med, 2011; 4:921–4. CrossRef
Murti K, Kumar U. Antimicrobial activity of Ficus benghalensis and Ficus racemosa roots L. Am J Microbiol, 2011; 2:21–4. CrossRef
Murugesu S, Selamat J, Perumal V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants, 2021; 10:2749. CrossRef
Naik R, Venugopalan V, Kumaravelayutham P, Krishnamurthy Y. Ethnoveterinary uses of medicinal plants among the Lambani community in Chitradurga district, Karnataka, India. Asian Pac J Trop Biomed, 2012; 2(2):470–S6. CrossRef
Nambiar VK, Sasidharan N, Renuka C, Balagopalan M. Studies on the medicinal plants of Kerala Forests. Division of Botany (Taxonomy), Kerala Forest Research Institute, Thrissur, Kerala, 1985.
Naquvi KJ, Ali M, Ahamad J. Two new phytosterols from the stem bark of Ficus benghalensis L. J Saudi Chem Soc, 2015; 19:650–4. CrossRef
Natarajan, inventor. Natarajan (In), assignee. Herbal compositions comprising coldenia procumbens for treatment or prevention of udder sore. World International Patent Organisation Application. WO2012131649A1. 2012-03-31.
Navanath MS, Naikwade NS, Mule SN, Krishna PP. Evaluation of anti-inflammatory activity of Cassia fistula and Ficus benghalensis. J Pharm Res, 2009; 2(8):1304–6.
Nayagam V, Melchias G, Kumaravel P. Ficus benghalensis mediates synthesis of silver nanoparticles: the green approach yields NPs that are its anti-bacterial and anti-oxidant. World J Pharm Sci, 2016; 4:1–12.
Nayak D, Ashe S, Rauta PR, Kumari M, Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng, 2016; 58:44–52. CrossRef
Nookaraju (In), inventor. Nookaraju (In), assignee. Synergistic herbal hair oil. Indian Patent. IN-CHE-2014-01011A. 2014-02-28.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract, 2017; 128:40–50. CrossRef
Omvati R, Geeta R, inventors. Rathora Geeta, Rathore Omvati (Sehore, Madhya Pradesh, India), assignee. A synergistic oral composition for treating mastitis infection and a method for preparation thereof. Indian Patent. 201721018163A. 2017-05-24.
Omóbòwálé TO, Oyagbemi AA, Adejumobi OA, Orherhe EV, Amid AS, Adedapo AA, Nottidge HO, Yakubu MA. Preconditioning with Azadirachta indica ameliorates cardiorenal dysfunction through reduction in oxidative stress and extracellular signal regulated protein kinase signalling. J Ayurveda Integr Med, 2016; 7:209–17. CrossRef
Otunola GA, Afolayan AJ. A review of the antidiabetic activities of ginger. Ginger cultivation: its antimicrobial and pharmacological potentials. IntechOpen, Rijeka, Croatia, 2019.
Palshetkar A, Pathare N, Jadhav N, Pawar M, Wadhwani A, Kulkarni S, Singh, KK. In vitro anti-HIV activity of some Indian medicinal plant extracts. BMC Complement Med Ther, 2020; 20:1–11. CrossRef
Panday DR, Rauniar GP. Effect of root-extracts of Ficus benghalensis (Banyan) in pain in animal models. J Neurosci Rural Pract, 2016; 07:210–5. CrossRef
Parameswari SA, Saleem T, Chandrasekar K, Chetty CM. Protective role of Ficus benghalensis against isoniazid-rifampicin induced oxidative liver injury in rat. Rev Brasil Farmacogn, 2012; 22:604–10. CrossRef
Pareek A, Trivedi PC. Ethnobotanical studies on medicinal plants of Kaladera region of Jaipur District. Indian J Fundam Appl Life Sci, 2011; 1:5.
Parmar DN, Patel NK. Sacred and medicinal plant diversity of vandiol sacred grove of Sabarkantha District (N.G.). Life Sci Leaflets, 2013; 5:34–49.
Patel CB, inventor. Patel Chunilal Bhanjibhai (In), assignee. Ayurvedic medicinal composition for treatment of asthma. Indian Patent. 202021010871A. 2020-03-13.
Patel R, Gautam P. Medicinal potency of Ficus benghalensis: a review. Int J Med Chem Anal, 2014; 4:53–8.
Patel SM, Venkata KCN, Bhattacharyya P, Sethi G, Bishayee A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin Cancer Biol, 2016; 40(41):100–15. CrossRef
Patil DA. Native phytomedicines in the perspective of doctrine of signatures in buldhana District (Maharashtra: India). Deccan Curr Sci, 2010; 3(2):104–11.
Patil V. Evaluation of the antidiarrheal activity of the plant extracts of Ficus species. Chin J Integr Med, 2012; 10:347–52. CrossRef
Patil VV, Patil VR. A comparative evaluation of anti-inflammatory activity of the bark of Ficus benghalensis in plants of different age. J Basic Clin Pharm, 2010; 1(2):107. CrossRef
Patil VV, Pimprikar RB, Patil VR. Pharmacognostical studies and evaluation of anti-inflammatory activity of Ficus benghalensis. J Young Pharm, 2009; 1:49. CrossRef
Philip JM, Mahalakshmi K, Abraham HM, inventors. Bharath Institute of Higher Education & Research (Chennai, India), assignee. Azadirachta indica extract imparting anticandidal effect to acrylic denture base resin. Indian Patent. 202041052438A. 2020-12-02.
Ptchelintsev DS, inventor. Avon Products Inc, assignee. Use of Tiliacora triandra in cosmetics and compositions thereof. Spanish Patent. 2741874T3. 30-06-2010.
Pushpangadan P, Rao CV, Raghavan (In), Rawat AKS, Srivastava SK, Deb B, Subramanian S, inventors. CSIR (In), Godfrey Philips India Ltd (In), assignee. Functional aphrodisiac rolled herbal bidis and cigarettes. World International Patent Organisation Application. WO2006111830A2. 2006-04-20.
Raheel R, Saddiqe Z, Iram M, Afzal S. In vitro antimitotic, antiproliferative and antioxidant activity of stem bark extracts of Ficus benghalensis L. S Afr J Bot, 2017; 111:248–57. CrossRef
Rahman A, Anisuzzaman M, Haider S, Ahmed F, Islam A, Naderuzzaman A. Study of medicinal plants in the graveyards of Rajshahi city. Res J Agric Biol Sci, 2008; 4(1):70–4.
Rahman AHMM, Khanom A. Taxonomic and ethno-medicinal study of species from Moraceae (Mulberry) family in Bangladesh flora. Res Plant Sci, 2013; 1:53–7.
Rahman AHMM. Graveyards angiosperm diversity of Rajshahi City, Bangladesh with emphasis on medicinal plants. Am J Life Sci, 2013; 1:98. CrossRef
Raisagar A, Kaur CD, Sawarkar HA, Kumar L, Raisagar A, Karmakar A, Sahu M. Comparative study of wound-healing effect of bark extracts of Ficus religiosa & Ficus benghalensis by mice model. J Pharmacogn Phytochem, 2019; 8:1815–21.
Rajalakshmi G, Tamilarasi TA. Study on phytochemical screening and anti-arthritics activity of Ficus benghalensis bark extract. World J Sci Res, 2019; 4:1–6.
Rajdev K, Jain S, Mahendra CH, Bhattacharaya SK. Antinociceptive effect of Ficus bengalensis bark extract in experimental models of pain. Cureus, 2018; 10(3):e2259. CrossRef
Rakhi M, Bag BG, Manisha D. Ficus benghalensis (Vata) aerial root extract mediated green synthesis of polyshaped gold nanoparticles and its application in catalytic reduction. Int J Res Chem Environ, 2013; 3(4):52–60.
Rangeela M, Jeevitha M, Rajeshkumar S, Jayaraman S. Preparation of mouthwash using Ficus benghalensis assisted silver nanoparticles and a comparative analysis of its antimicrobial activity. J Pharm Res Int, 2021; 33(60B):3669–78. CrossRef
Rao KVB, Ojha V, Preeti Kumar G, Karthik L. Phytochemical composition and antioxidant activity of Ficus benghalensis leaf extract. J Biol Act Prod Nat, 2014; 4:236–48. CrossRef
Ravindra G, Mali Anita A, Mehta A. Review on anthelmintic plants. Nat Prod Radiance, 2008; 7(5):466–75.
Ravindra S, Mohan YM, Reddy NN, Raju KM. 2010. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via green approach. Colloids Surf A Physicochem Eng Asp, 2010; 367(1–3):31–40. CrossRef
Renata-Maria V. Determination of acute and subacute toxicity of six plant extracts. Medico Res Chronicles, 2019; 6:64–7.
Rupani R, Chavez A. Medicinal plants with traditional use: ethnobotany in the Indian subcontinent. Clin Dermatol, 2018; 36:306–9. CrossRef
Saifi A, Namdeo KP, Chauhan R, Dwivedi J. Standardization and evaluation for antidiabetic activity of Ficus bengalensis Linn. stem bark. Indo Am J Pharm Res, 2014; 4(4):1960–7.
Salave A, Reddy PG, Diwakar P. Ethno pharmaceutical claims by the vanjaris from Pathardi Tahasil in Ahmednagar district (MS) India. Asian J Exp Biol Sci, 2011; 2(1):69–74.
Saloni J, Sakthivel J. Evaluation of antioxidant and anticancer potential of flavonoids from aerial roots of Ficus benghalensis Linn. Int J Pharm Sci Res, 2019; 11:12–20.
Sandeep PA, Surjan SR, inventors. SSR Pharma Pvt Ltd (Mumbai, Maharashtra, India), assignee. Fuel supplement to reduce harmful emissions. Indian Patent. 201621023550A. 2016-07-09.
Saraswathi S, Senthamarai R, Sundari S. Antidiabetic activity of leaves extract of Ficus benghalensis Linn on alloxan induced diabeteic rats. Int J Pharmacol Biol Sci, 2013; 7(3):394–400.
Sarojini, inventor. Sarojini (In), assignee. Anti-Inflammatory and anti-arthritic herbal composition and method of preparation thereof. Indian Patent. 201741039013A. 2017-11-02.
Satish A, Punith Kumar R, Rakshith D, Satish S, Ahmed F. Antimutagenic and antioxidant activity of Ficus benghalensis stem bark and Moringa oleifera root extract. Int J Chem Anal Sci, 2013; 4(2):45–8. CrossRef
Saware K, Sawle B, Salimath B, Jayanthi K, Abbaraju V. Biosynthesis and characterization of silver nanoparticles using Ficus benghalensis leaf extract. Int J Res Eng Technol Sci, 2014; 3(05):867–74. CrossRef
Sawarkar HA, Kumar SM, Kumar PA, Deepak B. In vitro anthelmintic activity of Ficus benghalensis, Ficus carica and Ficus religosa: a comparative study. Int J Pharm Pharm Sci, 2011; 3(2):152–3.
Saxena A, Tripathi RM, Zafar F, Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater Lett, 2012; 67(1):91–4. CrossRef
Shah A, Marwat SK, Gohar F, Khan A, Bhatti KH, Amin M, Din NU, Ahmad M, Zafar M. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am J Plant Sci, 2013; 04:98–116. CrossRef
Sharma M, inventors. Maya Sharma (Delhi, India), assignee. Ramban Cough Aushadi. Indian Patent. 201811048303A. 2018-12-20.
Sharma S, Chaturvedi M, Edwin E, Shukla S, Sagrawat H. Evaluation of the phytochemicals and antidiabetic activity of Ficus bengalensis. Int J Diabetes Dev Ctries, 2010; 27(2):56–9. CrossRef
Sharma S, Sharma GK, Mehta A. Antimutagenic protection of Ficus benghalensis extract against cyclophosphamide induced toxicity in rat bone marrow. Asian J Pharm Clin Res, 2012; 5(1):0974–2441.
Shekhawat MS, Ravindran CP, Manokari M. A green approach to synthesize the zinc oxide nanoparticles using aqueous extracts of Ficus benghalensis L. Int J Res Biosci Agric Techn, 2015; 6(1):1.
Shinde HM, Bhosale TT, Gavade NL, Babar SB, Kamble RJ, Shirke BS, Garadkar KM. Biosynthesis of ZrO2 nanoparticles from Ficus benghalensis leaf extract for photocatalytic activity. J Mater Sci Mater Electron, 2018; 29(16):14055–64. CrossRef
Shinde M, Shete RV, Kore KJ, Attal AR. Hepatoprotective activity of Ficus bengalensis Linn leaves. J Curr Pharm Res, 2012; 2(2):503–7 CrossRef
Shukla R, Anand K, Prabhu KM, Murthy PS. Hypocholesterolemic effect of water extract of the bark of Banyan tree, Ficus bengalensis. Indian J Clin Biochem, 1995a; 10(1):14–8. CrossRef
Shukla R, Anand K, Prabhu KM, Murthy PS. Hypolipidemic effect of water extract of Ficus bengalensis in alloxan induced diabetes mellitus in rabbits. Indian J Clin Biochem, 1995b; 10:119–21. CrossRef
Shukla R, Gupta S, Gambhir J, Prabhu K, Murthy P. Antioxidant effect of aqueous extract of the bark of Ficus bengalensis in hypercholesterolaemic rabbits. J Ethnopharmacol, 2004; 92:47–51. CrossRef
Singh A, Singh MK, Singh R. Traditional medicinal flora of the district Buxar (Bihar, India). J Pharmacogn Phytochem, 2013; 2(2):41–9.
Singh C, inventor. Singh C (New Delhi, India), assignee. Herbal mosquito insect repellent cow dung cake. Indian Patent. 201611011815A. 2016-04-04.
Singh RK, Geeta W. Antimicrobial potential of Ficus bengalensis aerial roots. Int J Pharma Bio Sci, 2010; 1(3):BS11.
Singh RK, Mehta S, Jaiswal D, Rai PK, Watal G. Antidiabetic effect of Ficus bengalensis aerial roots in experimental animals. J Ethnopharmacol, 2009; 123(1):110–4. CrossRef
Singh U, inventor. Singh Umesh (Sheohar, Bihar, India), assignee. Herbal preparation for therapeutic and prophylactic management of neurological disorders. Indian Patent. 356907A1. 2017-01-18.
Somabhai PT, inventor. Somabhai PT(In), assignee. Herbal compositions for the promoting or enhancing growth and yield in plants. Indian Patent. IN-MUM-2013-00663A. 2013-03-05.
Sonali, Siddiqui MA, Gupta A, Singh A, Kumar N. Formulation and evaluation of Ficus benghalensis emulgel for its anti-rheumatoid arthritis effect. J Innov Appl Pharm Sci, 2021; 6(3):31–6. CrossRef
Sowbarania S, Lakshmi T. Comparative antimicrobial activity of copper nanoparticles and selenium nanoparticles synthesized using Ficus benghalensis against wound pathogens. J Complement Med Res, 2021; 12(3):94–4. CrossRef
Spiegler V, Liebau E, Hensel A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat Prod Rep, 2017; 34:627–43. CrossRef
Sreeramulu N, Suthari S, Ragan A, Raju VS. Ethno-botanico-medicine for common human ailments in Nalgonda and Warangal districts of Telangana, Andhra Pradesh, India. J Plant Sci, 2013; 2(07):220–9.
Sridevi G, Gopkumar P, Shastry CS, Ashoka Shenoy M. Evaluation of the immunomodulatory activity of growing arial root tips of Ficus benghalensis. Pharmacology, 2009; 1:1158–68.
Sudhakar TPB, Premkumar JAADK, Sapkota R, Rijal S. Antimicrobial activity of silver nanoparticles synthesized from Ficus benghalensis against human pathogens. Res J Pharm Tech, 2017; 10(6):1635–40. CrossRef
Tabassum K, Pratima T, Satish G. Bioactivity guided fractionation of the aqueous extract of the indian banyan and evaluation of immunomodulatory activity-validating the traditional use. J Homeopathy Ayu Med, 2014; 3(158):158. CrossRef
Tani K, Hashimoto H, Yamashita R, Ayusawa M, inventors. B & C Lab Inc, assignee. Antioxidant. Japanese Patent. 2010159213A. 2009-01-06.
Taur D, Nirmal S, Patil R, Kharya M. Antistress and antiallergic effects of Ficus benghalensis bark in asthma. Nat Prod Res, 2007a; 21:1266–70. CrossRef
Taur DJ, Nirmal SA, Patil RY. Effect of various extracts of Ficus bengalensis bark on clonidine and haloperidol-induced catalepsy in mice. Pharmacologyonline, 2007b; 3:470–7.
Taur DJ, Patil RY. Effect of bio-fractions isolated from Ficus bengalensis bark on clonidine induced catalepsy. J Pharm Res, 2009; 2(11):1676–7.
Thakare VN, Suralkar AA, Deshpande AD, Naik SR. Stem bark extraction of Ficus benghalensis Linn for anti-inflammatory and analgesic activity in animal models. Indian J Exp Biol, 2010; 48(1):39–45.
Tharini P, Sivaraj C, Arumugam P, Manimaran A. Antioxidant activities and GCMS analysis fruits of Ficus benghalensis L. J Pharmacogn Phytochem, 2018; 7:518–23.
Thirumagal K, Jeevitha M, Rajeshkumar S, Jayaraman, S. Controlling oral pathogens using Ficus benghalensis mediated silver nanoparticles. J Pharm Res Int, 2021; 33:98–105. CrossRef
Thite AT, Patil RR, Naik SR. Anti-arthritic activity profile of methanolic extract of Ficus bengalensis: comparison with some clinically effective drugs. Biomed Aging Pathol, 2014; 4(3):207–17. CrossRef
Tkachenko H, Buyun L, Terech-Majewska E, Osadowski Z. In vitro antimicrobial activity of ethanolic extracts obtained from Ficus spp. leaves against the fish pathogen Aeromonas hydrophila. Fish Aquatic Life, 2016; 24(4):219–30. CrossRef
Tripathi RM, Hameed P, Rao RP, Shrivastava N, Mittal J, Mohapatra S. Biosynthesis of highly stable fluorescent selenium nanoparticles and the evaluation of their photocatalytic degradation of dye. BioNanoScience, 2020; 10:389–96. CrossRef
Tripathi RM, Pudake RN, Shrivastav BR, Shrivastav A. Antibacterial activity of poly (vinyl alcohol)—biogenic silver nanocomposite film for food packaging material. Adv Nat Sci Nanosci Nanotechnol, 2018a; 9(2):025020. CrossRef
Tripathi RM, Rao RP, Tsuzuki T. Green synthesis of sulfur nanoparticles and evaluation of their catalytic detoxification of hexavalent chromium in water. RSC Advances, 2018b; 8:36345–52. CrossRef
Tulasi C, Narasu ML, Saida L. Cytotoxic effect of Ficus religiosa and Ficus benghalensis latex extracts on MCF-7 cell line. Int J Res Biol Sci, 2018a; 5:96–100. CrossRef
Tulasi CDSLN, Lakshmi NM, Saida L. Cell viability assay of Ficus benghalensis latex solvent extracts on different cell lines. Asian J Pharm Clin Res, 2018b; 11:335–9. CrossRef
Tuse T, Bidkar AA, Bhale SA, Patankar RD. In-vitro anthelmintic activity of aerial roots of Ficus benghalensis. Int J Pharm Res, 2011; 1:10–3. CrossRef
Uma B, Prabhakar K, Rajendran S. In-vitro antimicrobial activity and phytochemical analysis of Ficus religiosa L. and Ficus benghalensis L. against diarrhoeal enterotoxigenic E. coli. Ethnobot Leafl, 2009; 2009(4):7.
Vankar SD, inventor. Somabhai Dhulabhai Vankar (In), assignee. Herbal formulation for the treatment of bloat. Indian Patent. IN-MUM-2014-03737A. 2014-11-26.
Varija D, Kumar KP, Reddy KP. Antinociceptive properties of Ficus bengalensis (bark) in alloxan-induced diabetic mice. J Appl Anim Res, 2011; 39(1):49–52. CrossRef
Verma OP, Poonam S, Kamin A. Phytochemical screening of Ocimum sanctum (Tulsi), Azadirachta indica (Neem) and Phyllanthus emblica (Amla). Int J Curr Microbiol Appl Sci, 2019; 8:682–6. CrossRef
Verma VK, Sehgal N, Prakash O. Characterization and screening of bioactive compounds in the extract prepared from aerial roots of Ficus benghalensis. Int J Pharm Sci Res, 2015; 6:5056.
Vignesh M, Priya VV, Gayathri R. Antistress activity of methanolic extract of Ficus benghalensis. Drug Invent Today, 2019; 12(6):1281–3.
Wanjari M, Kumar P, Umathe SN. Anti-inflammatory effect of ethanolic extract of Ficus bengalensis Linn. in carrageenan induced paw edema in rats. Pharmacogn J, 2011; 3(23):96–9. CrossRef
Watson JD, Baker DA, Bell SP, Gann A, Levine M, Losick R. Molecular biology of the gene. 5th edition, Pearson/Benjamin, San Francisco, CA, 2004.
Yadav PK, Upadhyay RK, Kumar D, Bano D, Chandra S, Jit S, Hasan SH. Synthesis of green fluorescent carbon quantum dots from the latex of Ficus benghalensis for the detection of tyrosine and fabrication of Schottky barrier diode. New J Chem, 2021; 45(28):12549–56. CrossRef
Yadav S, Kulshreshtha M, Goswami M, Rao CV Sharma V. Elucidation of analgesic and antipyretic activities of Ficus benghalensis Linn. leaves in rats. J Appl Pharm Sci, 2011a; 01(01):38–41.
Yadav S, Rani N, Saini K. Green synthesis of ZnO and CuO NPs using Ficus benghalensis leaf extract and their comparative study for electrode materials for high performance supercapacitor application. Mater Today Proc, 2022; 49(5):2124–30. CrossRef
Yadav YC, Srivastava DN, Saini V, Singhal S, Seth AK, Kumar S. In-vitro antioxidant activity of methanolic extraction of Ficus benghalensis L. latex. Pharmacology, 2011b; 1:140–8.
Yaqoob SB, Adnan R, Khan R, Muhammad R, Mohammad R. Gold, silver, and palladium nanoparticles: a chemical tool for biomedical applications. Front Chem, 2020; 3(8):376. CrossRef
Zerega NJ, Clement WL, Datwyler SL, Weiblen GD. Biogeography and divergence times in the mulberry family (Moraceae). Mol Phylogenet Evol, 2005; 37(2):402–16. CrossRef