INTRODUCTION
Early childhood caries (ECC) is defined as “the presence of one or more decayed (noncavitated or cavitated lesions), missing (due to caries), or filled surfaces, in any primary teeth of a child under age six” and as “tooth decay in preschool children which is common, mostly untreated and can have a profound impact on children’s lives” (Tinanoff et al., 2019). The main etiological factors causing dental decay are Streptococcus mutans and Streptococcus sorbrinus, but a fungus, Candida albicans, also plays a role in ECC.
Caries usually follows the emergence of S. mutans in dental cavities after 6–24 months. The presence of glucose, sucrose, and fructose forms of extracellular polysaccharides is a vital cariogenicity factor of S. mutans. The Candida species colonizes with other microflora in the oral cavity, depending upon systemic and oral conditions, and may shift to pathogenic microorganisms.
Candida albicans make the environment acidic and decrease the pH by secreting organic acids, which are more effective than the lactic acid secreted by S. mutans (Forssten et al., 2010). Several studies have reported Candida species in ECC and severe early childhood caries. Therefore, C. albicans can cause dental decay.
Few antimicrobial agents have been found to be effective clinically and suitable against microbial growth, and recently formulated agents against microbial growth include essential oils, plant extracts, metals, and chlorhexidine (CHX).
CHX is considered the gold standard, and other antimicrobial agents’ efficacy is compared against it. CHX is a digluconate agent developed in 1940 in search of an antiviral agent, but it was found to possess antibacterial properties (Sajjan et al., 2016).
CHX can be used as bacteriostatic or a bactericidal, depending on its dose. In higher concentrations, it causes increased cell permeability leading to cell lysis; in lower concentrations, spore formation causes damage to the cellular transference of bacterial cells (Sajjan et al., 2016).
The study aimed to determine the antimicrobial effect of 0.2% CHX on C. albicans and S. mutans isolated from children with ECC.
MATERIALS AND METHODS
This study was approved by the Institution Review Board of the university (Project Number - IRB – AIMS – 2019 – 151). It was performed in the tertiary care hospital, Department of Nanosciences and Molecular Medicine, and Department of Microbiology.
Patients aged 3–6 years with ECC who presented to the tertiary care hospital’s Outpatient Department of Pediatric and Preventive Dentistry were chosen for the study. Children and their parents were informed about the oral examination. They were given an informed consent form that explained the study’s purpose and the voluntary nature of their participation. This study excluded children who were on antibiotics and antifungals, treated with local or systemic fluorides, or had systemic diseases. Caries experience was diagnosed using the ICDAS II. A total of 20 samples that tested positive for C. albicans and S. mutans were collected from carious portions of the teeth using sterile cotton swabs and then transferred to the microbiology laboratory in conical tubes containing sabouraud dextrose medium.
The sabouraud’s dextrose agar (SDA) plates were prepared and supplemented with chloramphenicol to inhibit bacterial overgrowth. The materials were inoculated for culture. Colony morphology on SDA plates was used to identify isolates. Growth emerged as smooth, convex pasty colonies, creamy, with a moldy odor after 1–2 days, and if no growth was seen after incubating it for 72 hours, the culture was considered to be negative. The Candida species were identified using CHROMagar, germ tube test, and VITEK II.
Streptococcus mutans strains were cultured on blood agar plates and the species were identified using the catalase test and VITEK II.
Antifungal susceptibility test
The antimicrobial property of 0.2% CHX (Hexidine 0.2%, Icpa Health products Ltd, India) against C. albicans and S. mutans was investigated using Kirby Bauer’s disk diffusion technique and the agar well diffusion method as per the Clinical and Laboratory Standards Institute (CLSI) M44, 3rd ed, and M02, 13th ed, guidelines. The suspensions were made using the direct colony suspension method using an 18–24-hour-old agar plate. They were adjusted to McFarland turbidity of 0.5 and confirmed using a photometric device. Then, a sterile swab was inserted into the inoculum tube, spun several times, and pressed against the tube’s wall (above the fluid level) while applying firm pressure to remove excess fluid. The Mueller Hinton agar plate was swabbed across the whole surface by the inoculum. The uniform spread of inoculum was achieved, and this technique was repeated two more times, streaking each time by rotating the plate by around 60 degrees. Separately, 6-mm filter paper disks (13 μl/disk) were coated with 0.2% CHX and allowed to dry. With forceps, the disk of CHX was placed on the agar surface at equivalent distances and placed in an incubator for 24 hours at 35°C ± 2°C. In the agar well diffusion method, the microbial inoculum was diffused throughout the agar plate surface to inoculate it. The materials to be tested were placed within 6-mm-diameter hole that had been punched into the agar plate using a tip. The plates were incubated in an appropriate environment. The zones were measured to the closest millimeter with a scale, taking the diameter of the disk and well into account. The diameter was measured and rounded to the nearest millimeter.
This method was used to examine 20 C. albicans and S. mutans isolates.
Data analysis
IBM SPSS version 20 was used for statistical analysis. The median was used to represent numerical variables (Q1, Q3). The Mann–Whitney U test was used to determine the statistical significance of the comparison of zones of inhibition of CHX on S. mutans and C. albicans. Statistical significant difference was defined as p-value < 0.05.
RESULTS
The research included 33 children with an average age of 4.81 ± 0.98 years. Candida albicans incidence was determined to be 60.6% among the children, having 20 children testing positive. Candida albicans was recovered from the whole surface of the carious tooth.
Candida albicans was susceptible to CHX (Fig. 1) with the mean zone of inhibition of 12.4 ± 0.598 mm (Table 1) using the agar well diffusion method; no zone of inhibition was observed when Kirby Bauer’s disk diffusion method was used. Streptococcus mutans cultured in the blood agar plate for 48 hours at 37°C were identified by hard raised, convex, undulate, opaque, frosty glass appearance, and final confirmation was done using VITEK II. Streptococcus mutans was susceptible to CHX (Fig. 2) and the mean zone of inhibition was 20.85 ± 1.18 mm.
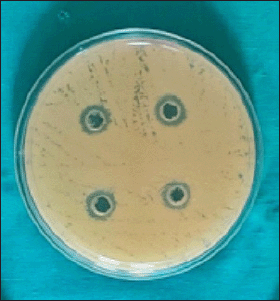 | Figure 1. CHX showing zones of inhibition against C. albicans. [Click here to view] |
 | Table 1. Comparison between zones of inhibition of CHX on C. albicans (Group 1) and S. mutans (Group 2). [Click here to view] |
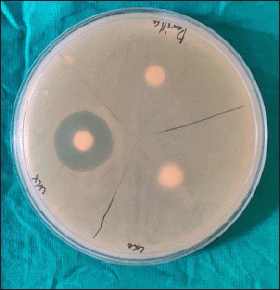 | Figure 2. Zone of inhibition observed with CHX on S. mutans. [Click here to view] |
The comparison of CHX showed the mean of CHX against C. albicans (Group 1) was 12.4 mm ± 0.598 and median was 12; Q1: 12 – Q3: 13 mm, and against S. mutans (Group 2) mean was 20.85 mm ± 1.182 and median was 21; Q1: 20 – Q3: 22 mm. The mean and median were high in S. mutans (Group 2), which was statistically significant (p-value < 0.001) (Table 1).
DISCUSSION
In ECC, the presence of S. mutans and C. albicans is a unique microbial feature observed (Ellepola et al., 2019). Streptococcus mutans has been the major etiological cause of caries for decades. However, recent research has revealed a high prevalence of S. mutans in biofilms harboring C. albicans, showing the interaction between the species and promoting caries development (Barbieri et al., 2007; Jarosz et al., 2009).
According to research by Xiao et al. (2016), mothers of children with ECC had significantly more numbers of C. albicans and the same C. albicans strains were observed in most child–mother dyads.
Candida albicans is the most common Candida species detected in the oral cavity. Identification of Candida strains is crucial since they range substantially in their potential to infect and their sensitivity to antimicrobial medications. As a result, the current research was carried out to detect C. albicans and S. mutans and investigate the antibacterial activity of 0.2%CHX.
Among the ECC children, the growth of Candida was observed to be 60.6%. This is consistent with past research by previous studies (de Carvalho et al., 2006; Ghasempour et al., 2011; Hossain et al., 2003; Lozano Moraga et al., 2017; Marchant et al., 2001; Rozkiewicz et al., 2006; Thomas et al., 2016; Xiao et al., 2016;). However, in the study by Neves et al. (2015) there was no correlation between the prevalence of Candida and carious lesions. This might be due to changes in content, buffering capacity, salivary flow rate, and the duration and amount of plaque that is present on the tooth surface as long-standing plaque will contain more Candida species, and in ECC, the amount of tooth surface remaining also plays a role in biofilm formation all of which affect the caries progression in the tooth. It was also observed that instead of making cultures, which is considered the gold standard for yeast identification, investigators employed other methods to detect Candida in the carious tooth.
In this study, the candidal prevalence is lower than that in the previous investigations (de Carvalho et al., 2006; Ghasempour et al., 2011; Lozano Moraga et al., 2017; Marchant et al., 2001; Thomas et al., 2016) where it was observed that the frequency of C. albicans in infants with ECC was 85.7%, 70.8%, 100%, 63%, and 100%, respectively.
Individual variability in the living environment, oral cavity ecological environment, geographical variance, and dietary preferences may have impacted the variation in candidal carriage rates between studies. In addition, the prevalence of yeast differs due to variations in age, physiological fluids, mucosal surface, hard tissue, and natural barriers to colonization.
CHX digluconate has a broad range of antibacterial action and is often used as an antiseptic mouthrinse inhibiting S. mutans and candidal adherence resulting in biofilm. It acts as a fungicide by coagulating nucleoproteins in cell walls, allowing cytoplasmic components to escape via the plasmalemma. Antimicrobial action was seen against S. mutans, with a mean zone of inhibition of 20.85 ± 1.18 mm, and the mean zone of inhibition observed against C. albicans was 12.4 ± 0.59 mm, which was more than the findings of Tirali et al. (2013) but was lesser than observed by Shino et al. (2016). A study conducted by Moeintaghavi et al. (2012) where 0.2% CHX was used did not show any zone of inhibition against C. albicans.
The research design might be to blame for the disparity in results. Antibacterial investigations using agar plates are frequent. However, the microbial inhibition zone depends on the test substance’s solubility and ability to permeate through agar; thus, it may not be fully effective.
It was observed that CHX has more antibacterial activity (20.85 ± 1.18 mm) than antifungal activity (12.4 ± 0.59 mm), as observed by the zone of inhibition exhibited. Therefore, CHX can slow down the carious process as it will affect the attachment of pioneer species like S. mutans and disturb the biofilm formation, adversely affecting Candida’s attachment to the tooth surface and other microorganisms.
in-vivo research is recommended for more exact results because the current study was conducted under restricted laboratory circumstances.
CONCLUSION
Within the limitations and the experimental conditions of this study, the following conclusions can be made: CHX’s antifungal effectiveness is proportional to its concentration, and 0.2% CHX is effective against the microorganisms tested and showed more antibacterial than antifungal activity.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study was approved by the Institution Review Board of the university (Project Number - IRB – AIMS – 2019 – 151).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
Barbieri DdSAV, Vicente VA, Fraiz FC, Lavoranti OJ, Svidzinski TIE, Pinheiro RL. Analysis of the in vitro adherence of Streptococcus mutans and Candida albicans. Braz J Microbiol, 2007; 38:624–31. CrossRef
de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DMP. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol, 2006; 51(11):1024–8. CrossRef
Ellepola K, Truong T, Liu Y, Lin Q, Lim TK, Lee YM, Cao T, Koo H, Seneviratne CJ. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-Candida albicans mixed-species biofilms. Infect Immun, 2019; 87(10):e00339–19. CrossRef
Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients, 2010; 2(3):290–8. CrossRef
Ghasempour M, Sefidgar SAA, Eyzadian H, Gharakhani S. Prevalence of C.albicans in dental plaque and caries lesion of early childhood caries (ECC) according to sampling site. Casp J Intern Med, 2011; 2(4):304.
Hossain H, Ansari F, Schulz-Weidner N, Wetzel WE, Chakraborty T, Domann E. Clonal identity of C.albicans in the oral cavity and the gastrointestinal tract of preschool children. Oral Microbial Immunol, 2003; 18(5):302–8. CrossRef
Jarosz LM, Deng DM, van der Mei HC, Crielaard W, Krom BP.Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell, 2009; 8:1658–64. CrossRef
Lozano Moraga CP, Rodriguez Martinez GA, Lefimil Puente CA, Morales Bozo IC, Urzúa Orellana BR. Prevalence of C.albicans and carriage of Candida non-albicans in the saliva of preschool children, according to their caries status. Acta Odontol Scand, 2017; 75(1):30–5. CrossRef
Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. The predominant microflora of nursing caries lesions. Caries Res, 2001; 35(6):397–406. CrossRef
Moeintaghavi A, Arab H, Khajekaramodini M, Hosseini R, Danesteh H, Niknami H. In vitro antimicrobial comparison of chlorhexidine, persica mouthwash and miswak extract. J Contemp Dent Pract, 2012; 13:147–52. CrossRef
Neves AB, Lobo LA, Pinto KC, Pires ES, Requejo ME, Maia LC, Antonio AG. Comparison between clinical aspects and salivary microbial profile of children with and without early childhood caries: a preliminary study. J Clin Pediatr Dent, 2015; 39(3):209–14. CrossRef
Rozkiewicz D, Daniluk T, Zaremba ML, Cylwik-Rokicka D, Stokowska W, Pawi?ska M, Dabrowska E, Marczuk-Kolada G, Waszkiel D. Oral C.albicans carriage in healthy preschool and school children. Adv Med Sci, 2006; 51:187–90.
Sajjan P, Laxminarayan N, Kar PP, Sajjanar M. Chlorhexidine as an antimicrobial agent in dentistry – a review. Oral Health Dent Manag, 2016; 15(2):93–100.
Shino B, Peedikayil FC, Jaiprakash SR, Bijapur GA, Kottayi S, Jose D. Comparison of antimicrobial activity of chlorhexidine, coconut oil, probiotics, and ketoconazole on C.albicans isolated in children with early childhood caries: an in vitro study. Scientifica, 2016; 2016:7061587. CrossRef
Tirali RE, Bodur H, Sipahi B, Sungurtekin E. Evaluation of the antimicrobial activities of chlorhexidine gluconate, sodium hypochlorite and octenidine hydrochloride in vitro. Aust Endod J, 2013; 39(1):15–8. CrossRef
Thomas A, Mhambrey S, Chokshi K, Chokshi A, Jana S, Thakur S, Jose D, Bajpai G. Association of oral C.albicans with severe early childhood caries-a pilot study. J Clin Diagn Res, 2016; 10(8):ZC109–12. CrossRef
Tinanoff N, Baez RJ, Guillory CD, Donly KJ, Feldens CA, McGrath C, Phantumvanit P, Pitts NB, Seow WK, Sharkov N, Songpaisan Y, Twetman S. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int J Paediatr Dent, 2019; 29(3):238–48. CrossRef
Xiao J, Moon Y, Li L, Rustchenko E, Wakabayashi H, Zhao X, Feng C, Gill SR, McLaren S, Malmstrom H, Ren Y, Quivey R, Koo H, Kopycka-Kedzierawski DT. C.albicans carriage in children with severe early childhood caries (S-ECC) and maternal relatedness. PloS One, 2016; 11(10):e0164242. CrossRef