INTRODUCTION
Malaria is caused by the Plasmodium parasite and transmitted by a mosquito bite of an infected female Anopheles mosquito. The first symptoms of the disease are fever, headache, and chills, and if left untreated, Plasmodium falciparum malaria can lead to death (WHO, 2020).
Cerebral malaria (CM) is the leading cause of death associated with P. falciparum malaria (Bruneel, 2019). The course of CM is not necessarily lethal, although survivors may have permanent neurological damage like behavioral abnormalities, epilepsy, and impaired motor functions (Nishanth and Schluter, 2019).
Chloroquine (CQ; molecular weight = 319.87 g mol−1) is commercially available as a diphosphate salt (molecular weight = 515.86 g mol−1) with a pKa of 8.4 and 10.8 (20°) (Castro e Souza et al., 2020; Tariq and Al-Badr, 1984). It is an aminoquinoline with antimalarial activity and generally acts as a schizonticide in malarial infections (Macedo et al., 2016). Currently, few strains of P. falciparum are sensitive to CQ, mainly due to its wide prophylactic use (Coban, 2020). Furthermore, this drug’s primary reported adverse effects are nausea, vomiting, diarrhea, headache, blurred vision, and acute cardiovascular toxicity (Chukanchitipat and Na-Bangchang, 2017; Vieira et al., 2020).
Colchicine (CC) is widely used to treat acute attacks of gout prophylaxis and familial Mediterranean fever (Slobodnick et al., 2018); it has been shown to suppress caspase-1 activation and IL-1β and IL-18 release. Moreover, this mechanism has been attracting attention in treating other diseases, including coronary heart disease (Martinez et al., 2015). The main problems reported in using CC are microtubule disassembly leading to cell death, low oral bioavailability, and low therapeutic index (Angelidis et al., 2018).
Given the problems that may arise regarding the use of such drugs, new strategies are necessary to treat malaria, and one notable alternative includes incorporating these drugs into nanoparticulate systems. The incorporation of antimalarials such as quinine (Haas et al., 2009; Michels et al., 2019), artemisinin (Ibrahim et al., 2015), and CQ (Muga et al., 2018) has shown promising results. Co-encapsulating CQ and CC may improve the physicochemical and pharmacokinetic characteristics of free drugs and promote synergistic effects to treat severe malaria.
CQ- and CC -loaded nanocapsules (NCs) were developed and characterized for particle size, polydispersity, pH, encapsulation rate, and dosage. High-performance liquid chromatography (HPLC) is generally used for the drug content and encapsulation rate of drugs, and, to date, no analytical technique to simultaneously quantify CQ and CC in pharmaceutical products has been reported. Therefore, this study aimed to develop and validate a method to simultaneously quantify CQ and CC in an innovative NCs formulation.
MATERIALS AND METHODS
Chemical and reagents
The ultrapurified water was obtained using a Milli-Q Plus system from Millipore (Bedford, USA). The chemical solvents, HPLC-grade acetonitrile (ACN), and methanol (MeOH) were acquired from Tedia (Fairfield, USA). The drugs, CQ and CC, were acquired from Sigma-Aldrich Ltd (São Paulo, Brazil).
Instrumentations
Analyses were performed on HPLC Shimadzu (Kyoto, Japan), coupled with a pump model LC-20AT, degasser model DGU-20A3, a detector photodiodes (DAD) model SPD-M20A, autosampler model SIL-20A, system controller model CBM-20A, and LC Solution software (Release 1.22 SP1). The chromatographic column utilized was RP-18, 4.6 mm × 300 mm × 5 μm (Waters), and utilized guard column (4 × 3 mm id).
Chromatographic conditions
Chromatographic analyses were carried out at a controlled oven temperature (30°C) and the mobile phase (MP) utilized was ACN, MeOH, and 0.01% triethylamine (pH 3, adjusted with orthophosphoric acid; 17:18:65, v/v/v). After preparation, MP was filtered using a 0.45 μm membrane and degassed by ultrasound for 15 minutes. The flow rate was one ml minutes−1, the wavelength to detect CQ and CC was 350 nm, and the injection volume was 20 μl.
CQ-and CC -loaded NCs preparation and characterization
NCs were prepared using the nanoprecipitation method and were named NCcqcc. The organic phase contained Eudragit® RS100 (EUD), Poloxamer® F127, caprylic/capric acid triglyceride, CQ (1 mg ml−1), CC (0.5 mg ml−1), and ethanol. The aqueous phase contained distilled water and polysorbate 80. The two phases were mixed and remained under stirring for 10 minutes, and the excess solvent was evaporated. A formulation without the drugs (namely white nanocapsules - NCb) was also prepared.
The diameter was determined by the dynamic light scattering technique and the NCs zeta potential was determined by the electrophoretic migration technique, both through using the NanoBrook 90Plus PALS equipment (Brookhaven®), and the pH was determined using a previously calibrated potentiometer (HANNA®) (Velasques et al., 2018). The drug content and encapsulation efficiency are described in the applicability section.
Standard solution and sample preparation
The CQ standard solution (1,000 μg ml−1) was prepared with 16 mg of CQ, value corrected by the molecular weight of CQ (319,872 g/mol), and CQ diphosphate (515.16 g/mol) to reach the desired concentration of CQ, in 10 ml of water:ACN (50:50) in a volumetric flask. The CC standard solution (1,000 μg/ml) was prepared with 10 mg of CC in 10 ml of ACN in a volumetric flask. Both standard solutions were placed in ultrasound for 2 minutes. From the stock solutions, an intermediate solution of 100 μg ml−1 was prepared.
Validation of the method
The method was validated following the guidelines of the International Conference on Harmonization (ICH), the Food and Drug Administration (FDA), and the National Health Surveillance Agency (ANVISA) (ANVISA: Agência Nacional de Vigilância Sanitária, 2017; ICH, Guidelines Q2B: Validation of Analytical Procedures: Methodology, 1996; ICH, Harmonized Tripartite Guideline: Validation of Analytical Procedure. Text and Methodology Q2 (R1), 2005; FDA, United States FDA, 2005; FDA, United States FDA, 2015).
Specificity
Specificity was performed to verify if the excipients used to prepare the NCs interfered with quantifying the drugs. Analyses of the NCb were performed in triplicate after the same procedure described to quantify the drugs in the NC. The chromatograms were compared with those containing free drugs.
Linearity
For linearity, three curves were prepared and evaluated on three different days. The curve concentrations for CQ were 5, 7.5, 10, 12.5, 15, and 17.5 μg ml−1, whereas 1, 2.5, 5, 7.5, 10, and 12.5 μg ml−1 were used for CC. Curve concentrations were prepared from the same 1,000 μg.ml−1 standard solutions.
Limits of detection (LOD) and quantification
The results of linearity were evaluated by analysis of variance (ANOVA) and linear regression analysis. (LOD; Equation 1) was calculated using the factor 3.3 multiplied by the intersection of the calibration curve (σ) divided by the slope of the mean(s). For the limit of quantification, the factor used was 10 (Eq. (2)).
LOD = [3.3×(σ/s], (1)
LOQ = [10×(σ/s]. (2)
Precision
Intraday precision was evaluated by preparing six samples of NCcqcc (CQ = 10 μg ml−1 and CC = 5 μg ml−1; n = 6) on only 1 day. For interday precision, the six samples were prepared and evaluated on three different days (n = 18). The results were expressed as SD and coefficient of variation relative standard deviation (RSD%) of intra- and interday precisions.
Accuracy
To assess the accuracy of the method, three known concentrations of CQ (8, 10, and 12 μg ml−1) and CC (4, 5, and 6 μg ml−1) solutions were added to the NCb, so 100 μl of NCb and known drug concentrations (80%, 100%, and 120%) was added to a 10 ml volumetric flask and made up with ACN:MeOH (70:30). Then, this remained in ultrasound for 2 hours, and the same procedure was used for NCs containing the drugs. Method accuracy results are expressed as recovery percentage and calculated from drug recovery.
Robustness
The robustness of the method was evaluated through small changes in the proposed method. The changes made were flow (0.9 and 1.1 ml minutes−1) and oven temperature (29°C and 31°C) and pH of the aqueous solvent of the MP (pH 2.9 and 3.2); each parameter was changed individually. The results were evaluated from the (RSD%) between the values obtained with the alteration of the parameters.
Applicability
Drug-loaded NCs quantification and applicability
To quantify the drugs in the NCs samples (Equation 3), 100 μl of the NC (1 mg ml−1 of CQ and 0.5 mg ml−1 of CC) was transferred to a volumetric flask and completed with ACN:MeOH (70:30). Then, this remained under ultrasound for 2 hours for drug extraction. The samples were filtered using a filter of 0.45 μm and quantified by HPLC coupled to a diode array detector (DAD) with the methodology described in the previous section.
The encapsulation efficiency (EE) of the CQ and CC was determined using Ultrafree® (Millipore). HPLC-DAD quantified the samples and the EE of CQ and CC was determined using Equation (4).
In vitro release
The in vitro release of CQ and CC was performed using the dialysis bag diffusion technique. 5 ml of the NCcqcc formulation (NC–CQ and NC–CC) or free CQ and CC (F-CQ and F-CC) solutions was added to dialysis bags (25 × 16 mm; cut-off = 12,000–14,000 Da, Sigma-Aldrich, USA), which were immersed in 250 ml of pH 7.4 buffer. The release medium was kept under constant stirring at 50 rpm and at a temperature of 37°C ± 1°C. Aliquots of the dissolution medium were collected at predetermined times, and the same volume was replaced with fresh medium at each collection. Samples were quantified by HPLC-DAD. The experiment was carried out in triplicate.
Statistical analysis
The results for the LOD and quantification were evaluated by ANOVA and linear regression analysis.
RESULTS AND DISCUSSION
NCs characterization and chromatographic method: development and optimization
The formulations were characterized for diameter, zeta potential, and pH. After validation, the formulations were characterized for encapsulation rate and drug content. As for the diameter, NCb had 230 ± 2.52 nm and NCcqcc had 247 ± 2.65 nm. The polydispersity index was less than 2.0 for both formulations. As a comparison, NCs developed with co-encapsulation of the drugs having a particle diameter of 206 ± 2.00 nm with quinine and curcumin and 288 ± 2.00 nm with co-encapsulation of curcumin and meloxicam (Nakama et al., 2020; Velasques et al., 2018).
The pH of the formulations was 4.91 ± 0.03 and 4.99 ± 0.03 for NCcqcc and NCb, respectively. The acid pH for these formulations is related to the functional structure of the Eudragit® RS100 EUD (Yin et al., 2016); notably, De Santos et al. (2021) also observed this behavior (de Santos et al., 2021). The zeta potential of the formulations was positive: 15.78 ± 1.82 mV for NCcqcc and 12.68 ± 1.01 mV for NCb.
Different chromatographic conditions were tested during method development to obtain a method capable of separating the drugs with good resolution: stationary phases with different characteristics (carbon charge, length, particle size, and internal diameter), MP with different proportions of organic solvent (ACN and/or MeOH), and water with and without different triethylamine concentrations in a wide range of pH (3.0–5.0).
The best chromatographic condition was achieved using a controlled oven temperature (30°C) with a MP composed of ACN, MeOH, and 0.01% triethylamine (pH 3, with orthophosphoric acid; 17:18:65). A flow rate of 1.0 mL minutes−1 and a total run time of 11 minutes were necessary to detect the CQ and CC at 350 nm.
As a comparison, Miranda et al. (2015) developed an analytical method by HPLC-DAD to simultaneously quantify CQ and primaquine. The authors used a column with a shorter length, different brands of columns (C18: 100 × 4.6 mm id × 5 μm), and a lower oven temperature (25°C). As for the MP, the authors used two solvents (ACN and 0.1% aqueous triethylamine) with a triethylamine concentration 10 times higher; the proportion of the MP was gradient ranging from 10%–40% of ACN with a 5-minutes runtime. Another difference in our study was the detection wavelength of 260 nm (Miranda et al., 2015).
Tantawy et al. (2021) developed a method to simultaneously quantify probenecid and CC. The authors used an MP composed of phosphate buffer pH 5 and ACN in a 70:30 (v/v) ratio, which is different from our approach, in which the pH used was higher but in a similar proportion to the aqueous solvent in the MP. For these drugs, the authors used a detection wavelength of 254 nm (Tantawy et al., 2021).
Validation of the method
Specificity
Specificity was evaluated by determining the peak purity of the analytes by analyzing the drug solution. The NC excipients did not interfere with the chromatographic peak of the drug, demonstrating that the method was specific for CQ and CC analysis. Peak purity for both drugs was 99.99%; the chromatograms are shown in Figure 1.
Linearity
The linearity of the drugs (CQ and CC) was evaluated using calibration curves with ranges of 5–17.5 and 1–12.5 μg ml−1 for CQ and CC, respectively. The calibration curves for drugs were linear, with a correlation coefficient of 0.999, presenting the straight-line equation y = 63,085 × – 80,433 for CQ and y = 53,312 × – 10,129 for CC, where x represents the concentration and y the area of the chromatograms.
For CC quantification, Hadad et al. (2013) used a calibration curve of 0.5 and 20 μg ml−1; the method was developed using a C18 Phenomenex column (250 × 4.6 mm id × 5 μm), a size closer to that used in our study. The MP was composed of ACN and sodium phosphate buffer pH 3 at a 35:65 (v/v) ratio, the same ratio as the aqueous solvent used in our method (Hadad et al., 2013). For CQ, Miranda et al. (2015) used a calibration curve from 90 to 210 μg ml−1(Miranda et al., 2015).
Precision
The precision results are listed in Table 1. A low RSD% was obtained for intraday precision, which was below 2% for CQ and CC. Likewise, the interday precision also presented RSD (%) below 2%: 1.31% for CQ and 1.64% for CC. With the lower RSD% values in the interday and intraday precisions, they confirmed the good precision of the method to quantify co-nanoencapsulated drugs, thereby being in accordance with the ICH, FDA, and ANVISA recommendations.
Accuracy
The CQ and CC recoveries were determined for accuracy after adding three known concentrations (80%, 100%, and 120%) to the NCb samples. Drug recovery was 100% for all analyses. The method proved accurate and presented an RSD (%) of 1.45 and 0.17% for CQ and CC, respectively (Table 2), indicating that the assay is accurate and in accordance with the ICH, FDA, and ANVISA guidelines.
Robustness
The FDA, ICH, and ANVISA guidelines suggest several typical variations for liquid chromatography for evaluation of the robustness; among them, we chose oven temperature and flow rate. Analyses were performed in triplicate for each change, and one change was performed at a time.
In the development phase of the analytical method, some parameters were observed, such as tailing factor, retention factor, theoretical plate number, and RSD between sample replicates (RSD%). These parameters are used to demonstrate that the system is suitable for quantitative analysis, and thus it must meet some preestablished parameters. These parameters are also used to assess the robustness of the analytical method (ANVISA, 2017; FDA, 2005 2015; ICH, 1996 2005).
All changes led to slight variations. The quantification of the drugs showed no significant difference between the changes (RSD < 2.0%; Table 3). None of the changes had significant effects on drug quantifications in the NCs, indicating the method’s robustness and that it is in accordance with the ICH, FDA, and ANVISA recommendations (ANVISA, 2017; FDA, 2005, 2015; ICH, 1992 2005).
Applicability
Drug-loaded NCs quantification
The CQ and CC drug content was 100%, and the encapsulation rates were 99.47% ± 0.01% and 99.71% ± 0.03% for CQ and CC, respectively. Moreover, ACN and MeOH (70:30) were used to quantify the drugs in the NCs samples. The solvents were tested alone or mixed. The sample showed turbidity when using only MeOH, which was discarded and was not analyzed by HPLC. When only ACN was used, the samples showed clarity but with a low drug content. Thus, different mixture proportions of these solvents were tested until reaching the 70:30 ratio, which was clear and presented a high drug content.
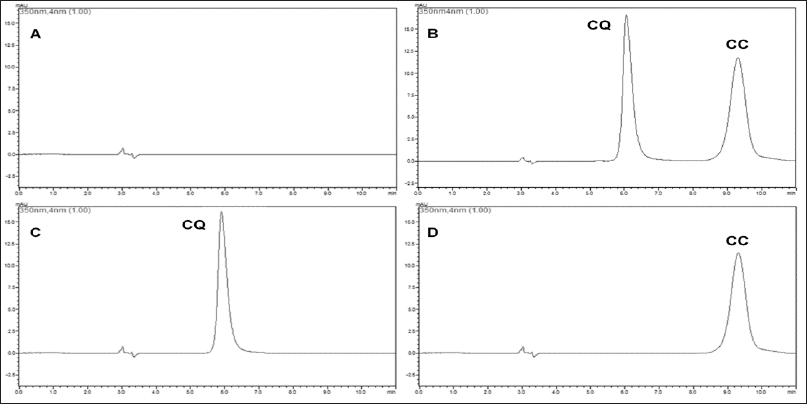 | Figure 1. Chromatograms of (A) blank formulations, (B) 17.5 μg.ml−1 CQ and 12.5 μg.ml−1 CC-loaded nanocapsules, (C) 17.5 μg.ml−1 CQ, and (D) 12.5 μg.ml−1 CC at a 350 nm wavelength. [Click here to view] |
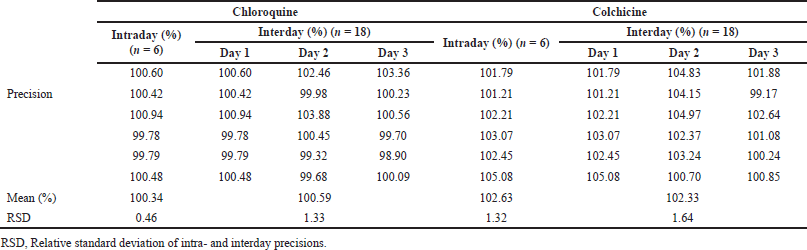 | Table 1. Intra- and interday precisions (%) of the method to quantify CQ and CC in NC. [Click here to view] |
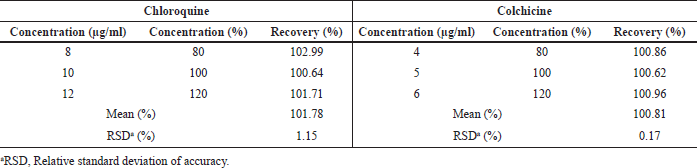 | Table 2. Method accuracy for CQ and CC quantification. [Click here to view] |
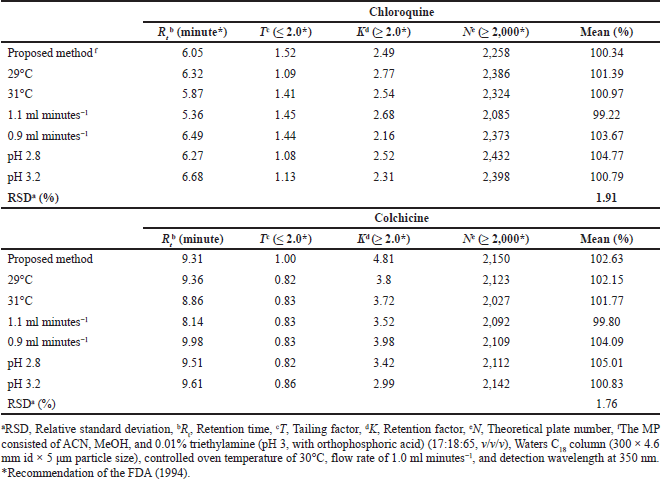 | Table 3. Robustness results. [Click here to view] |
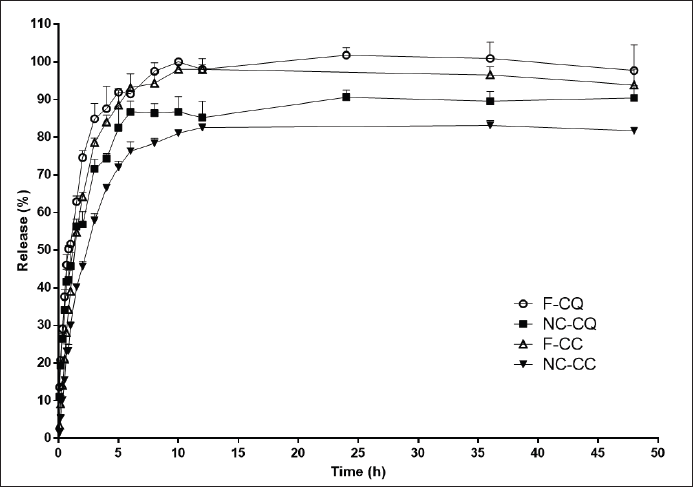 | Figure 2. In vitro release profiles of NCcqcc encapsulated drugs (NC-CQ and NC-CC) and free CQ (F-CQ) and free CC (F-CC) in a pH 7.4 buffer medium (n = 3). [Click here to view] |
The drugs used (CQ and CC) have different solubility characteristics. CQ has a higher polarity (Daneshfar et al., 2009), making solubilization difficult in only ACN, while CC was more polar and made solubilization difficult in only MeOH (Molad, 2002). Therefore, the best option for solubilization and recovery of both drugs was the solvent mixture.
In Vitro release
The in vitro release profile was performed using the dialysis bag diffusion technique. Samples were quantified by the same HPLC-DAD validated method.
Figure 2 shows the cumulative percentage of CQ and CC released from the NCs over time. The maximum release of F-CQ was around 100%, while for CQ released from NCs was 90.4% ± 5.7%. When observing the profile of CQ release during the 48 hours evaluation, it is observed that the profile of CQ released from the NCs was always below the free CQ. This same behavior was observed for CC; throughout the evaluation time, the concentrations of CC released from NCs were lower than free CC. The maximum concentration of free CC released was 96.3% ± 8.4%, while CC released from NCs was 81.7% ± 1.1%.
Nanoencapsulation sustained the release of CQ and CC due to the reservoir characteristics of the NCs, as observed by Contri et al. (2011). The nanoencapsulation of curcumin also sustained the release of the compound (Santos et al., 2021).
CONCLUSION
Based on the results, the method for quantification of CQ and CC simultaneously in NCs was developed and validated. The method is sensitive, linear, precise, accurate, and robust. In addition, the method could simultaneously quantify CQ and CC in an innovative NCs formulation. Nanoencapsulation sustained the release of free drugs.
ACKNOWLEDGMENTS
This study was financed by the Coordination for the Improvement of Higher Education Personnel, Brazil (CAPES); the Rio Grande do Sul Science Foundation (FAPERGS) with grants 88881.506652/2020-01 and 19/2551-0001970-0; the National Counsel of Technological and Scientific Development (CNPq) #309401/2020-8); student scholarships; and Atlas Assessoria Linguística.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Angelidis C, Kotsialou Z, Kossyvakis C, Vrettou AR, Zacharoulis A, Kolokathis F, Kekeris V, Giannopoulos G. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des, 2018; 24(6):659–63. CrossRef
ANVISA. Agência Nacional de Vigilância Sanitária. Resolução da diretoria colegiada–rdc nº 166 de 24 de julho de, 2017.
Bruneel F. Human cerebral malaria: 2019 mini review. Rev Neurol (Paris), 2019; 175(7–8):445–50. CrossRef
Chukanchitipat K, Na-Bangchang K. A review of clinical pharmacokinetics of CQ and primaquine and their application in malaria treatment in thai population. Afr J Pharm Pharmacol, 2017; 11:475–90. CrossRef
Coban C. The host targeting effect of CQ in malaria. Curr Opin Immunol, 2020; 66:98–107. CrossRef
Contri RV, Kaiser M, Poletto FS, Pohlmann AR, Guterres SS. Simultaneous control of capsaicinoids release from polymeric nanocapsules. J Nanosci Nanotechnol, 2011; 11(3):2398–406. CrossRef
Daneshfar A, Khezeli T, Manafi MH. Determination of anti-malaria agent chloroquine using single drop liquid-liquid-liquid microextraction. J Sep Sci, 2009; 32(4):511–6. CrossRef
FDA. United States Food and Drug Administration. Analytical procedures and methods validation for drugs and biologics. CDER and CBER, US Department of Health and Human Services, 2005.
Haas SE, Bettoni CC, de Oliveira LK, Guterres SS, Costa TD. Nanoencapsulation increases quinine antimalarial efficacy against plasmodium berghei in vivo. Int J Antimicrob Agents, 2009; 34(2):156–61. CrossRef
Hadad GM, Badr JM, El-Nahriry K, Hassanean HA. Validated hplc and hptlc methods for simultaneous determination of colchicine and khellin in pharmaceutical formulations. J Chromatogr Sci, 2013; 51(3):258–65. CrossRef
Ibrahim N, Ibrahim H, Sabater AM, Mazier D, Valentin A, Nepveu F. Artemisinin nanoformulation suitable for intravenous injection: preparation, characterization and antimalarial activities. Int J Pharm, 2015; 495(2):671–9. CrossRef
ICH. Guidelines Q2B: validation of analytical procedures: Methodology. Guidelines q2b: Validation of analytical procedures: Methodology. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 1996.
ICH. Harmonized tripartite guideline: validation of analytical procedure. Text and methodology Q2(R1). Harmonized tripartite guideline: Validation of analytical procedure. Text and methodology q2(r1) . International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 2005.
Macedo TS, Colina-Vegas L, Da Paixao M, Navarro M, Barreto BC, Oliveira PCM, Macambira SG, Machado M, Prudencio M, D’Alessandro S, Basilico N, Moreira DRM, Batista AA, Soares MBP. Chloroquine-containing organoruthenium complexes are fast-acting multistage antimalarial agents. Parasitology, 2016; 143(12):1543–56. CrossRef
Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, Celermajer DS, Patel S. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J Am Heart Assoc, 2015; 4(8):e002128. CrossRef
Castro e Souza MA, Reis NFA, de Souza Batista L, da Costa Cesar I, Fernandes C, Pianetti GA. An easy and rapid spectrophotometric method for determination of chloroquine diphosphate in tablets. Current Pharmaceutical Analysis, 2020, 16(1):5–11. CrossRef
Michels LR, Maciel TR, Nakama KA, Teixeira FEG, de Carvalho FB, Gundel A, de Araujo BV, Haas SE. Effects of surface characteristics of polymeric nanocapsules on the pharmacokinetics and efficacy of antimalarial quinine. Int J Nanomedicine, 2019; 14:10165–78. CrossRef
Miranda TA, Silva PH, Pianetti GA, Cesar IC. Simultaneous quantitation of chloroquine and primaquine by uplc-dad and comparison with a hplc-dad method. Malar J, 2015; 14:29. CrossRef
Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep, 2002; 4(3):252–6. CrossRef
Muga JO, Gathirwa JW, Tukulula M, Jura WGZO. In vitro evaluation of chloroquine-loaded and heparin surface-functionalized solid lipid nanoparticles. Malar J, 2018; 17(1):133. CrossRef
Nakama KA, dos Santos RB, da Rosa Silva CE, Izoton JC, Savall ASP, Gutirrez MEZ, Roman SS, Luchese C, Pinton S, Haas SE. Establishment of analytical method for quantification of anti-inflammatory agents co-nanoencapsulated and its application to physicochemical development and characterization of lipid-core nanocapsules. Arab J Chem, 2020; 13(1):2456–69. CrossRef
Nishanth G, Schluter D. Blood-brain barrier in cerebral malaria: pathogenesis and therapeutic intervention. Trends Parasitol, 2019; 35(7):516–28. CrossRef
De Santos RB, Nakama KA, Pacheco CO, de Gomes MG, de Souza JF, de Souza Pinto AC, de Oliveira FA, da Fonseca AL, Varotti F, Fajardo AR, Haas SE. Curcumin-loaded nanocapsules: influence of surface characteristics on technological parameters and potential antimalarial activity. Mater Sci Eng C Mater Biol Appl, 2021; 118:111356. CrossRef
Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine. Rheumatology (Oxford), 2017, 2018; 57(suppl_1):i4–i11. CrossRef
Tantawy MA, Wahba IA, Saad SS, Ramadan NK. Stability-indicating chromatographic methods for the simultaneous determination of probenecid and colchicine in their combined tablet. J Chromatogr Sci, 2021; 59(10):956–63. CrossRef
Tariq M, Al-Badr AA. Chloroquine. In: Florey K (ed.). Analytical profiles of drug substances, Academic Press, Cambridge, MA, pp 95–125, 1984. CrossRef
Velasques K, Maciel TR, de Castro Dal Forno AH, Teixeira FEG, da Fonseca AL, de Pilla Varotti F, Fajardo AR, Avila DS, Haas SE. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against plasmodium falciparum and decreases their toxicity to caenorhabditis elegans. Eur J Pharm Sci, 2018; 118:1–12. CrossRef
Vieira MVDF, Mello AGCN, De Sena LWP, Vieira JLF. Absence of gender influence on the pharmacokinetics of chloroquine combined with primaquine in malaria vivax patients. J São Paulo Inst Trop Med, 2020; 62:e83. CrossRef
WHO. World health organization. Treat malaria. WHO, Geneva, Switzerland, 2020.
Yin J, Xiang C, Song X. Nanoencapsulation of psoralidin via chitosan and Eudragit s100 for enhancement of oral bioavailability. Int J Pharm, 2016; 510(1):203–9. CrossRef