INTRODUCTION
Clomiphene is chemically designated as (E,Z) -2-(4 (2-chloro-1, 2-diphenylethenyl) phenoxy)-N,N-diethylethanamine. Its molecular mass and chemical formula (Fig. 1) are 405.97 g/mol and C26H28ClNO correspondingly. Clomiphene, belonging to the tertiary amines class, is a nonsteroidal ovulatory stimulant that is taken by mouth and acts as a selective estrogen receptor modulator (Hughes et al., 2010; Yilmaz et al., 2018). Clomiphene can cause multiple ovulation, which makes it more likely that a woman will have twins. There may be a higher chance of getting ovarian cancer and gaining weight (Rodrigez et al., 2016). Clomiphene can interact with tissues that have estrogen receptors, like the pituitary, hypothalamus, endometrium, ovary, cervix and vagina. It may compete with estrogen for binding sites on estrogen receptors and slow down the restocking of estrogen receptors inside cells. Clomiphene starts a chain of hormonal events that end with a rise in preovulatory gonadotropin and the rupture of follicles (Bach et al., 2016; Trost et al., 2014; Trabert et al., 2013). As a result of taking clomiphene, the pituitary gland releases more gonadotropins. This is the first change in the endocrine system. This starts the processes of steroidogenesis and folliculogenesis, which cause the ovarian follicle to grow and the level of estradiol in the blood to rise. Plasma levels of progesterone and estradiol rise and fall after ovulation, just like they do in a usual ovulatory cycle.
Clomiphene has both anti-estrogenic and estrogenic effects, but the exact way it works is still not known. Clomiphene stops the release of gonadotropin, follicle-stimulating hormones, and luteinizing hormones. This causes ovarian follicles to grow and mature, ovulation to happen, and the corpus luteum to grow and work, which leads to pregnancy. Gonadotropin release can happen when the hypothalamic-pituitary axis is directly stimulated or when estrogens have less effect on the hypothalamic-pituitary axis thru opposing with endogenous estrogens in the hypothalamus, pituitary, or uterus (DiGiorgio et al., 2016; Miller et al., 2019). Clomiphene does not seem to have any progestational, androgenic, or antiandrogenic effects. It also does not seem to mess with the way the pituitary, adrenals, or thyroid work (Nordt et al., 2008).
 | Figure 1. Clomiphene chemical structure. [Click here to view] |
Literature on clomiphene discloses that only 2 analytical methodologies on high performance liquid chromatography (HPLC) for clomiphene citrate (Atul et al., 2021) and LC-tandem mass spectrometric (Crewe et al., 2007) for its isomers were designated for the determination of clomiphene in unknown solutions. So, the present study was pointed to develop a specific, linear and precise LC–MSMS procedure for the determination of clomiphene in plasma samples.
MATERIAL AND METHODS
Reagents and chemicals
The clomiphene (99.71% pure) standard and nilotinib (99.91% pure) were acquired from Hetero Drugs, Bollaram, Telangana, India. Methyl alcohol and acetonitriles of HPLC level grade were attained from Merck, Vikhroli, Maharashtra, India. In the present research, the water of LC-grade purity was produced from the Milli-Q instrument, USA.
LC–MS/MS instrument and parameters
The LC–MSMS instrument consists of an Agilent 3,200 liquid chromatography system with two pumps (dual-SL) and an Agilent/6164 mass triple quadrupoles spectrometric detector with the source of electrospray ionization (CA, America). Chromatography statistics were executed thru MassHunter software. YMC-Pack C18-AM (3µm; 4.60 mm i.d × 50.0 mm) stationary phase was utilized to achieve chromatography elution, through a flowing rate of 0.70 ml/minute. Isocratic elution was done using methanol, acetonitrile, and 0.10% formic acid in the fraction of 70:15: 15 as the mobile phasic system. A triple quadrupole mass detector was employed for the quantification of ions. Electrospray ionization in a positive ionizing method, which was executed in (multiple reaction monitorings) (MRM) with parent/product ionic transition of m/z 406.18→100.11 for clomiphene and 530.70→289.50 for Nilotinib internal standard. The MSMS parameters were optimized as capillary voltage at 5.0 kV, source temperature at 450°C; dryer gas (N2) ?ow at 10 l/minute and nebulization gas at 40 psi. The autosampler temperature and infusion volumes were kept at 8.0°C and 15 µl correspondingly; 18 eV of collisional energy was employed in the chromatography elution.
Standard quality controls (QCs)
1,000 µg/ml clomiphene and nilotinib stock solutions were employed in the mobile phase (as diluent) individually. The resultant clomiphene solutions were processed for a series of dilutions with mobile phase to make working standard controls. Nilotinib’s internal standard working standard at 150 ng/ml was processed accordingly to get in all the clomiphene quality. The prepared QCs were monitored at −20°C till the sample analysis.
Linearity QCs of clomiphene (12.5, 21.0, 45.0, 95.0, 190.0, 290.0, 390.0, and 500.0 ng/ml) were achieved by the method of spiking to plasma blanks. QC solutions at lower, medium, and higher concentrations (35.0, 250.0, and 375.0 ng/ml) were employed individually in the same manner.
Sample preparation method
A 300µl blank plasma solution was relocated into a 10.0 ml tube for processing. Drug and 100.0 µl of internal standard solutions were added to tubes, so as to get the required concentration in the final dilution to be infused. The mixture was added to 5ml of ethyl acetate for the liquid–liquid extraction method and employed for centrifugation (20 minutes). The organic solvent phase that was left over was moved to a new glass tube, and nitrogen steam was used to evaporate it. The resultant dried residue was put back together with 100µl of a movable solvent, and 15.0µl aliquots were put into LC–MSMS equipment to be looked at.
Method validation
The developed analytical method was subjected to validation according to the rules of the USFDA for variable validation parameters to fulfill the requirements (FDA, 2001; EMA, 2011).
RESULTS AND DISCUSSION
Mass system optimization
During the developing stage, fresh clomiphene solutions were injected to make sure that the product and parent ions were working at their best. In the positive ionization method, a precursor ion with a value of 406.18 m/z was found. When the precursor ion broke apart, pieces with masses of 192.1, 125.12, and 100.11 were found. At 100.11 m/z, the most intense value was found for the daughter ion of clomiphene. Nilotinib has similar physical and chemical properties to clomiphene, which makes it a good choice as an internal standard for this bioanalytical methodology development and good recoveries throughout the sample and validation processing. MRM scans were used to find the daughter and molecular ionic components for both drug constituents. The final transitions for clomiphene were m/z 406.18–100.11 and for nilotinib internal standard they were m/z 530.70→289.50.
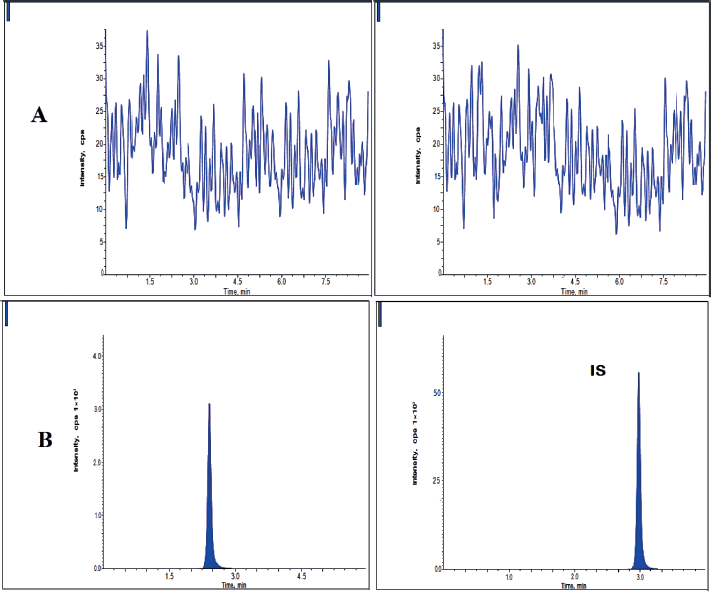 | Figure 2. Clomiphene A) plasma blank chromatogram and B) LLOQQC chromatogram. [Click here to view] |
Speci?city
Blank plasma and plasma spiked at lower limit of quantification (LLOQ) level (12.5 ng/ml), of clomiphene and nilotinib were infused into an LC-MSMS instrument and the resulting chromatograms were given in Figure 2. Sample plasmas of clomiphene and nilotinib did not show any peaks due to interference. Clomiphene and nilotinib were eluted from the system in 5 minutes. Clomiphene and nilotinib resided in the system for 2.38 minutes and 2.98 minutes, respectively (ICH, 2005).
Linearity and sensitivity
The signal/noise results were >10.0 at this concentration (12.5 ng/ml) level, and the accuracy and precision findings were 3.52% relative standard deviation (RSD) (Nirav et al., 2017), hence the LLOQQC of the clomiphene was set at 12.5 ng/ml. Every set of clomiphene plasma concentrations between 12.5 and 500.0.0 ng/ml was analyzed using rectilinear plots (Jaivik et al., 2017) (Table 1). Calculated from the mean findings of 6 replication calibration controls, an equation of rectilinear graph for clomiphene was determined to be: y = 0.00413x – 0.00854, where “x” stands for plasma concentration and “y” for peaks ratio, or clomiphene/Nilotinib.
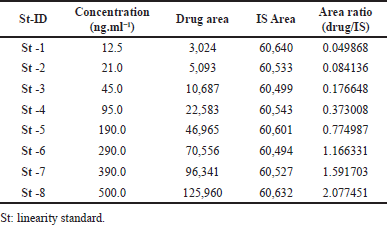 | Table 1. Clomiphene calibration QCs. [Click here to view] |
Accuracy, precision, and recovery
Interday and intraday precision and accuracy outcomes were shown in Figure 3 and Table 2. Precision findings in a day were present between %RSD of 2.02% and 4.12% for clomiphene, where the accuracy outcomes (Patel et al., 2011). were present between the relative error of −3.25%–4.79%. Similarly, in between different experimental days, precision values were varied in the limits of 1.91%–3.89% (RSD) for clomiphene, whereas the accuracy was present between the relative error of −1.79%–4.38%.
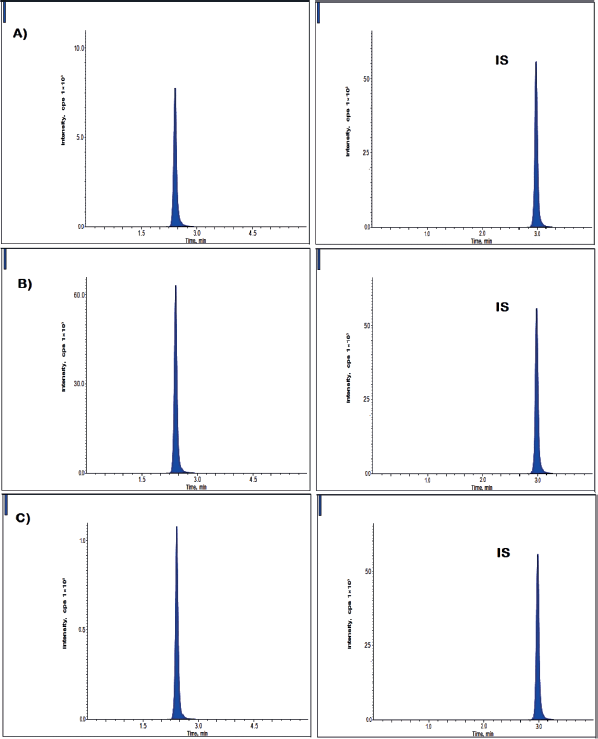 | Figure 3. Clomiphene outcomes at A) low-QC B) median-QC and C) high-QC level. [Click here to view] |
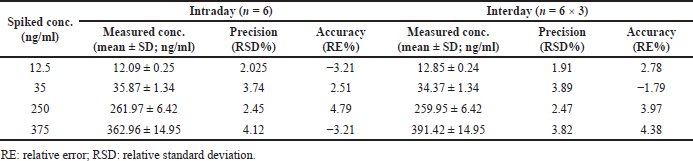 | Table 2. Intra-day and interday precision and accuracy of clomiphene. [Click here to view] |
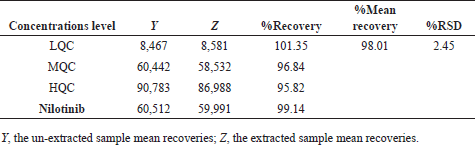 | Table 3. Clomiphene and nilotinib recovery study. [Click here to view] |
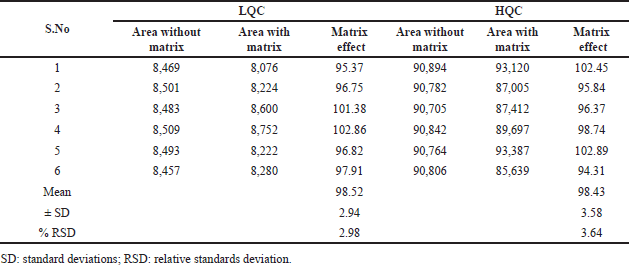 | Table 4. Clomiphene matrix effects at low-QC and high-QC levels. [Click here to view] |
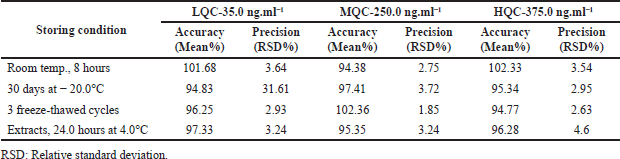 | Table 5. Clomiphene stability studies at variable environmental conditions (n = 3). [Click here to view] |
Clomiphene average recoveries were exist in between the limits of 95.82% to 101.35% at three-QCs (Table 3). The processed extraction technique for sample solution evidenced that clomiphene and nilotinib (99.14%) were improved (Titier et al., 1997) with high percentage outcomes from blank plasma.
Matrix effects
The peak response ratios of clomiphene/nilotinib in blank plasma extract to those with diluent was present between 95.37% and 102.86% for clomiphene (Table 4) at low-QC level (Jaivik et al., 2017) and 94.31%–102.89% at high QC level.
Stability study
Clomiphene stability was proven by executing the control samples to variable storage environments. The exposed environments comprise long-time stabilities subsequently storage of samples at −20°C for 30 days (FDA, 2001), short-time stabilities at room temperature for up to 8 hours, and 3 completely freeze-thawed cycles (frozen at −20.0°C for 12.0 hours) and treated (extracts) samples stabilities after 24 hours at 4.0°C (Nirav et al., 2017). Table 5 shows the results for stability for control sample concentrations in the plasmas. According to regulatory requirements, the clomiphene drug’s evaluated accuracy levels, which ranged from 93.64% to 103.84%, were acceptable.
CONCLUSION
A specific, linear and precise liquid chromatographic—tandem mass spectrometric method was established and subjected to the validation for the quantitation of clomiphene in the plasma sample. YMC-Pack C18-AM (3 µm; 4.60 mm i.d × 50.0 mm) stationary phase was utilized to achieve chromatography elution, through a flowing rate of 0.70 ml/minute. Electrospray ionization in a + ve ionizing method, which was executed in MRM (multiple reaction monitoring) with parent/daughter ionic transition of m/z406.18→100.11 for clomiphene and 530.70→289.50 for nilotinib internal standard. A calibration graph was executed between the concentrations of 12.5–500.0 ng/ml and the resulting equation was y = 0.00413x − 0.00854 with an r2 value of more than 0.99. Clomiphene recovery values were found more than 95.82%, and its accuracy measured in terms of relative error was in the range 3.25%–4.79%. Lastly, the method made was within the guidelines for bioanalytical methodology validation and can be used to measure the amount of clomiphene in different biological samples.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Sayare AS, Undre PB, Ghode PD, Singh SV, Ghode SP. Development and validation of RP-HPLC method for the estimation of clomiphene citrate in pharmaceutical dosage form. Res J Pharm Technol, 2021; 14(7):3483–8.
Bach PV, Najari BB, Kashanian JA. Adjunct management of male hypogonadism. Curr Sex Health Rep, 2016; 8(4):231–9.
Trost LW, Khera M. Alternative treatment modalities for the hypogonadal patient. Curr Urol Rep, 2014; 15(7):417.
Crewe HK, Ghobadi C, Gregory A, Rostami-Hodjegan A, Lennard MS. Determination by liquid chromatography-mass spectrometry of clomiphene isomers in the plasma of patients undergoing treatment for the induction of ovulation. J Chromatogr B, 2007; 847(2):296–9.
DiGiorgio L, Sadeghi-Nejad H. Off label therapies for testosterone replacement. Transl Androl Urol. 2016; 5(6):844–9.
European Medicines Agency, Guideline on bioanalytical method validation 2011.
FDA Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM) May 2001.
Hughes E, Brown J, Collins JJ, Vanderkerchove P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Revi, 2010; (1):CD000057.
ICH guidelines for validation of analytical procedures: text and methodology. Q2(R1) ICH, Geneva; 2005. p. 1-14.
Shah JV, Shah PA, Shah PV, Sanyal M, Shrivastav PS. Fast and sensitive LC–MS/MS method for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma. J Pharm Anal, 2017; 7:163–9.
Miller GD, Moore C, Nair V, Hill B, Willick SE, Rogol AD, Eichner D. Hypothalamic-pituitary-testicular axis effects and urinary detection following clomiphene administration in males. J Clin Endocrinol Metab, 2019; 104(3):906–14.
Patel NP, Sanyal M, Sharma N, Patel DS, Shrivastav PS, Patel BN. Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study. J Pharm Anal, 2017; 6:145–50.
Nordt CA, DiVasta AD. Gynecomastia in adolescents. Curr Opin Pediatr, 2008; 20(4):375–82.
Patel DS, Sharma N, Patel MC. Development and validation of a selective and sensitive LC–MS/MS method for determination of cycloserine in human plasma: application to bioequivalence study. J Chromatogr B, 2011; 879:2265–73.
Rodriguez KM, Pastuszak AW, Lipshultz LI (August 2016). Enclomiphene citrate for the treatment of secondary male hypogonadism. Exp Opin Pharmacother, 2016; 17(11):1561–7.
Kulkarni RM, Bhamare VS, Santhakumari B.. Oxidative transformation of antiretroviral drug zidovudine during water treatment with permanganate: reaction kinetics and pathways. Desalin Water Treat, 2016; 57(52);24999–5010.
Kulkarni RM, Bhamare VS, Santhakumari B. Mechanistic and spectroscopic investigations of Ru3+-catalyzed oxidative degradation of azidothymidine by heptavalent manganese at environmentally relevant pH. Desalin Water Treat, 2016; 57(58):28349–62.
Titier K, Castaing N, Le-Deodic M. Quantification of tricyclic antidepressants and monoamine oxidase inhibitors by high-performance liquid chromatography-tandem mass spectrometry in whole blood. J Anal Toxicol, 1997; 21:200–7.
Trabert B, Lamb EJ, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, Brinton LA. Ovulation-inducing drugs and ovarian cancer risk: results from an extended follow-up of a large United States infertility cohort. Fertil Steril, 2013; 100 (6):1660–6.
Bhamare VS, Kulkarni RM. Palladium (II)-catalyzed oxidation kinetics of azidothymidine by heptavalent manganese during water treatment: kinetics, mechanism, and degradation. Desalin Water Treat, 2019; 144:211–23.
Yilmaz S, Yilmaz Sezer N, Gönenç ?M, ?lhan SE, Yilmaz E. Safety of clomiphene citrate: a literature review. Cytotechnology, 2018; 70(2):489–95.