INTRODUCTION
Metoprolol (MET), (Fig. 1) a common beta-blocker drug, offers a range of benefits in cardiovascular health. Primarily used for hypertension and angina, MET works by blocking stress hormones from affecting the heart. This lowers heart rate and blood pressure, easing the heart’s workload and improving function [1]. Beyond cardiovascular benefits, MET can improve survival rates after a heart attack. It also shows promise in preventing migraines by reducing their frequency and severity. Additionally, MET can help manage symptoms of hyperthyroidism, a condition of an overactive thyroid gland [2]. While generally well-tolerated, some patients may experience fatigue, dizziness, or a slow heart rate. It is crucial to consult a doctor before taking MET, especially for those with certain heart conditions, allergies, or other medical issues. Following proper dosage and usage instructions under medical supervision ensures optimal effectiveness and safety for each patient [3,4].
Several methods exist for determining MET in human plasma, HPLC with UV or fluorescence detection, GC-MS, and HPLC with MS [5–23]. LC-MS/MS is a beneficial, robust, and sensitive procedure used for analysis of a wide variety of small molecules [24–26]. These methods often rely on expensive extraction techniques like solid-phase extraction or require large volumes of plasma. This study presents a simpler, more cost-effective, and novel approach for MET analysis in human plasma. We utilize a combination of diethylether and dichloromethane for extraction, achieving higher efficiency compared to previously reported solvents. The method requires only 100 ml of plasma and boasts a short run time, allowing for faster processing of large sample sets. Our method employs electrospray ionization with single reaction monitoring (SRM) to enhance sensitivity and selectivity. This approach adheres to US FDA validation guidelines [27].
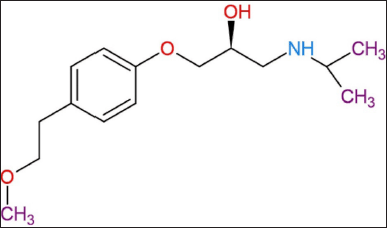 | Figure 1. Chemical structure of MET (source: https://niainnovation.in/product/r-MET/). [Click here to view] |
MATERIALS AND METHODS
Chemicals and reagents
The study utilized MET and MET d7 (IS) pure standards from Vivan Life Sciences, Mumbai, India. HPLC-grade methanol, acetonitrile, and ethyl acetate, along with analytical-grade formic acid, from Merck Specialities, Mumbai, India. Freshly collected K2 EDTA human plasma was procured from the local blood bank.
Liquid chromatography (LC) and MS operating conditions
The analysis was performed using an HPLC system from Shimadzu coupled with a tandem mass spectrometry (MS/MS) system from Thermo. The separation of MET and the internal standard (ISTD) was achieved on a Phenomenex LUNA C8 column; with a mixture of acetonitrile, methanol, and 0.1% formic acid; at a flow rate of 0.6 ml per minute in isocratic mode at 40°C. For optimal detection, specific source parameters were set for both the drug and the ISTD. These parameters included spray voltage, vaporizer temperature, and various gas pressures. The system utilized SRM mode to detect specific ions. The transitions monitored for the drug and ISTD were their respective precursor and product ion masses. LC Quan software version 2.6 controlled all aspects of the HPLC and MS/MS operation.
Preparation of solutions
Prepare a stock solution of MET by accurately weighing around 2.9 mg and transferring it to a 10-ml volumetric flask. Add 5 ml of methanol and mix thoroughly using a vortex mixer. Fill the flask to the mark with methanol, seal it, and gently shake to ensure complete mixing. Label the solution with the preparation date and batch number, then store it in a refrigerator between 2°C and 8°C. This stock solution should be used within 18 days of preparation. Prepare calibration standard solutions by spiking diluent with the MET stock solution to achieve a concentration range of 17.467 ng/ml to 0.025 ng/ml. Similarly, prepare quality control (QC) spiking solutions in the same concentration range. Prepare an ISTD dilution solution by adding a small amount of the ISTD stock solution (around 100 ng/ml) to a diluent mixture of water and methanol (20:80 v/v). Store this ISTD solution in a refrigerator between 2°C and 8°C and use it within 7 days of preparation.
Sample preparation
After thawing, the tubes were mixed thoroughly using a vortex mixer. Pre-labeled tubes were arranged according to the processing order. For each sample (except the standard blank), 500 ml of plasma was pipetted into a tube, followed by the addition of 50 ml of the ISTD working solution. Standard blanks only received 50 ml of diluent instead of the ISTD solution. All tubes were vortexed to ensure proper mixing. Next, 200 ml of a pre-treatment solution (2% ammonia in water) was added to each tube, followed by another round of vortexing. Then, 2.5 ml of the extraction solvent (a mixture of diethylether and dichloromethane in 70:30 ratio) was transferred to all tubes, shaken at 2,500 rpm using a vibrating shaker; centrifuged at 4,000 rpm and 10°C for 5 minutes. After centrifugation, approximately 2 ml of the clear upper layer (supernatant) was collected from each tube. The collected solvent was then evaporated and dried residues were then reconstituted with 300 ml of the mobile phase solution and mixed thoroughly using a vortex mixer. Finally, a small volume (10 ml) of the reconstituted sample was injected.
Method validation
The method was rigorously validated following US FDA guidelines for bioanalytical method validation.
Selectivity & system suitability
To ensure the method’s selectivity, we analyzed human plasma samples from six different sources. This step investigates any potential interference from other substances in the blood that might appear at the same time as MET or IS during analysis of the LC system. Additionally, a test at the beginning was performed, this involved injecting the same standard solution six times in a row. The test assesses the system’s overall performance and consistency by ensuring the coefficient of variation (% CV) of these injections falls within acceptable limits.
System performance and carryover effect
To assess the system’s performance throughout analysis, one sample prepared at the lower limit of quantification (LLOQ) with the ISTD was injected at the beginning of each batch. Additionally, the auto sampler’s potential for sample carryover was investigated. This involved injecting a specific sequence of solutions at the beginning of validation and under other circumstances. The sequence included blank solutions, high and low concentration standards, and extracted samples at similar concentrations. This test helps to ensure that leftover material from previous samples doesn’t contaminate subsequent injections.
Specificity
To verify the method’s ability to distinguish MET from other blood components, we tested blank samples (without adding MET) from ten different commercially available human plasma sources. These sources included seven normal plasma samples with the anticoagulant K2EDTA, one sample of lipemic plasma (containing high-fat levels) with K2EDTA anticoagulant, one sample of hemolyzed plasma (containing red blood cell breakdown products) with K2EDTA anticoagulant, one sample of plasma using heparin as the anticoagulant.
Calibration curve
To establish a relationship between the amount of MET present and the signal from the detector, calibration curves were created. The blank and zero samples help to assess background noise and potential interferences. The accuracy of the calculated MET concentrations in the standards was evaluated. Generally, these values should be within 15% of the expected concentrations. However, a slightly larger deviation of up to 20% was allowed for the LLOQ.
Recovery & matrix effect
To evaluate how efficiently the method extracts MET and the IS from human plasma, we compared the detector response for these analytes in extracted samples at LQC, MQC, and HQC levels to the response from unextracted plasma standard samples with the same concentrations. Additionally, we investigated whether components in the plasma might affect how MET and the IS are detected. This was done by comparing the detector response from extracted QC plasma samples to the response from solutions containing the same concentrations of MET and IS in pure water (aqueous samples). This comparison helps to assess any potential influence of plasma components on the measurement. The effect of plasma constituents on ionization, which is the process enabling detection, was evaluated for both MET and the IS at the same concentration levels used in the extraction efficiency experiment. The results are furnished in Table 1.
Precision and accuracy
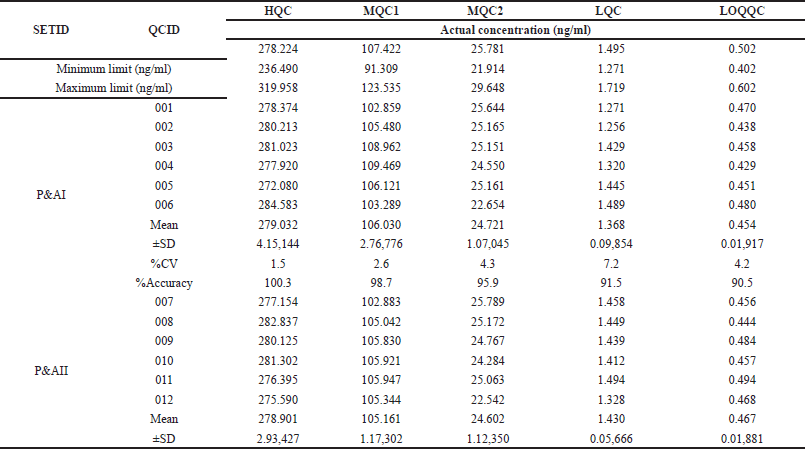
The method’s repeatability was assessed by analyzing six samples at the LLOQ of MET, along with three samples at different QC levels. This analysis was repeated on multiple days to determine the method’s reproducibility (inter-assay precision and accuracy). Both repeatability and reproducibility were evaluated within the same batch (intra-batch) and across different batches (inter-batch). The data were considered acceptable if the calculated concentration of MET was within 15% of the expected value (accuracy) and the variation between measurements was less than 15%. The results are furnished in Table 2.
 | Table 1. Recovery of MET QC samples. [Click here to view] |
Ruggedness
The ruggedness was evaluated by analyzing samples across three different scenarios. In the first scenario, different chromatography columns were used to assess if the column selection significantly impacts the results. In the second scenario, different analysts performed the analysis to determine if analyst variability affects the measurements. This helps ensure the method can be reliably used by different personnel. Finally, the method was tested on different analytical instruments to verify its consistency across equipment variations. The results are furnished in Table 3.
Dilution integrity
We tested diluted samples without altering the measured MET concentration. This is called dilution integrity. To assess this, we prepared dilute solutions with MET and then further diluted five and ten times more with additional blank plasma. This process was repeated six times for each dilution level. The analysis of these diluted samples ensured two key aspects: precision (variation between the six measurements) should be less than 15% and accuracy (closeness to the expected concentration) should be within 100% ± 15%. The results are furnished in Table 4.
Stability experiments
To assess how stable MET and IS are in storage solutions over short periods, we prepared stock solutions following the standard operating procedure for the method. Six samples of each stock solution were stored at room temperature (between 17°C and 28°C) for approximately 1.14 days. Six additional samples were stored in a refrigerator (2°C–8°C) for 1.11 days for MET and the IS. The stability was evaluated by the average response ratio obtained from the stored stock solutions to the response ratio obtained from the freshly prepared solutions (considered the baseline at 0.00 hours).
Effect of potential interfering drugs
The potential interference of other drugs commonly used during clinical trials was investigated. Stock solutions were prepared for each potentially interfering drug (acetaminophen, ibuprofen, cetirizine, caffeine, domperidone, ondansetron, and diclofenac). These stock solutions were then diluted to reach the maximum concentration expected in the blood (Cmax) when the drug is at its peak level. A blank human plasma sample (free of MET) was processed along with each potential interfering drug individually. Additionally, 6 replicates LLOQ were spiked with a mixture containing all the potential interfering drugs. These LLOQ samples were then analyzed alongside the other samples to assess any impact on MET measurement.
RESULTS
Sample preparation and chromatographic conditions
Optimizing sample preparation is crucial in bioanalysis. To isolate MET from human plasma samples, we explored various techniques. For chromatographic separation of MET, a combination of acetonitrile, methanol, and 0.1% formic acid (in specific ratios) was used on a Phenomenex LUNA C8; 40°C; at 0.6 ml per minute. Based on chromatographic behavior and ionization properties, deuterated MET (d7MET) was chosen as the ISTD due to its close resemblance to MET. Diethylether and dichloromethane (70:30 ratio) were selected as the most effective extraction solvents for MET using liquid–liquid extraction. This method produced clean chromatograms with minimal interference from other blood components. The entire analysis process takes approximately 2 minutes, with MET and d7MET eluting from the column at around 0.6 minutes. MS analysis revealed that both MET and the IS formed precursor ions at specific mass-to-charge ratios (m/z). In positive mode electrospray ionization, these parent ions were efficiently generated for both MET and d7MET. Further optimization was performed to achieve the strongest signal for the fragment ions by adjusting the required parameters.
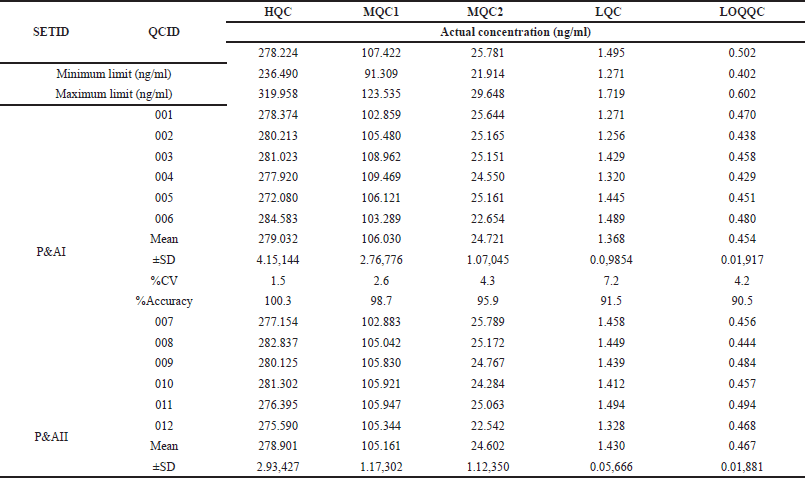 | Table 2. Intra and inter-day precision of MET QC samples. [Click here to view] |
 | Table 3. Ruggedness results (CC) of MET. [Click here to view] |
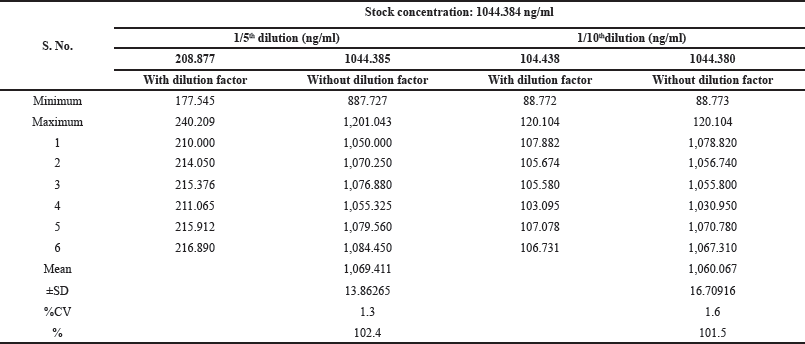 | Table 4. Dilution integrity. [Click here to view] |
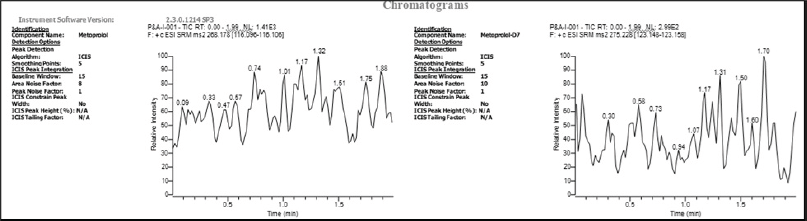 | Figure 2. Chromatogram of STD blank (Analyte and ISTD). [Click here to view] |
Method validation
Figure 2 and Figure 3 illustrate the selectivity, no interfering substances from the blood (endogenous components) were detected near MET or the IS peaks in the chromatograms of blank plasma extracts from various batches. Additionally, injecting the IS at its highest detectable concentration showed no interference with the MET peak during analysis. The retention times for both MET and the IS were highly reproducible, with CV (0.50%–0.81%). Similarly, the area ratio between MET and the IS showed good precision, with a CV between 0.54% and 1.58%. The lowest amount of MET that can be reliably measured in human plasma samples (limit of quantification) is 0.501 ng/ml. At this concentration, the analysis yielded a precise (5.81% CV) and accurate (100.40%) result for MET. The relationship between the concentration of MET and the detector response was best described by a mathematical equation that assigns more weight to lower concentrations. This equation compares the ratio of drug concentration to IS concentration.
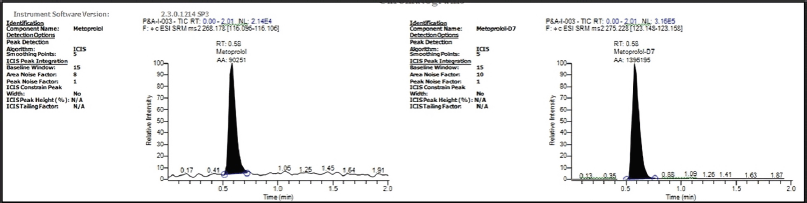 | Figure 3. Chromatogram of STD (Analyte and ISTD). [Click here to view] |
To assess recovery rates, extracted MET samples were compared to unextracted samples. The response of the IS in extracted samples at LQC, MQC2, and HQC was consistent across all eighteen QC samples. The average recovery rate for MET was around 79.4%, with variations between 1.14% and 2.88%. The IS recovery rate was similar (79.31%) with a variation of 1.86%–3.15%. This indicates that the extraction process efficiently recovered MET and the IS from plasma samples with minimal losses. No significant matrix effect, where blood components might influence the measurement, was observed in any of the analyzed samples. The ISTD normalization factor had good precision (around 1.3%) at both low and high QC levels. To test dilution integrity, samples were diluted fivefold (1/5th) and tenfold (1/10th). The diluted samples were then compared to undiluted samples (batch-2). The appropriate dilution factor was chosen based on the QC sample concentrations. The analysis showed that MET can be reliably diluted fivefold and tenfold without compromising accuracy (over 100%) or precision (less than 2%).
Stability studies
We investigated how long MET remains stable in various storage conditions. This included testing its stability in the instrument injector (1.35 days), on the laboratory bench (10.42 hours), and in a freezer at –70°C (with a range of ±15°C) for at least 17.56 days. MET levels remained consistent throughout these tests, with good precision and recovery rates. Overall, these results demonstrate that plasma samples containing MET can be frozen, thawed multiple times, and analyzed using this optimized method without compromising the accuracy and reliability of the MET measurements.
DISCUSSION
We developed a fast and sensitive method to measure MET levels in human plasma. Our analysis showed that MET and the IS were detected better in positive ion mode. For optimal chromatography, we tested various organic solvents and found methanol to be the most suitable choice. Methanol provided sharper peaks and better sensitivity for both MET and IS. The mobile phase in the chromatography system consisted of a mixture of acetonitrile, methanol, and 0.1% formic acid in specific ratios. Under these optimized conditions, both MET and the IS were detected with high sensitivity. A major challenge in bioanalysis is co-elution, where unwanted substances from blood (endogenous materials) interfere with the target analyte (MET). This interference can affect how efficiently molecules are ionized, leading to lower accuracy and reproducibility and potentially causing the analysis to fail specific detection. To minimize such interference, we carefully selected and optimized the solvent used for extraction. The low matrix effect values observed in our study indicate that the chosen extraction process effectively isolates MET with minimal interference from other blood components.
CONCLUSION
The developed new method for MET in human plasma offers several advantages over previously reported methods. They are, faster and more sensitive—our method can detect MET over a wider range of concentrations 0.501–349.342 ng/ml compared to existing methods, making it suitable for various analysis. Simpler sample preparation- the method utilizes liquid-liquid extraction for sample preparation, which is a straight forward, efficient technique, and cost-effective—this approach is less expensive than previously reported methods. The LC-MS/MS technique with selected reaction monitoring mode precisely detects MET by targeting specific transitions between parent and daughter ions for both MET and the ISTD. In conclusion, this new method is a simple, sensitive, and reliable tool for measuring MET concentrations in human plasma. This makes it well-suited for monitoring MET levels in clinical pharmacokinetic studies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of V. V. Institute of Pharmaceutical Sciences, Andhra Pradesh, India with approval number 1847/PO/Re/S/16/CPCSEA on December 1st, 2020.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. https://pubchem.ncbi.nlm.nih.gov/compound/MET-Succinate
2. https://go.drugbank.com/salts/DBSALT000863
3. https://www.scbt.com/p/MET-succinate-98418-47-4
4. https://www.chemspider.com/Chemical-Structure.56654.html
5. Albers S, Elshoff JP, Volker C, Richter A, Laer S. HPLC quantification of MET with solid-phase extraction for the drug monitoring of pediatric patients. Biomed Chromatogr. 2005;19(3):202–7. CrossRef
6. Delamoye M, Duverneuil C, Paraire F, De Mazancourt P, Alvarez JC. Simultaneous determination of thirteen beta-blockers and one metabolite by gradient highperformance liquid chromatography with photodiode-array UV detection. Forensic Sci Int. 2004;141(1):23–31. CrossRef
7. Yilmaz B, Asci A, Arslan S. Determination of MET in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Sep Sci. 2010;33(13):1904–8. CrossRef
8. Fang J, Semple HA, Song J. Determination of MET, and its four metabolites in dog plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;809(1):9–14. CrossRef
9. Chiu FC, Damani LA, Li RC, Tomlinson B. Efficient high-performance liquid chromatographic assay for the simultaneous determination of MET and two main metabolites in human urine by solid-phase extraction and fluorescence detection. J Chromatogr BBiomed Sci Appl. 1997;696(1):69–74. CrossRef
10. Boralli VB, Coelho EB, Cerqueira PM, Lanchote VL. Stereoselective analysis of MET and its metabolites in rat plasma with application to oxidative metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;823(2):195–202. CrossRef
11. Mistry B, Leslie J, Eddington, NE. A sensitive assay of MET and its major metabolite alpha-hydroxy MET in human plasma and determination of dextromethorphan and its metabolite dextrorphan in urine with high performance liquid chromatography and fluorometric detection. J Pharm Biomed Anal. 1998;16(6):1041–9. CrossRef
12. Mistry B, Leslie JL, Eddington ND. Enantiomeric separation of MET and alpha-hydroxyMET by liquid chromatography and fluorescence detection using a chiral stationary phase. J Chromatogr B Biomed Sci Appl. 2001;758(2):153–61. CrossRef
13. Musch G, Buelens Y, Massart DL. A strategy for the determination of beta blockers in plasma using solid-phase extraction in combination with high-performance liquid chromatography. J Pharm Biomed Anal. 1989;7(4):483–97. CrossRef
14. Li S, Wang X, Peng K, Ma Z, Zhang X, Fu S, et al. Rapid and sensitive LC-MS/MS method for the determination of MET in beagle dog plasma with a simple protein precipitation treatment and its pharmacokinetic applications. Molecules. 2012;17(3):2663–74. CrossRef
15. Rao Z, Ma Y, Qin H, Wang Y, Wei Y, Zhou Y, et al. Development of a LC-MS/MS method for simultaneous determination of MET and its metabolites, α-hydroxyMET and O-desmethylMET, in rat plasma: application to the herb–drug interaction study of MET and breviscapine. Biomed Chromatogr. 2015;29(9):1453–60. CrossRef
16. Quarterman CP, Kendall MJ, Jack DB. Determination of MET metabolites in plasma and urine by electron-capture gas-liquid chromatography. J Chromatogr. 1980;183(1):92–8. CrossRef
17. Li S, Liu G, Jia J, Liu Y, Cheng P, Chen Y, et al. Simultaneous determination of ten antiarrhythmic drugs and a metabolite in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847(2):174–81. CrossRef
18. Gowda KV, Mandal U, Selvan PS, Solomon WDS, Ghosh A, Sarkar AK, et al. Liquid chromatography tandem mass spectrometry method for simultaneous determination of MET tartrate and ramipril in human plasma, J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858(1-2):13–21. CrossRef
19. Jensen BP, Sharp CF, Gardiner SJ, Begg EJ. Development and validation of a stereoselective liquid chromatography-tandem mass spectrometry assay for quantification of S-and R-MET in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;865(1-2):48–54. CrossRef
20. Sarkar AK, Ghosh D, Das A, Selvan PS, Gowda KV, Mandal U, et al. Simultaneous determination of MET succinate and amlodipine besylate in human plasma by liquid chromatography-tandem mass spectrometry method and its application in bioequivalence study, J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873(1):77–85. CrossRef
21. Selvan PS, Pal TK. Chromatography-tandem mass spectrometry method for the simultaneous quantitation of MET succinate and simvastatin in human plasma. J Pharm Biomed Anal. 2009;49(3):780–5. CrossRef
22. Venkateswarlu P, Kumar BN, Seshaiah K, Prasad VVV. Selective and sensitive method for the determination of MET in human plasma using liquid chromatography coupled with tandem mass spectrometry. Acta Pharm. 2010;60(2):177–84. CrossRef
23. Yilmaz B, Arslan S. Development and validation of GC-MS method for determination of MET in human urine. J Chromatogr Sci. 2010;48:613–7. CrossRef
24. Venkateswara Rao P, Lakshmana Rao A, Prasad SVUM. Development and validation of a method for simultaneous estimation of sitagliptin and ertugliflozin in rat plasma by LC-MS method. Curr Pharm Anal. 2021;17:1060–74. CrossRef
25. Mukkanti Eswarudu M, Lakshmana Rao A, Vijay K. Development and validation of a LC-MS/MS method for simultaneous quantification of ivabradine and MET in rat plasma. J Pharmacol Toxicol Methods. 2022;116:107186. CrossRef
26. Mukkanti Eswarudu M, Lakshmana Rao A, Vijay K. Novel validated LC-MS/MS method for simultaneous estimation of celecoxib and amlodipine in rat plasma and its application to a pharmacokinetic study. J Pharm Negat Results. 2022;13(9):3882–95. CrossRef
27. Bioanalytical method validation, Guidance for industry. Rockville, MD: U.S. Department of Health and Human services Food and Drug Administration; May 2001.