INTRODUCTION
Periodontitis is a provocative inflaming disorder of the oral cavity, affecting the periodontium’s hard and soft supporting tissues, catalyzed by the subgingival hoarding of anaerobic Gram-negative microbes (Könönen et al., 2019; Martínez-García and Hernández-Lemus, 2021). While a variety of individual concomitant health features, including inheritance, diabetes mellitus (DM), and tobacco consumption, have an impact on the consequences and advancement of the diseases process, bacteria play the primary role in the growth and evolution of periodontal illness (Bhuyan et al., 2022; Könönen et al., 2019; Nazir, 2017).
Gum-related illnesses have been identified and investigated for under 5,000 years (Highfield, 2009). The fundamental model for diagnosing periodontal disease in clinical practice today continues to be the current clinical diagnostic parameters (Kakar et al., 2022), which were introduced more than a few decades ago (Committee on Diagnostic Error in Health Care et al., 2015; Highfield, 2009; Ko et al., 2021; Listgarten, 1986). They contain radiographs that measure alveolar bone levels and probing pocket depths (PPDs), bleeding on probing, clinical attachment levels (CALs), plaque index, and degrees of clinical attachment (Khashaba et al., 2020; Kim et al., 2023; Lang and Bartold, 2018; Preshaw, 2015). The procedure is simple to handle, cost-efficient, and comparably non-intrusive. The periodontal probe’s clinical attachment loss appraisal also estimates the injury from prior ruin events. Moreover, the procedure is able to estimate up to 2–3 mm substantial difference (Giannobile et al., 2009; Ko et al., 2021; Taba et al., 2005).
Research on diagnosing oral and periodontal diseases advances toward techniques that identify and measure periodontal risk using biomarkers (Taba et al., 2005). In 1998, the National Institutes of Health Biomarkers Definitions Working Group (2001) defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”
Antibodies to several pathogenic microbes have been found in the local area in the periodontal tissue and systemically in the bloodstream (Martínez-García and Hernández-Lemus, 2021). Periodontal infections that cause periodontitis also cause an inborn or inherent humoral immune response (Stathopoulou et al., 2015). According to studies, the preponderance of the main periodontopathogens has hemolytic activity, which dissolves erythrocytes and results in elevated iron levels (Chu et al., 1991). Such periodontal bacteria may produce excessive amounts of iron, raising local iron concentrations, and eventually producing iron disorders (Guo et al., 2018).
Iron is a fundamental element for virtually all life forms as it takes part in oxidation–reduction reactions necessary for several fundamental biologic processes (Abbaspour et al., 2014; Dev and Babitt, 2017). Among human beings, iron is incorporated into proteins (Ems et al., 2022). These iron-containing proteins are required for cellular and organismic purposes, including O2 transportation, xenobiotic metabolism, nucleic acid replication or duplication, and host protection (Dev and Babitt, 2017; Ward and Cloonan, 2019).
Several types of dietary iron are absorbed, including ferritin, heme, and inorganic forms (Abbaspour et al., 2014; Ems et al., 2022). Ferritin performs a variety of specialized tasks, including reprocessing iron in macrophages, momentary and long-standing iron repositories in hepatocytes, and typical intracellular tasks, which conserve iron for cytochromes, nitrogenase, hemoglobin (Hb), myoglobin, and other anabolic enzymes as well as potential detoxification if too much iron enters the cells (Kotla et al., 2022; Sukhbaatar and Weichhart, 2018). As a result, ferritin is typically thought of as a protein that stores iron inside cells (Chiou and Connor, 2018). Commonly people’s extracellular fluids include ferritins as well. Clinical conditions, such as cancer, chronic infection, hepatocellular injury, and acute inflammation, result in high serum ferritin levels (Sandnes et al., 2021).
Ferritins are high in infection without signaling body iron overload. As ferritin is an amyloid precursor protein, it is frequently higher in the course of disease. Evidence suggests that smoking, obesity, triglycerides, diabetes, and periodontal disease have an association with serum ferritin levels. It is suggested that patients with periodontitis show changes in cellular and molecular components of peripheral blood due to inflammatory changes in the periodontal tissues.
The purpose of this study was to measure the concentrations of serum ferritin from dental biofilm-induced gingivitis and Stage 3 Grade A Periodontitis patients before and after non-surgical periodontal therapy and test whether these concentrations correlate with clinical parameters associated with periodontal disease.
OBJECTIVES OF THE STUDY
The objectives of this research are to measure the serum ferritin level among dental biofilm-induced gingivitis and Stage 3 Grade A Periodontitis patients and also to evaluate the effect of non-surgical periodontal therapy on serum ferritin levels in Stage 3 Grade A Periodontitis patients. Also, along with serum ferritin level, the Hb level was evaluated in dental biofilm-induced gingivitis and Stage 3 Grade A Periodontitis patients and also to evaluate the effect of non-surgical periodontal therapy on Hb level in chronic periodontitis patients.
MATERIALS AND METHODS
Study design
The biochemical analysis was carried out in the Department of Periodontics at Karnavati School of Dentistry, Uvarsad, Gandhinagar, Gujarat 382422, India. After dividing the patients into their respective groups (Fig. 1), baseline clinical parameters were recorded, and blood samples were obtained to measure serum ferritin levels. Only oral hygiene instructions were given to those in the control group. At the same time, thorough scaling and root planning were carried out for those in the experimental group, and oral hygiene instructions were given. After 3 months, the patients were called back for reevaluation of clinical parameters and serum ferritin levels. All patients met the inclusion criteria: for the control group, no evidence of interproximal attachment loss and probing depth ≤3 mm in all the teeth; for the test group (Eke et al., 2012), two or more interproximal sites with attachment loss ≥4 mm and two or more interproximal sites with probing depth ≥5 mm, not on the same tooth those without a history of any systemic disease or condition, those who were not on anti-inflammatory or antibiotic drugs, mouthwash, or vitamin supplements within a previous 3-month period. After confirming the Hb level (≥12 g/dl), patients were included in the study. The exclusion criteria included pregnant and lactating women and patients who smoked or consumed tobacco products. The patients explained the procedure well, and informed consent was taken before enrollment into the study.
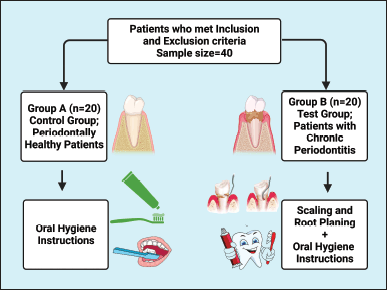 | Figure 1. A flowchart illustrating the study group. This figure has been drawn utilizing the premium version of BioRender with the Licence number (RJ25DQU2R5). Image Credit: Susmita Sinha. [Click here to view] |
Clinical parameters
The Oral Hygiene Simplified Index (OHI-S) suggested by Greene and Vermillion (1964) (Sosiawan et al., 2022) was recorded in the patients of both groups. After that, the gingival index (GI), first coined by Löe-Silness (Gehlot et al., 2022; Löe, 1967; Marçal et al., 2022; McClanahan, 2001), was recorded. PPDs and CAL were measured using a UNC-15 probe in all teeth. The control group had PPDs ≤3 mm with no attachment loss, whereas PPDs defined the test group ≥5 mm and/or attachment loss ≥4 mm at a minimum of two interproximal sites.
Collection of blood from the subjects
The skin disinfection at the venous puncture site was conducted with surgical spirit before each sample collection. Strapping with the strip of cloth or a band of rubber 3–4 inches above the antecubital fossa, a blood sample of 5 ml was gathered from the antecubital vein of the forearm using a 25-gauge needle and 5 ml syringe. The collected blood was divided into two parts. The first part was transferred to an aseptic vacuum tube containing an anticoagulant to estimate Hb. The second part was transferred to a sterile vacuum tube with no supplemental anticoagulant to assess the serum ferritin level. The collected blood was kept at room temperature to allow clot formation. Then, serum was segregated from blood by centrifuging at 2,700–3,300 rpm for 15 minutes, and extracted serum was immediately transported to the vial for serum ferritin level measurement (Fig. 2).
Statistical analysis
Clinical and laboratory parameters were recorded for both groups at baseline and 3 months intervals. The mean and standard deviation of the collected data were calculated for both the groups and between. The within-group comparison was made using repeated measured analysis of variance (ANOVA) by adjusting the model with age and sex. The biological parameters of each of the 20 patients were followed at two time points, during enrollment and 3 months later. Therefore, an autocorrelation was created between the responses of each patient. To assess the group effects on changes in biomarkers over time by considering the patient’s autocorrelation, a generalized estimating equation (GEE) model was performed, considering an exchangeable correlation matrix. The treatment effects were adjusted by covariates, such as age, sex, and time. Statistical analysis was performed using STATA-15, and charts were prepared using GraphPad Prism 8.3.2 (Fig. 4).
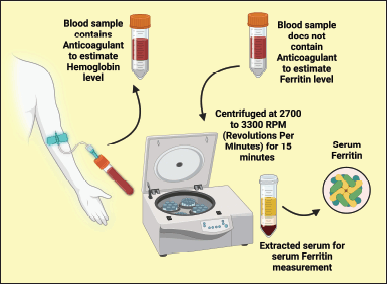 | Figure 2. Illustrating the blood collection method. This figure has been drawn utilizing the premium version of BioRender with the Licence number (OQ255QGN01). Image Credit: Susmita Sinha. [Click here to view] |
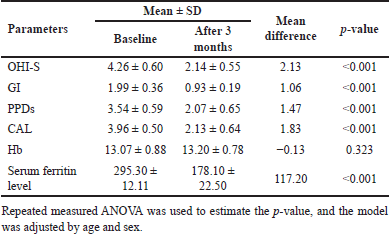 | Table 1. Within-group difference in the test group between baseline and follow-up after 3 months. [Click here to view] |
Ethical approval
This study obtained ethical approval from the School of Dentistry, Karnavati University, Uvarsad-Adalaj Road At.&PO.: Uvarsad, Dist, Gandhinagar, Gujarat 382422, India with Reference No.: KSDEC/17-18/Apr/20, Dated:04-Dec-2017.
RESULTS
The study comprised 40 patients aged 18–45 years: 20 in the control group and 20 in the test group. In the control group, out of 20 patients, 10 were male and 10 were female, with a mean age of 38.7. In the test group, out of 20 patients, 12 were male, and 8 were female, with a mean age of 40.64.
Clinical and laboratory parameters in test group patients
In the control group, significant changes were observed. Except for the Hb level, all other clinical and laboratory parameters declined to exceptional values, as shown below (Table 1 and Fig. 3A and B).
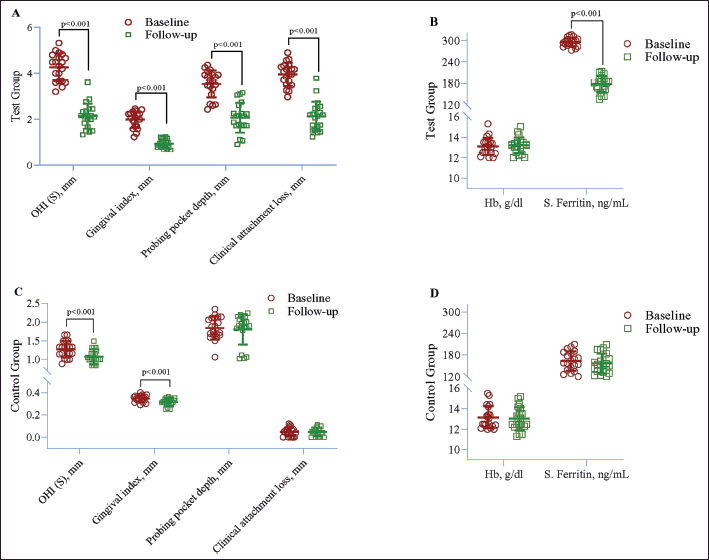 | Figure 3. Within-group difference in case (A and B) and control (C and D) between baseline and follow-up after 3 months. Repeated measured ANOVA was used to estimate the p-value. [Click here to view] |
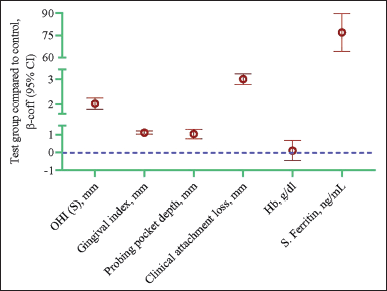 | Figure 4. Longitudinal changes in biological parameters in the test group compared to the control group. Data are presented as a β coefficient with 95% confidence intervals in parentheses and adjusted for age, sex, and duration. Statistical analysis was performed using the GEE model. The p-value of <0.05 is significant. [Click here to view] |
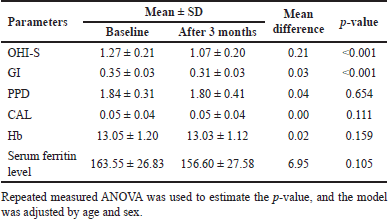 | Table 2. Within-group difference in the control group between baseline and follow-up after 3 months. [Click here to view] |
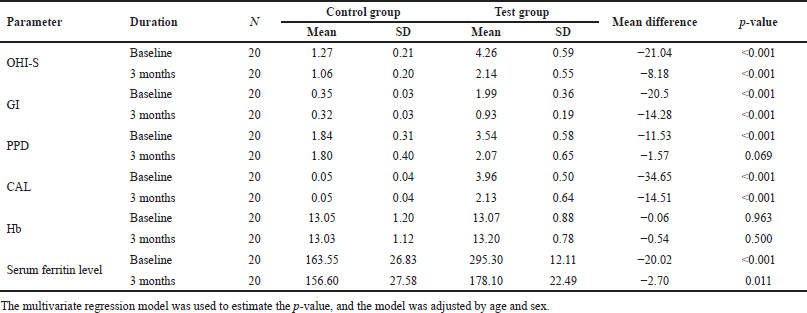 | Table 3. Biological parameters differ between control and test groups at baseline and follow-up. [Click here to view] |
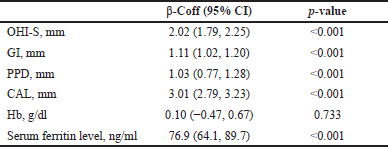 | Table 4. Longitudinal changes in biological parameters in the test group compared to the control group. [Click here to view] |
Oral Hygiene Simplified Index (mm) = OHI-S; Gingival index (mm) = GI; Probing pocket depths (mm) = PPDs; Clinical attachment level (mm) = CAL; Hemoglobin (g/dl) = Hb, serum ferritin level (ng/ml).
Clinical and laboratory parameters in control group patients
In the control group, a significant decline was noted in OHI-S (p < 0.001) and GI (p < 0.001). However, no other changes were found in clinical and laboratory parameters (Table 2 and Fig. 3C and D).
Oral Hygiene Simplified Index (mm) = OHI-S; Gingival index (mm) = GI; Probing pocket depths (mm) = PPDs; Clinical attachment level (mm) = CAL; Hemoglobin (g/dl) = Hb, serum ferritin level (ng/ml).
Intergroup comparison of clinical and laboratory parameters
When intergroup comparison was made at intervals of 3 months, the mean values of oral hygiene, GI, CAL, and serum ferritin level showed a better reduction in the test group compared to the control group, while no statistically significant values were noted for PPD and Hb level (Table 3).
Oral Hygiene Simplified Index (mm) = OHI-S; Gingival index (mm) = GI; Probing pocket depths (mm) = PPDs; Clinical attachment level (mm) = CAL; Hemoglobin (g/dl) = Hb, serum ferritin level (ng/ml).
Analysis by the GEE method exhibited a significant increase in OHI-S (β = 2.02, p < 0.001), GI (β = 1.11, p < 0.001), PPD (β = 1.03, p < 0.001), CAL (β = 3.01, p < 0.001), and serum ferritin (β = 76.9, p < 0.001) in the test group compared to the control group. However, no significant difference was noted in Hb (Table 4 and Fig. 3).
DISCUSSION
Periodontitis is a long-standing inflammatory illness caused by infection of the facilitating tissues around the teeth (Hoare et al., 2019; Könönen et al., 2019). A limited group of primarily Gram-negative anaerobic bacteria and spirochetes, including Treponema denticola, Tannerella forsythia, and Porphyromonas gingivalis, colonize and increase to start the infection (Hiranmayi et al., 2017; Zhu et al., 2013). These bacteria expand apically along the surface of the tooth roots, embedded with various other species in biofilms, to encourage the development of periodontal pockets and the obliteration of the alveolar bone and collagenous attachment fibers of the periodontal ligament (Lasserre et al., 2018).
It is reported that ferritin is an acute-phase reactant; it is increased in conditions, such as liver disease, persistent infection, autoimmune disorders, and inflammation (Kell and Pretorius, 2014; Mahroum et al., 2022). Fever, leukocytosis, thrombocytosis, metabolic abnormalities, changes in the concentration of some plasma proteins, and changes in metabolism are among the clinical and metabolic characteristics of the acute phase response (Chakraborty and Burns, 2023). Several plasma proteins, including ferritin, alter because of infection (Kernan and Carcillo, 2017).
Ferritin is essential in iron storage and recycling, apart from its aspect as an acute-phase protein (Kotla et al., 2022). Ferritin plays a critical role in the host immune response (Ganz and Nemeth, 2015), as is conspicuous from its raised level at the same time as infection to hindering infective agents that attempt to bind iron from the host tissue (Gehrer et al., 2023).
The factors which control ferritin expression are iron and proinflammatory cytokines (Moreira et al., 2020). Proinflammatory cytokines like that of tumor necrosis factor (TNF), interleukin (IL)-1, and IL-6 play a significant role in periodontitis (Gomes et al., 2016; Ramadan et al., 2020). Clinical studies have shown that TNF-α and IL-1a stimulate ferritin expression by including transcription of the heavy chain ferritin (H-ferritin) gene in mouse adipocytes and human muscle cells, which suggests that ferritin is involved in the immune response to inflammation (Moreira et al., 2020). Lipopolysaccharide of the extracellular membrane of Gram-negative bacteria provokes various reactions involving ferritin (Farhana and Khan, 2022; Maldonado et al., 2016), thereby resulting in upraised serum ferritin in circulation, suggesting that ferritin is possibly embroiled in the host’s immune response to bacterial infections (Kernan and Carcillo, 2017). In gingival tissue disruption, local inflammatory arbitrators derived from the host activate and are overexpressed when exposed to pathogenic microbes and their outgrowth (Cekici et al., 2014; Silva et al., 2015). The above mode of action is involved in the correlation between serum ferritin and chronic periodontitis (Thounaojam, 2019).
Periodontitis is a multifactorial disease, and periodontal therapy depends on several factors that can modify host response in patients with generalized infections, those with diabetes, those on immunosuppressive therapy, and other debilitating diseases (Preshaw, 2015; Simpson et al., 2015). Among people with diabetes, individuals’ pathogenic microbial deterrent mechanisms are often impaired. The vulnerability toward pathogens is realized and potentiated through high blood glucose levels associated with DM and the level of control of DM (Nagendra et al., 2022). Diabetic patients have an exceptional likelihood of infective disorders (Abu-Ashour et al., 2017).
One of the censorious difficulties pregnant and lactating ladies often face is inadequate iron intake, leading to anemia. However, it is also important to note that pregnant and lactating women may have different nutritional needs and may require additional interventions to address iron deficiency anemia compared to non-pregnant and non-lactating individuals. Therefore, it is possible that excluding this population was necessary to achieve the research objectives and avoid confounding factors. That is why we have excluded pregnant and lactating women (including criteria Hb ≥ 12 g/dl) in this study (Kadry et al., 2018).
Various types of mouthwash are regularly used in the current lifestyle (Joshipura et al., 2017; Macfarlane et al., 2011). Every mouthwash acts as a bacteriostatic or bactericidal on the oral mucosa and gingival tissue (Radzki et al., 2022; Rajendiran et al., 2021). These properties of mouthwashes can alter the result of this study’s parameters (Kumar and Varghese, 2020; Rajendiran et al., 2021). Considering this, we have excluded patients who use mouthwashes within 3 months.
Smoking is the most potent behavioral hazardous feature for the incidence and advancement of periodontitis (Silva, 2021). It deleteriously affects all aspects of the periodontium (Jiang et al., 2020; Leite et al., 2018). Tobacco users have been shown to retort to non-surgical therapeutic intervention for periodontitis less than non-smokers (Kanmaz et al., 2021). Smoking can compromise the immune response, impair healing, and exacerbate inflammation in the gums, all of which can contribute to the evolution and progression of periodontitis (Zhang et al., 2019). Based on this evidence, we have excluded them from this research.
In the present study, the intergroup comparison of the mean value of the oral hygiene index at 3 months showed a better reduction in the test group than in the control group. These results agree with those of Chakraborty et al. (2014).
In the present study, the intergroup comparison of the mean value of the GI at 3 months showed a better reduction in the test group than in the control group. The GI score was low throughout the 3 months of the study period. This may be because of proper oral hygiene guidance. However, the better reduction in the GI score in the test group suggests that the intervention provided to this group effectively improved gingival health beyond what can be achieved through good oral hygiene practices alone. These results follow the study conducted by Latha et al. (2015).
In the current research, the intergroup comparison of PPD at 3 months between two groups showed a mean difference of −1.57, which was statistically nonsignificant because in the test group, the mean value after phase-1 therapy reduced and reached up to the 3-month mean value of the control group. Ali and Ahmed (2018) conducted a similar observational study and found statistically significant results in GI, PPD, and CAL.
The mean clinical attachment loss at 3 months between the two groups showed a mean difference of −14.51, which was statistically significant. Thounaojam (2019) did a 1-month intervention study on the effects of chronic periodontitis on serum ferritin levels. He found a mean reduction in serum ferritin levels from the reference point and 1 month after non-surgical periodontal therapeutic intervention in CP patients and a substantial decline in clinical attachment loss.
No marked difference in Hb level was observed in both, and the groups and mean differences between both groups were statistically not significant. Several studies have examined the correlation between Hb levels and chronic periodontitis. A study conducted by Kolte et al. (2014) revealed no considerable correlation linking Hb level and the severity of inflammation. In lieu, several researchers (Anumolu et al., 2015; Gokhale et al., 2010; Naik et al., 2010; Patel et al., 2013; Parihar et al., 2019; Pradeep and Anuj, 2011; Shetty et al., 2014) showed a significant correlation between Hb level and severity of inflammation, i.e., reduction in Hb level as the severity of inflammation gets higher.
The current research shows a substantial association linking serum ferritin and chronic periodontitis with multiple earlier studies (Bhavya et al., 2017; Chakraborty et al., 2014; Thounaojam, 2019). In all this research and the present study, the result shows a significant depletion in serum ferritin levels and, subsequently, medical periodontal treatment as the level of inflammation gets reduced. Latha et al. (2015), and Ali and Ahmed (2018) did an observational study on the interrelationship uniting serum ferritin levels and chronic periodontitis. They found a significant correlation between serum ferritin and chronic periodontitis. Contrary to all these studies, Prakash et al. (2012) stated that no correlation between serum ferritin level and long-standing periodontitis was found in all these studies.
Based on the results achieved in the present research, it must be emphasized that the present study shows a significant association betwixt serum ferritin levels and the ferocity of inflammation.
However, it is important to note that the study’s sample size, demographic characteristics, and other factors may have influenced the results. Additionally, the duration of the study period may not be long enough to fully capture the effects of the intervention over a more extended period. Overall, the findings of this study highlight the potential for effective interventions to improve periodontal health. However, additional studies are required to fully understand the impact of the intervention on clinical attachment loss and its potential for widespread application.
LIMITATIONS OF THE STUDY
1. The sample size is small. Thereby, we planned another randomized control multicenter clinical trial with larger sample sizes over a longer duration so that study results can be generalized for India.
2. A single investigator in the current investigation carried out clinical parameters and treatment techniques. This experiment was not double-blinded, so the data gathering may have been biased. Therefore, it could be advantageous for future research to be double-blinded to avoid an opinionated introduction.
3. Ferritin sample was collected from serum; saliva can also be used to evaluate the ferritin level. The advantage of a salivary collection of ferritin would be its non-invasive collection technique.
4. IL 6, plasminogen activator inhibitor type 1, and the TNF-α are the additional inflammatory mediators, which can be measured using enzyme-linked immunosorbent assay kits and can be used to evaluate periodontal inflammation.
CONCLUSION
The findings from this comparative, clinical, and biochemical trial revealed that serum ferritin level is an essential factor in the pathogenesis of periodontal disease. It can be used as a diagnostic marker for periodontal disease or a prognostic marker for disease progression.
RECOMMENDATION
More real-life clinical research studies must be carried out to recognize and realize the beneficial aspect of LAB on human health. Also, governments and academic institutions need to play an active role in incorporating LAB use for human health. Further refinement of delivery means to ensure LAB cells’ survival needs to be done. Researchers should further investigate and use probiotics as therapeutics for various illnesses in the best possible ways.
ACKNOWLEDGMENTS
The graphical abstract has been drawn utilizing the premium version of BioRender with the Licence number (ZV255FGULR). Image Credit: Shreya Gajja.
AUTHORS’ CONTRIBUTIONS
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study obtained ethical approval from the School of Dentistry, Karnavati University, Uvarsad-Adalaj Road At.&PO.: Uvarsad, Dist, Gandhinagar, Gujarat 382422, India with Reference No.: KSDEC/17-18/Apr/20, Dated:04-Dec-2017.
FUNDING
There is no funding to report.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
DATA AVAILABILITY
This is an original manuscript and all data are available for only research purposes from principal investigators.
REFERENCES
Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci, 2014; 19(2):164–74.
Abu-Ashour W, Twells L, Valcour J, Randell A, Donnan J, Howse P, Gamble JM. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res Care, 2017; 5(1):e000336; doi: 10.1136/bmjdrc-2016-000336 CrossRef
Ali CJ, Ahmed MAA. Evaluation of serum ferritin, hemoglobin, mean cell volume, mean corpuscular hemoglobin concentration, and mean corpuscular hemoglobin levels in blood from patients with different severities of periodontal diseases. Res J Pharm Biol Chem Sci, 2018; 9(1):593–600.
Anumolu VN, Srikanth A, Paidi K. Evaluation of the relation between anemia and periodontitis by estimation of blood parameters: a cross-sectional study. J Indian Soc Periodontol, 2016; 20(3):265–72; doi: 10.4103/0972-124X.176392 CrossRef
Bhavya B, Ashwini S, Shruthi KR. Estimation of hemoglobin and serum ferritin concentration from females with chronic periodontitis before and after non-surgical periodontal therapy: an interventional study. Int J Recent Sci Res, 2017; 8(9):20276–9; doi:10.24327/ijrsr.2017.0809.0863 CrossRef
Bhuyan R, Bhuyan SK, Mohanty JN, Das S, Juliana N, Juliana IF. Periodontitis and its inflammatory changes linked to various systemic diseases: a review of its underlying mechanisms. Biomedicines, 2022; 10(10):2659; doi: 10.3390/biomedicines10102659 CrossRef
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther, 2001; 69(3):89–95; doi: 10.1067/mcp.2001.113989 CrossRef
Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000, 2014; 64(1):57–80; doi: 10.1111/prd.12002 CrossRef
Chakraborty RK, Burns B. Systemic inflammatory response syndrome. StatPearls, Treasure Island, FL, 2023. Available via https://www.ncbi.nlm.nih.gov/books/NBK547669/ (Accessed 24 March 2023).
Chakraborty S, Tewari S, Sharma RK, Narula SC. Effect of non-surgical periodontal therapy on serum ferritin levels: an interventional study. J Periodontol, 2014; 85(5):688–96; doi: 10.1902/jop.2013.130107 CrossRef
Chiou B, Connor JR. Emerging and dynamic biomedical uses of ferritin. Pharmaceuticals (Basel), 2018; 11(4):124; doi: 10.3390/ph11040124 CrossRef
Chu L, Bramanti TE, Ebersole JL, Holt SC. Hemolytic activity in the periodontopathogen Porphyromonas gingivalis: kinetics of enzyme release and localization. Infect Immun, 1991; 59(6):1932–40; doi: 10.1128/iai.59.6.1932-1940.1991 CrossRef
Committee on Diagnostic Error in Health Care, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine, Balogh EP, Miller BT, Ball JR, editors. Improving diagnosis in health care. National Academies Press, Washington, DC, 2015. Available via https://www.ncbi.nlm.nih.gov/books/NBK338593/ (Accessed 24 March 2023).
Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int, 2017; 21(Suppl 1):S6–20; doi: 10.1111/hdi.12542 CrossRef
Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol, 2012; 83(12):1449–54; doi: 10.1902/jop.2012.110664 CrossRef
Ems T, St Lucia K, Huecker MR. Biochemistry, iron absorption. StatPearls, Treasure Island, FL, 2022. Available via https://www.ncbi.nlm.nih.gov/books/NBK448204/ (Accessed 24 March 2023).
Farhana A, Khan YS. Biochemistry, lipopolysaccharide. StatPearls, Treasure Island, FL, 2023. Available via https://www.ncbi.nlm.nih.gov/books/NBK554414/ (Accessed 24 March 2023).
Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol, 2015; 15(8):500–10; doi: 10.1038/nri3863 CrossRef
Gehlot M, Sharma R, Tewari S, Kumar D, Gupta A. Effect of orthodontic treatment on periodontal health of periodontally compromised patients. Angle Orthod, 2022; 92(3):324–32; doi: 10.2319/022521-156.1 CrossRef
Gehrer CM, Mitterstiller AM, Grubwieser P, Meyron-Holtz EG, Weiss G, Nairz M. Advances in ferritin physiology and possible implications in bacterial infection. Int J Mol Sci, 2023; 24(5):4659; doi: 10.3390/ijms24054659 CrossRef
Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000, 2009; 50:52–64; doi: 10.1111/j.1600-0757.2008.00288.x CrossRef
Gokhale SR, Sumanth S, Padhye AM. Evaluation of blood parameters in patients with chronic periodontitis for signs of anemia. J Periodontol, 2010; 81(8):1202–6; doi: 10.1902/jop.2010.100079 CrossRef
Gomes FI, Aragão MG, Barbosa FC, Bezerra MM, de Paulo Teixeira Pinto V, Chaves HV. Inflammatory cytokines interleukin-1β and tumour necrosis factor-α—novel biomarkers for the detection of periodontal diseases: a literature review. J Oral Maxillofac Res, 2016; 7(2):e2; doi: 10.5037/jomr.2016.7202 CrossRef
Greene Jc, Vermillion Jr. The simplified oral hygiene index. J Am Dent Assoc, 1964; 68:7–13; doi: 10.14219/jada.archive.1964.0034 CrossRef
Guo LN, Yang YZ, Feng YZ. Serum and salivary ferritin and Hepcidin levels in patients with chronic periodontitis and type 2 diabetes mellitus. BMC Oral Health, 2018; 18(1):63; doi: 10.1186/s12903-018-0524-4 CrossRef
Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J, 2009; 54 Suppl 1:S11–26; doi: 10.1111/j.1834-7819.2009.01140.x CrossRef
Hiranmayi KV, Sirisha K, Ramoji Rao MV, Sudhakar P. Novel pathogens in periodontal microbiology. J Pharm Bioallied Sci, 2017; 9(3):155–63; doi: 10.4103/jpbs.JPBS_288_16 CrossRef
Hoare A, Soto C, Rojas-Celis V, Bravo D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediators Inflamm, 2019; 2019:1029857; doi: 10.1155/2019/1029857 CrossRef
Jiang Y, Zhou X, Cheng L, Li M. The impact of smoking on subgingival microflora: from periodontal health to disease. Front Microbiol, 2020; 11:66; doi: 10.3389/fmicb.2020.00066 CrossRef
Joshipura KJ, Muñoz-Torres FJ, Morou-Bermudez E, Patel RP. Over-the-counter mouthwash use and risk of pre-diabetes/diabetes. Nitric Oxide, 2017; 71:14–20; doi: 10.1016/j.niox.2017.09.004 CrossRef
Kadry S, Sleem C, Samad RA. Hemoglobin levels in pregnant women and its outcomes. Biom Biostat Int J, 2018; 7(4):326?36; doi: 10.15406/bbij.2018.07.00226 CrossRef
Kakar A, Blanchard S, Shin D, Maupomé G, Eckert GJ, John V. Periodontal diagnosis and treatment planning—an assessment of the understanding of the new classification system. J Dent Educ, 2022; 86(12):1573–80; doi: 10.1002/jdd.13037 CrossRef
Kanmaz M, Kanmaz B, Buduneli N. Periodontal treatment outcomes in smokers: a narrative review. Tob Induc Dis, 2021; 19:77; doi: 10.18332/tid/142106 CrossRef
Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics, 2014; 6(4):748–73; doi: 10.1039/c3mt00347g CrossRef
Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol, 2017; 29(9):401–9; doi: 10.1093/intimm/dxx031 CrossRef
Khashaba O, Alasfar A, Elgendy EA, Mowafey B. Clinical and radiographic diagnostic study of strontium ranelate andmetal-substituted hydroxyapatite bone graft materials in diabetes mellitus with chronic periodontitis. J Adv Periodontol Implant Dent, 2020; 12(2):51–7; doi: 10.34172/japid.2020.015 CrossRef
Kim HN, Kim K, Lee Y. Intra-oral photograph analysis for gingivitis screening in orthodontic patients. Int J Environ Res Public Health, 2023; 20(4):3705; doi: 10.3390/ijerph20043705 CrossRef
Ko TJ, Byrd KM, Kim SA. The chairside periodontal diagnostic toolkit: past, present, and future. Diagnostics (Basel), 2021; 11(6):932; doi: 10.3390/diagnostics11060932 CrossRef
Kolte RA, Kolte AP, Deshpande NM. Assessment and comparison of anemia of chronic disease in healthy subjects and chronic periodontitis patients: a clinical and hematological study. J Indian Soc Periodontol, 2014; 18(2):183–6; doi: 10.4103/0972-124X.131321 CrossRef
Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med, 2019; 8(8):1135; doi: 10.3390/jcm8081135 CrossRef
Kotla NK, Dutta P, Parimi S, Das NK. The role of ferritin in health and disease: recent advances and understandings. Metabolites, 2022; 12(7):609; doi: 10.3390/metabo12070609 CrossRef
Kumar KM, Varghese SS. Views on antioxidant mouthwashes as adjunct in periodontal therapy. Bioinformation, 2020; 16(12):1069-1079; doi: 10.6026/973206300161069 CrossRef
Lang NP, Bartold PM. Periodontal health. J Periodontol, 2018; 89(Suppl 1):S9–16; doi: 10.1002/JPER.16-0517 CrossRef
Lasserre JF, Brecx MC, Toma S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials (Basel), 2018; 11(10):1802; doi: 10.3390/ma11101802 CrossRef
Latha S, Thirugnanamsambandan S, Arun RT, Masthan KM, Malathi L, Rajesh E. Serum ferritin level and red blood cell parameters in healthy controls and chronic periodontitis patients. J Pharm Bioallied Sci, 2015; 7(Suppl 1):S184–9; doi: 10.4103/0975-7406.155896 CrossRef
Leite FRM, Nascimento GG, Scheutz F, López R. Effect of smoking on periodontitis: a systematic review and meta-regression. Am J Prev Med, 2018; 54(6):831–41; doi: 10.1016/j.amepre.2018.02.014 CrossRef
Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontol, 1986; 13(5):418–30; doi: 10.1111/j.1600-051x.1986.tb01485.x CrossRef
Löe H. The gingival index, the plaque index, and the retention index systems. J Periodontol, 1967; 38(6):Suppl:610–6; doi: 10.1902/jop.1967.38.6.610 CrossRef
Macfarlane TV, Kawecki MM, Cunningham C, Bovaird I, Morgan R, Rhodes K, Watkins R. Mouthwash use in general population: results from adult dental health survey in Grampian, Scotland. J Oral Maxillofac Res, 2011; 1(4):e2; doi: 10.5037/jomr.2010.1402 CrossRef
Mahroum N, Alghory A, Kiyak Z, Alwani A, Seida R, Alrais M, Shoenfeld Y. Ferritin—from iron, through inflammation and autoimmunity, to COVID-19. J Autoimmun, 2022; 126:102778; doi: 10.1016/j.jaut.2021.102778 CrossRef
Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev, 2016; 40(4):480–93; doi: 10.1093/femsre/fuw007 CrossRef
Marçal FF, Mota de Paulo JP, Barreto LG, de Carvalho Guerra LM, Silva PGB. Effectiveness of orthodontic toothbrush versus conventional toothbrush on plaque and gingival index reduction: a systematic review and meta-analysis. Int J Dent Hyg, 2022; 20(1):87–99; doi: 10.1111/idh.12511 CrossRef
Martínez-García M, Hernández-Lemus E. periodontal inflammation and systemic diseases: an overview. Front Physiol, 2021; 12:709438; doi: 10.3389/fphys.2021.709438 CrossRef
McClanahan SF, Bartizek RD, Biesbrock AR. Identification and consequences of distinct Löe-Silness gingival index examiner styles for the clinical assessment of gingivitis. J Periodontol, 2001; 72(3):383–92; doi: 10.1902/jop.2001.72.3.383 CrossRef
Moreira AC, Mesquita G, Gomes MS. Ferritin: an inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms, 2020; 8(4):589; doi: 10.3390/microorganisms8040589 CrossRef
Nagendra L, Boro H, Mannar V. Bacterial infections in diabetes. In: Feingold KR, Anawalt B, Blackman MR, et al. (eds.). Endotext, MDText.com, South Dartmouth, MA, 2000. Available via https://www.ncbi.nlm.nih.gov/books/NBK579762/ (Accesssed 24 March 2023).
Naik V, Acharya A, Deshmukh VL, Shetty S, Shirhatti R. Generalized, severe, chronic periodontitis is associated with anemia of chronic disease: a pilot study in urban, Indian males. J Investig Clin Dent, 2010;1(2):139–43; doi: 10.1111/j.2041-1626.2010.00028.x CrossRef
Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim), 2017; 11(2):72–80.
Parihar S, Sharma N K, Bhatnagar A, Kishore D, Parihar AV, Rahman F. Comparison of hematological parameters for signs of anemia among participants with and without chronic periodontitis: a cross-sectional study. J Indian Assoc Public Health Dent, 2019; 17:4–7; doi: 10.4103/jiaphd.jiaphd_49_18 CrossRef
Patel MD, Shakir QJ, Shetty A. Interrelationship between chronic periodontitis and anemia: a 6-month follow-up study. J Indian Soc Periodontol, 2014; 18(1):19–25; doi: 10.4103/0972-124X.128194 CrossRef
Pradeep AR, Anuj S. Anemia of chronic disease and chronic periodontitis: does periodontal therapy have an effect on anemic status? J Periodontol, 2011; 82(3):388–94; doi: 10.1902/jop.2010.100336 CrossRef
Prakash S, Dhingra K, Priya S. Similar hematological and biochemical parameters among periodontitis and control group subjects. Eur J Dent, 2012; 6(3):287–94.
Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health, 2015; 15 (Suppl 1):S5; doi: 10.1186/1472-6831-15-S1-S5 CrossRef
Radzki D, Wilhelm-W?glarz M, Pruska K, Kusiak A, Ordyniec-Kwa?nica I. A fresh look at mouthwashes-what is inside and what is it for? Int J Environ Res Public Health, 2022; 19(7):3926; doi: 10.3390/ijerph19073926 CrossRef
Rajendiran M, Trivedi HM, Chen D, Gajendrareddy P, Chen L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules, 2021; 26(7):2001; doi: 10.3390/molecules26072001 CrossRef
Ramadan DE, Hariyani N, Indrawati R, Ridwan RD, Diyatri I. Cytokines and chemokines in periodontitis. Eur J Dent, 2020; 14(3):483–95; doi: 10.1055/s-0040-1712718 CrossRef
Sandnes M, Ulvik RJ, Vorland M, Reikvam H. Hyperferritinemia-a clinical overview. J Clin Med, 2021; 10(9):2008; doi: 10.3390/jcm10092008 CrossRef
Shetty MK, Thomas B, Shetty AV. Comparative evaluation of hemoglobin level in anemic patients with chronic periodontitis before and after treatment. J Interdiscip Dentistry, 2014; 4:24–6; doi: 10.4103/2229-5194.135000 CrossRef
Silva H. Tobacco use and periodontal disease-the role of microvascular dysfunction. Biology (Basel), 2021; 10(5):441; doi: 10.3390/biology10050441 CrossRef
Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernández M, Gamonal J. Host response mechanisms in periodontal diseases. J Appl Oral Sci, 2015; 23(3):329–55; doi: 10.1590/1678-775720140259 CrossRef
Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev, 2015; 2015(11):CD004714; doi: 10.1002/14651858.CD004714.pub3 CrossRef
Sosiawan A, Wahjuningrum DA, Setyowati D, Suhartono M, Audrey NW, Mawantari TP, Setiawan F, Pawar AM. The relationship between parents’ oral hygiene knowledge and children with Down Syndrome’s oral hygiene via OHI-S. F1000Res, 2022; 11:374; doi: 10.12688/f1000research.87848.2 CrossRef
Stathopoulou PG, Buduneli N, Kinane DF. Systemic biomarkers for periodontitis. Curr Oral Health Rep, 2015; 2:218–226; doi:10.1007/s40496-015-0072-9 CrossRef
Sukhbaatar N, Weichhart T. Iron regulation: macrophages in control. Pharmaceuticals (Basel), 2018; 11(4):137; doi: 10.3390/ph11040137 CrossRef
Taba M Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am, 2005; 49(3):551–71, vi; doi: 10.1016/j.cden.2005.03.009 CrossRef
Thounaojam N. Effects of chronic periodontitis in serum ferritin levels before and 1 month after nonsurgical periodontal therapy: an intervention study. Int J Prev Clin Dent Res, 2019; 6:32–4; doi: 10.4103/INPC.INPC_29_19 CrossRef
Ward DM, Cloonan SM. Mitochondrial iron in human health and disease. Annu Rev Physiol, 2019; 81:453–82; doi: 10.1146/annurev-physiol-020518-114742 CrossRef
Zhang Y, He J, He B, Huang R, Li M. Effect of tobacco on periodontal disease and oral cancer. Tob Induc Dis, 2019; 17:40; doi: 10.18332/tid/106187 CrossRef
Zhu Y, Dashper SG, Chen YY, Crawford S, Slakeski N, Reynolds EC. Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One, 2013; 8(8):e71727; doi: 10.1371/journal.pone.0071727 CrossRef