INTRODUCTION
Soft coral, specifically Nepthea sp. from Southeast Sulawesi, Indonesia, was chosen as the sample to continue our research on the chemical and pharmacological aspects of marine natural resources. We had previously worked with sponges, and through the process of isolation and structural determination, we were able to get a new compound from Clathria sp., called clathruohate (Sahidin et al., 2018), as well as the chemical screening of various sponges (Sahidin et al., 2020). Antihyperlipidemic properties (Wahyuni et al., 2019), anti-inflammatory properties (Fristiohady et al., 2019), antioxidant properties, and acute toxicity (Fristiohady et al., 2020) are some of the biological activities of the sponges growing in Southeast Sulawesi.
The Indo-Pacific region (Seah et al., 2015), the Mauritius and Rodrigues Island (Jahajeeah et al., 2021), and the Brazilian Coast (Almeida et al., 2014) are all thought to be home to Nepthea sp. (Nephtheidae). According to the study, a derivate of tetraprenyl-benzoquinone generated by Nepthea sp. and a guaiane-based sesquiterpene discovered from Nepthea chabroli (Almeida et al., 2014), a molecule from Nepthea sp., called nephtoacetal, is cytotoxic to the HeLa cell lines (Zhang et al., 2013). Furthermore, erectasteroids A–H from Nepthea erecta are active against the HT-29 and P-388 cell lines (Cheng et al., 2007), and nebrosteroids A–H from N. chabroli have anti-inflammation activity (Huang et al., 2008).
However, no research on the chemical and pharmacological features of Nepthea growing in Southeast Sulawesi has been published yet. Soft corals whose studies have been reported from around the Sulawesi island include the following: soft coral from the South China Sea, Sarcophyton solidum, which produces diterpenoids (Zhu et al., 2015), Sinularia depressa from the South China Sea which generates sinulasterols A–C that play a role in cancer prevention through anti-inflammatory actions (Yang et al., 2020), and Lobophytum sp. from Selayar, South Sulawesi, the ethyl acetate extracts of which are active as antibacterials and antioxidants (Putra et al., 2016). Meanwhile, the mapping phase of a soft coral project in Southeast Sulawesi is still ongoing (Pedoja et al., 2018; Wanda et al., 2018).
This article describes the cytotoxicity and antioxidant properties of the ethyl acetate extract and its subfractions of soft coral Nepthea sp., as well as a chemical study of those samples using phytochemical screening, LC-MS/MS analysis, and total phenolic content (TPC) to learn more about soft corals from Southeast Sulawesi.
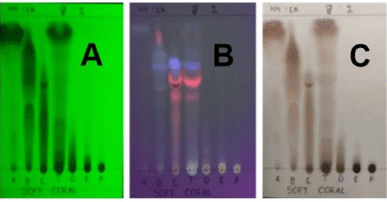 | Figure 1. TLC chromatogram of ethylacetate extract (T) and the fraction A–F. (A) λ 254 nm (short); (B) λ 366 nm (long); and (C) CeSO4 + heat. [Click here to view] |
MATERIALS AND METHODS
General procedures
Methanol, ethyl acetate, n-hexane, Aquades, and acetone were all analytical grade compounds. The other materials used were Kieselgel 60 F254 0.25 mm (Merck), Si-gel 60 GF254 p.a (Merck®), silica 60 G (Merck®), cerium sulfate (CeSO4) (Merck®), ascorbic acid (Merck®), gallic acid (Merck®), quercetin (Merck®), doxorubicin (Merck®), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). The LC-MS/MS study used Waters ACQUITY UPLC I–Class together with the Xevo G2-X2 Quadrupole Time-of-Flight Mass Spectrometer.
Collection of Nepthea sp.
Nepthea sp. was taken on the reef slopes of the Saponda Islands in Indonesia’s Province of Southeast Sulawesi. Scuba diving at a depth of 4–10 m was used to collect the sample. The material was collected and put in separate ice containers before being returned to the laboratory for further analysis. Soft coral identification was made by the specialist staff of Universitas Halu Oleo’s Faculty of Fisheries and Marine Science (Baru Sadarun, Ph.D.), and the specimen was placed at the Faculty of Pharmacy.
Fractionation and extraction
Fresh samples of Nepthea sp. (3 kg) were extracted with ethyl acetate at room temperature (3 × 10 l, 24 hours each time), and a dark brown extract was produced after being compressed under lower pressure. The extract was fractionated using vacuum liquid chromatography with Si-gel as an adsorbent and a combination of n-hexane: ethyl acetate (polarity rising) and 100% of methanol as eluent, yielding six fractions (A–F).
Screening of phytochemical contents
Screening of secondary metabolites in all the samples was done using the Harborne methods (Sadarun et al., 2022).
Total phenolic content
Total phenolic compounds were determined using the Folin–Ciocalteu reagent with slight adjustments to Singleton and Rossi’s description (Chandra et al., 2014).
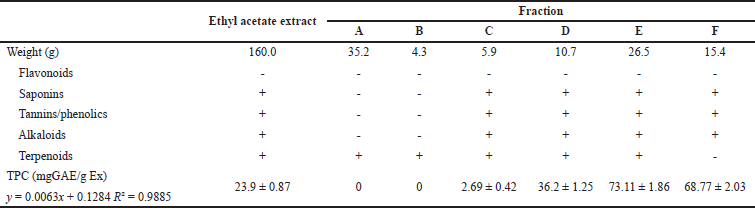 | Table 1. Weight and chemical profiles the samples. [Click here to view] |
LC-MS/MS analysis
The LC-MS/MS standard operating procedure was prepared for the chemical identification of the soft coral Nepthea sp. ethyl acetate extract and its subfractions (A–F). The UNIFI software was used to identify the mass-to-charge ratio (m/z) values of all peaks obtained from the LC-MS/MS analysis using the MSE identification method. The chemicals found were thoroughly examined using the Dictionary of Marine Natural Products (Blunt and Munro, 2008).
Antioxidant and cytotoxic activities
The antioxidant activity of those samples was measured by the DPPH radical (Sahidin et al., 2020) and the ABTS method (Wahyuni et al., 2021). The cytotoxicity property was evaluated toward the MCF-7 cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in vitro (Asasutjarit et al., 2021).
RESULTS AND DISCUSSION
The study of the chemical and pharmaceutical aspects of soft coral Nepthea sp. (3 kg) from the Southeast Sulawesi Sea, starting with sample extraction using ethyl acetate, yielded 160 g of the extract. The ethyl acetate extract of Nepthea sp. was fractionated into six fractions (A–F). The compounds profile of each fraction was analyzed using thin-layer chromatography (TLC), which is shown in Figure 1.
The chemical contents in the ethyl acetate extract and fractions A–F of Nepthea sp. are quite different, as shown by the TLC chromatogram in Figure 1. Qualitatively, terpenoids, alkaloids, and phenolics are compounds produced by Nepthea sp. based on phytochemical screening. Flavonoids are extremely rare and nearly undetectable in this sample. Fractions A and B do not contain phenolic compounds. Detailed data on the weight and chemical profile of samples are presented in Table 1.
Fraction C has the best cytotoxic potential against MCF-7 breast cancer cells lines compared to other samples with IC50 72.82 ± 1.30 mgl−1, and fraction E had the most antioxidant potential compared to other samples with IC50 67.39 ± 1.56 mgl−1 (DPPH) and IC50 54.12 ± 0.95 mgl−1 (ABTS) (Table 2 and Fig. 2). The difference in the biological activity of each fraction is strongly influenced by the content of its secondary metabolites.
Fractions C and E were studied in depth because they demonstrated the greatest promise for anticancer and antioxidant properties, respectively. Table 3 presents the chemicals identified and unidentified in the ethyl acetate extract, fractions C and F, of Nepthea sp. based on LC-MS/MS data. In addition, Figure 3 shows the identified structure of the identified compounds.
Figure 3 shows that the majority of the compounds found in Nepthea sp. are terpenoids, particularly sesquiterpenes and diterpenes, lactone, and nitrogenous compounds like 8 and 9. The number of terpenoids detected in Nepthea sp. is comparable to those found in other soft corals, such as sarcophine (Saleh et al., 2020), terpenoids from Lobophytum crassum, and steroids from Sarcophyton pauciplicatum (Florean et al., 2020; Saleh et al., 2020). The majority of the unidentified molecules are nitrogen compounds, and alkaloids are assumed to represent a type of secondary metabolites that include nitrogen atoms.
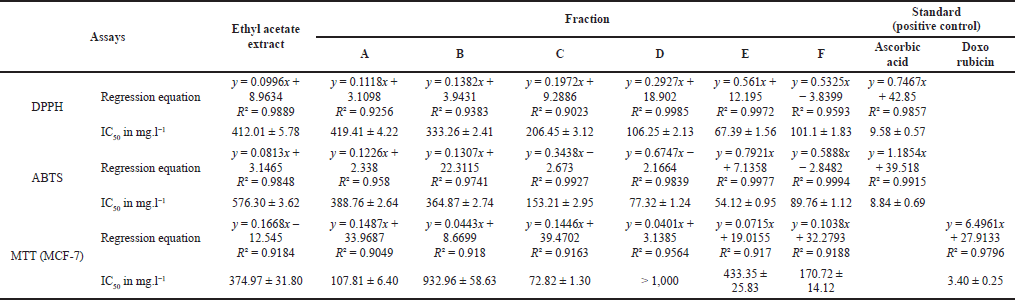 | Table 2. Biological properties of all samples. [Click here to view] |
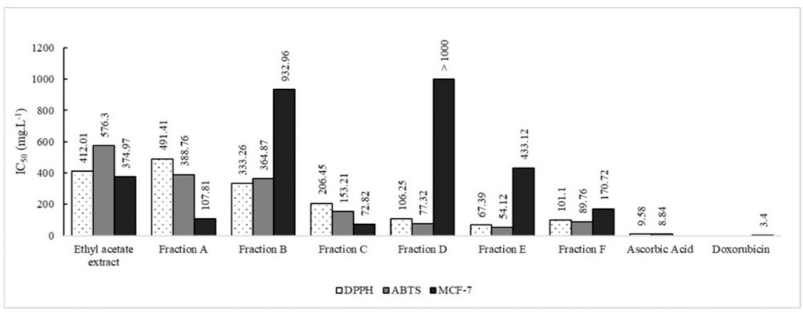 | Figure 2. Bwwiological activities of the samples. [Click here to view] |
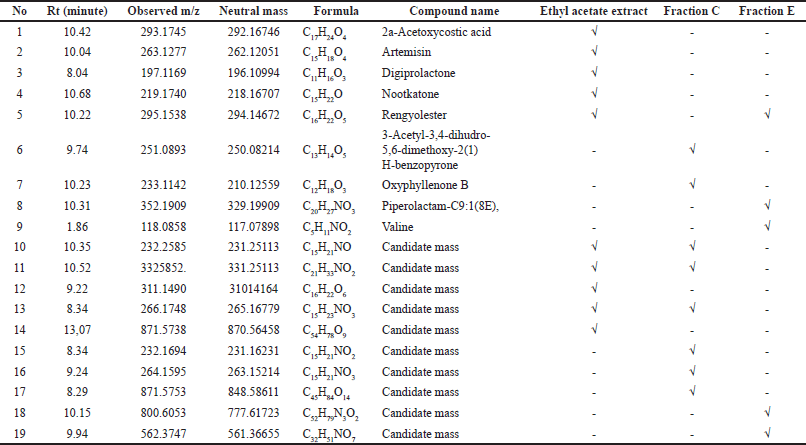 | Table 3. Chemical profile of ethylacetat extract, fraction C and F based on LC-MS/MS data. [Click here to view] |
When compared to other samples, the biological activity of fraction C revealed that it had the strongest cytotoxic capability against MCF-7 breast cancer cells lines (Table 2 and Fig. 2). Based on the cytotoxicity level (Sadarun et al., 2022), the extract is categorized as very active (IC50 < 10 mg.ml−1), active (10–100 mg.ml−1), and moderately active (100–500 mg.ml−1). Thus, the ethyl acetate extract and fraction E were categorized as moderately active, while fraction C was categorized as active against MCF-7 breast cancer cells lines. The component concentration of fraction C, which is primarily nitrogen compounds, is assumed to be the cause (Table 3). Doxorubicin, a nitrogen molecule with the chemical formula C27H29NO11, is used as a positive control. Tamoxifen is a nitrogen molecule with the chemical formula C26H29NO or 2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine, which is often used by breast cancer patients. Tamoxifen works by preventing estrogen from acting on the breast. The hormone estrogen is required for the development of several kinds of breast cancer. This mechanism of action can be employed in both the treatment and prevention of breast cancer in high-risk women (Thayyeb et al., 2020).
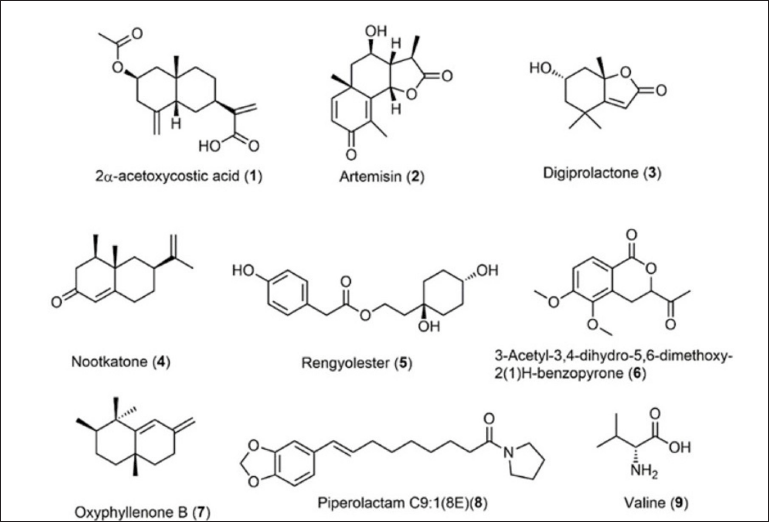 | Figure 3. Identified compound structures of ethyl acetate extract, fraction C and E. [Click here to view] |
In comparison to the other samples, fraction E had a maximum level of antioxidant capacity. The IC50 value of the DPPH and ABTS test findings is demonstrated in Table 2, and Figure 2 shows the classification of the antioxidant potential proposed by Blois, where Blois distinguishes it into four levels, namely very strong if IC50 < 50 mgml−1, strong if IC50 is between 50 and 100 _etl−1, moderate if IC50 is between 100 and 150 _etl−1, and weak if IC50 is more than 150 _orl−1 (Nur et al., 2021). According to the Blois criteria, the ethyl acetate extract and fraction C were categorized as weak antioxidants, while fraction E was included in the category of strong antioxidants. The quantity of phenolic compounds in fraction E influenced the strength of the antioxidant activity of the fraction quantitatively. The TPC value of fraction E was the greatest compared to the other samples, and the presence of flavonoids was detected in fraction E as well, as evidenced by the TFC value (Table 1). In terms of quality, phenolic compounds have conjugated double bonds with hydroxyl groups, allowing them to neutralize free radicals via resonance (Sahidin et al., 2014). Vitamin C (ascorbic acid), for example, has four hydroxyl groups, a conjugated double bond with a carbonyl unit, and an ester group, making it an excellent antioxidant substance. Rengyolester as the owner of this condition has a phenolic unit, an ester group, and two hydroxyl units that make up this chemical. Fraction E is regarded to have greater antioxidant effects than other samples due to the presence of this molecule. The ethyl acetate extract had rengyolester as well; however, fraction E had a larger mole fraction of rengyolester than the ethyl acetate extract.
CONCLUSION
The diversity of chemicals and biological activities of soft coral Nepthea sp. was clearly observed after fractionation. Fractionation of the ethyl acetate extract of soft coral Nepthea sp. produced six fractions (fractions A–F). Fraction C revealed that it had the strongest cytotoxic capability against MCF-7 breast cancer cells lines compared to other samples, which has an active category that is thought to be due to the high amount of nitrogenous chemicals. Meanwhile, the most antioxidant potential was shown by fraction E, which has a strong category that is caused by the content of phenolic compounds. As a result, the ethyl acetate extract and its subfraction from Nepthea sp., especially fractions C and E, can be used as a source of raw materials for anticancer agents and antioxidants, respectively.
ACKNOWLEDGMENTS
The Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia supported this project with a research grant under the Penelitian Dasar Unggulan Perguruan Tinggi (PDUPT) 2021 No. 21/UN29.20/PG/2021 scheme.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest in this study.
AUTHORS’ CONTRIBUTIONS
The idea was developed by I. Sahidin, B. Sadarun, and A. Fristiohady. Sample collection was arranged by B. Sadarun and A. W. M. Yodha. I. Sahidin, B. Sadarun, and A. W. M. Yodha worked on sample preparation, extraction, and fractionation. Evaluations of biological activities were done by A. Fristiohady and N. S. Rahmatika. A. Sundowo and I. Sahidin worked on the LC-MS/MS experiment and interpretation of the data. I. Sahidin, B. Sadarun, and A. Fristiohady contributed to manuscript preparation and revision.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Almeida MTR, Moritz MIG, Capel KCC, Perez CD, Schenkel EP. Chemical and biological aspects of octocorals from the Brazilian coast. Rev Bras Farmacogn, 2014; 24:446–67. CrossRef
Asasutjarit R, Sooksai N, Fristiohad A, Lairungruang K, Ng SF, Fuongfuchat A. Optimization of production parameters for andrographolide-loaded nanoemulsion preparation by microfluidization and evaluations of its bioactivities in skin cancer cells and UVB radiation-exposed skin. Pharmaceutics, 2021; 13(8):1290; doi:10.3390/pharmaceutics13081290 CrossRef
Blunt JW, Munro MHG. Dictionary of marine natural products with CD-ROM. Chapman and Hall/CRC, Taylor and Francis, Boca Raton, FL, 2018.
Chandra S, Khan S, Avula B, Lata H, Yang MH, El-Sohly MA, Khan IA. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Alternat Med, 2014; 2014:253875; doi:10.1155/2014/253875 CrossRef
Cheng S, Dai C, Duh C. New 4-methylated and 19-oxygenated steroids from the formosan soft coral Nepthea erecta. Steroids, 2007; 72:653–9; doi:10.1016/j.steroids.2007.05.001 CrossRef
Florean C, Dicato M, Diederich M. Immune-modulating and anti-inflflammatory marine compounds against cancer. Semin Cancer Biol, 2020; In Press.
Fristiohady A, Wahyuni, Malik F, Purnama LOMJ, Sahidin I. Anti-Inflammatory Activity of marine sponge Callyspongia sp. and its acute toxicity. Asian J Pharm Clin Res, 2019; 12(12):97–100. CrossRef
Fristiohady A, Sadrun B, Wahyuni, Malaka MH, Ahmad F, Malik F, Julian Purnama LOMJ, Sahidin I. Isolation and identification of secondary metabolite acetone extract Aaptos sp. and its antioxidant properties and acute toxicity. J Appl Pharm Sci, 2020; 10(6):081–9. CrossRef
Huang Y, Wen Z, Wang S, Hsu C, Duh C. New anti-inflammatory 4-methylated steroids from the Formosan soft coral Nepthea chabroli. Steroids, 2008; 73:1181–6; doi:10.1016/j.steroids.2008.05.007 CrossRef
Jahajeeah D, Bhoyroo V, Ranghoo-Sanmukhiya M. An assessment of soft coral community (Octocorallia; Alcyonacea) around Mauritius and Rodrigues Island—new record of soft coral. Reg Stud Mar Sci, 2021; 47:101976; doi:10.1016/j.rsma.2021.101976 CrossRef
Nur S, Aisyah AN, Lukitaningsih E, Rumiyati, Juhardi RI, Andirah R, Hajar AS. Evaluation of antioxidant and cytotoxic effect against cancer cell lines of Angiopteris ferox Copel tuber and its compounds by LC-MS analysis. J Appl Pharm Sci, 2021; 11(08):054–61.
Pedoja K, Husson L, Bezos L, Pastier AW, Imran AM, Arias-Ruiz C, Sarr AC, Elliot M, Pons-Branchu E, Nexer M, Regard V, Hafidz A, Robert X, Benoit L, Delcaillau B, Authemayou C, Dumoulin C, Choblet G. On the long-lasting sequences of coral reef terraces from SE Sulawesi (Indonesia): distribution, formation, and global significance. Quat Sci Rev, 2018; 188:37e57; doi:10.1016/j.quascirev.2018.03.033 CrossRef
Putra MY, Murniasih T, Swasono RT, Wibowo JT, Saputri ANC, Widhiana MR, Arlyza IS. Secondary metabolites and their biological activities in Indonesian soft coral of the genus Lobophytum. Asian Pac J Trop Biomed, 2016; 6(11):909–13. CrossRef
Sadarun B, Rahmatika NS, Yodha AWM, Fristiohady A, Sundowo A, Baharum SN, Sahidin I. Antioxidant and cytotoxic properties of soft coral Nepthea sp. Indones J Mar Sci, 2022; 27(1):29–36; doi:10.14710/ik.ijms.27.1.29-36 CrossRef
Sahidin S, Nohong N, Sani A, Manggau MA, Sukohar S, Widodo H, Baharum SN. Radical scavenging activity of triterpene steroids from stem of Polygonum pulchrum Bl. IJPPS, 2014; 6(8):350–4.
Sahidin I, Sabandar CW, Wahyuni, Hamsidi R, Malaka MH, Sadarun B, Aslan LO. A-nor sterols from an Indonesian marine sponge Clathria species. Malays J Anal Sci, 2018; 22(3):375–82. Available via http://www.ukm.my/mjas/mjas2018/; doi:10.17576/mjas-2018-2203-02
Sahidin I, Sabandar CW, Wahyuni, Hamsidi R, Mardikasari SA, Zubaydah WOS, Sadarun B, Musnina WOS, Darmawan A, Sundowo A. Investigation of compounds and biological activity of selected Indonesian marine sponges. Nat Prod J, 2020; 10(3):312–21. CrossRef
Saleh HA, Raafat KM, Temraz TA, Noureldin N, Breitinger HG, Breitinger U. Sarcophine and (7S, 8R)-dihydroxydeepoxysarcophine from the Red Sea soft coral Sarcophyton glaucum as in vitro and in vivo modulators of glycine receptors. Neurotoxicology, 2020; 80:105–11. CrossRef
Seah JZS, Yap NW, Tan LT, Goh BPL. Distribution and abundant of octocoral (Octocorallia, Alcyonacea) communities at three Southern Island of Singapore. Ocean Sci J, 2015; 50(2):299–306. CrossRef
Thayyeb H, Muis M, Murtala B. Gambaran Ultrasonografi Kelainan Endometrium pada Penderita Kanker Payudara yang Mendapat Terapi Hormonal di Rumah Sakit Wahidin Sudirohusodo. Nusantara Med Sci J, 2020; 5(2):13408; doi:10.20956/nmsj.v5i2.13408 CrossRef
Wanda E, sadarun B, Rahmadani. Keanekaragaman dan kepadatan karang lunak di perairan Waworaha Kecamatan Soropia. Sapa Laut, 2018; 3(1):9-15; doi:10.33772/jsl.v3i1.6504
Wahyuni, Fristiohady A, Malaka MH, Malik F, Yusuf MI, Leorita M, Sadarun B, Saleh A, Musnina WOS, Sabandar CW, Sahidin I. Effects of Indonesian marine sponges ethanol extracts on the lipid profile of hyperlipidemic rats. J Appl Pharm Sci, 2019; 9(10):008. CrossRef
Wahyuni, Diantini A, Ghozali M, Subarnas A, Julaeha E, Amalia R, Sahidin I. Phytochemical screening, toxicity activity, and antioxidant capacity of ethanolic extract of Etlingera alba rhizomes. Pak J Biol Sci, 2021; 24:807–14. CrossRef
Yang M, Cui WX, Li H, Li SW, Yao LG, Tang W. Sinulasterols A–C, three new bioactive oxygenated steroids from the South China Sea soft coral Sinularia depressa. Steroids, 2020; 157:08598; doi:10.1016/j.steroids.2020.108598 CrossRef
Zhang J, Li L, Wang K, Liao X, Deng Z, Xu S. Pentacyclic hemiacetal sterols with antifouling and cytotoxic activities from soft coral Nepthea sp. Bioorg Med Chem Lett, 2013; 23:1079–82; doi:10.1016/j. bmcl. 2012.12.012 CrossRef
Zhu JY, Li W, Bao JM, Zhang JS, Yin S, Tang GH. Diterpenoids from the South China Sea soft coral Sarcophyton solidum. Biochem Syst Ecol, 2015; 62:6e10. CrossRef