INTRODUCTION
Alpelisib chemically designated as (2S) -1- N- [4- Methyl -5- [2- (1,1,1- trifluoro-2-methylpropan-2-yl) pyridin-4-yl] -1,3-thiazol-2-yl] pyrrolidine-1, 2-dicarboxamide. Its chemical formula and molecular mass are C19H22F3N5O2S and 441.47 g/mol, respectively (Fig. 1). Alpelisib inhibits the enzyme, phosphatidylinositol 3-kinase (PI3K) and shows effective antitumor action (Konstantinopoulos et al., 2019; Yang et al., 2017). It acts by the inhibition of class-I PI3K p110α specifically, a lipid kinase which shows an important role in different biological processes, like survival, proliferation, metabolism, and differentiation (James et al., 2015; Rodon et al., 2018).
This drug is approved by the United States Food and Drug Administration (USFDA) in the month of May 2019, as the first PI3K inhibitor designated for human epidermal growth factor receptor 2-negative, treatment of hormone receptor-positive, advanced or metastatic breast cancer, PIK3CA-mutated in combination with fulvestrant for postmenopausal women and male patients. To start alpelisib treatment, it is essential that the existence of PIK3CA transmutation in tissue or liquid biopsy sample collection should be established through diagnostic tests approved by Food & Drug Administration (FDA). The drug is marketing under the Piqray trade name and is existing as oral tablets. Studies assessing the therapeutic efficiency of drug in other cancer types, such as colorectal cancer and ovarian cancer, are under continuing research (De Buck et al., 2014; James et al., 2017).
The literature on alpelisib reveals that no single method was reported on liquid chromatography-tandem mass spectrometry (LC–MS/MS) for the estimation of alpelisib in plasma (Seo et al., 2021). Current research work was directed to develop a new sensitive and specific technique for the estimation of alpelisib in human plasma. Few analytical methods on LC-MS/MS were reviewed for method development and validation.
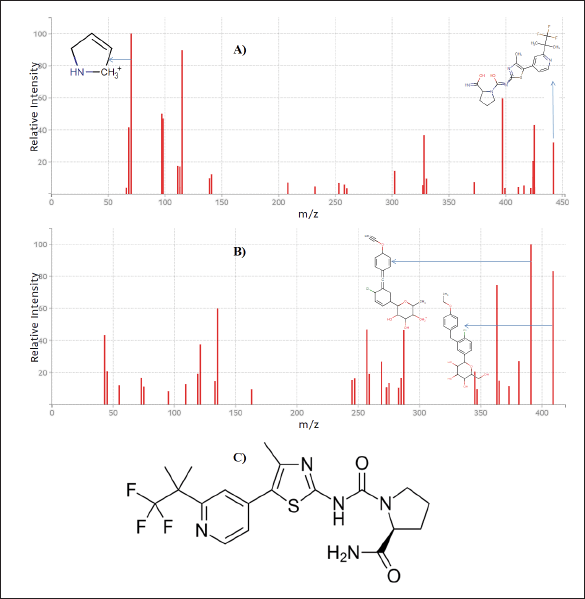 | Figure 1. Mass spectrum of A) Alpelisib, B) Dapagliflozin, and C) Chemical structure of Alpelisib. [Click here to view] |
MATERIALS AND METHODS
Reagents and chemicals
The standards of alpelisib (purity: 99.51%) and dapagliflozin (purity: 99.82%) utilized as internal standard (IS) were obtained from the Dr. Reddy’s Lab, Hyderabad, India. High Performance Liquid Chromatography (HPLC) grade methanol and acetonitrile were procured from AB enterprises, Mumbai, India. Deionized water is processed by a Milli-Q water system. Human plasma samples were procured from Vivekananda blood blank, Hyderabad, India.
Equipment and LC–MS/MS parameters
The LC–MS/MS instrument comprised of an Agilent1200 liquid chromatographic system with a SL-binary pump and 6460-Agilent triple-quadrupole mass system with electrospray ionization. Mass-Hunter software B0104 version was utilized for the data acquisition and for the controlling the LC-MS/MS system. The analyte elution was executed at 35.0°C through Zorbax C18 (50 × 4.6 mm, 5 μ) reverse phase analytical column. The mobile phase consisted of acetonitrile and 0.1% formic acid in the ratio of 90:10 (%V/V) with 0.90 ml/minute ?ow rate. The auto-sampler temperature was maintained at 10.0°C, and infusion volume was 10.0 μl. Mass system was ran in positive ionization mode. MS/MS study was organized under scan mode of multiple reaction monitoring (MRM). The MS/MS constraints were adjusted as: 5.0 kV capillary voltage; 400.0°C source temperature; 12.0 l/minute N2 drying gas ?ow and 40.0 psi nebulizer gas pressure. The optimized voltage for the fragmentation of both alpelisib and IS was 135.0 V. Data acquisition was processed in MRM mode utilizing transitions of m/z 442.15 → 70.06 for alpelisib with 12.0 eV collision energy and m/z 409.14 → 391.13 for IS with 15.0 eV collision energy.
Protocol for quality control (QC) and standard solutions
1.0 mg/ml alpelisib and IS individual stock solutions were processed in 80% acetonitrile (diluent) in water separately. The alpelisib stock solution was then subjected for serial dilution to acquire the working standards. The IS 500 ng/ml working solution was processed by diluting IS stock solution with diluent. Calibration curve standards of alpelisib (145, 410, 1,050, 1,950, 2,900, 3,900, 4,900, and 5,800 ng/ml) were processed by spiking the suitable working standards to blank plasma. QC samples at low, medium, and high concentrations (406, 2,900, and 4,350 ng/ml) were executed distinctly in the same way.
Protocol for sample solution
50.0 μl of sample plasma was employed in 10.0 ml plastic tube and into that 125.0 μl of IS working standard was added to all the sample solutions except blank. Mixture was subjected for extraction with 4.0 ml of diethyl ether by vortexing for 5.0 minutes and shaking for 25.0 minutes. Then, samples are subjected for centrifugation at 5.0°C for 15 minutes at 4,500 rpm. Organic layer is relocated to fresh glass tubing and vaporized to dryness with the help of stream of N2. The resulting residue was re-formed with 100.0 μl of movable solvent and a 10.0μl sample was infused in to LC–MS/MS instrument for the examination.
Pharmacokinetic study
Six male rabbits of about 2.50 to 3.0 kg were chosen for the pharmacokinetic study of alpelisib. Before 12 hours of a drug direction into the rabbits, food was evaded. Water has provided for the rabbits under the study throughout the work and 37.5 mg/kg drug dose has given to healthy rabbits and taken 0.60 ml of blood sample solutions from marginal ear vein of rabbits prior to dosing (zero-time) and at different time intervals between 0 and 24 hours. The resultant solution was exposed to 4,500 rpm in the centrifuge for 15.0 minutes and plasma samples were separated and relocated into labelled polypropylene tubings at −20.0°C (Patel et al., 2011; Ravi et al., 2021). The study was approved by Institutional animal ethical committee with No: 1447/PO/Re/8/11/CPCSEA/18/A.
Method validation
The developed analytical process was subjected for the validation as per the guidelines of FDA (EMA, 2005; Shankar and Bhikshapathi, 2021) to meet the acceptance limit.
RESULTS AND DISCUSSION
Method optimization
Current research work was aimed to develop a highly sensitive and selective LC–MS/MS method for the estimation of alpelisib in human plasma. Initially, a mobile phase consisting of methanol, acetonitrile and formic acid, in varying combinations were tried, but less resolution and low response was observed. Finally chromatographic separation was performed on a Zorbax C18 (50 × 4.6 mm id, 5 μm) analytical column with a simple isocratic mobile phase composed of 0.1% formic acid and acetonitrile, (10:90, v/v). The LC system was operated at a flow rate of 0.90 ml/minute with total single run time of 6.5 minutes. The column and auto-sampler temperatures were maintained at 40.0°C and 10.0°C, respectively.
Mass system optimization
During the optimization of product and parent ions in the mass instrument, neat alpelisib solution was injected under the positive ionization mode. Precursor ion was observed at m/z 442.15 and fragments of m/z 397.13, 115.08 and 70.06 were noticed upon parent ion fragmentation. Fragment of alpelisib ion having m/z 70.06 was identified with the utmost intensity. Alpelisib isotopes were not available commercially, so we have searched for variable possible IS, and elected dapagliflozin as IS. MRM transitions of m/z 442.15 → 70.06 for alpelisib, and m/z 409.14 → 391.13 for IS were monitored after optimization of the conditions.
Speci?city and selectivity
Interference peaks were not identified in the alpelisib and dapagliflozin samples of plasma. The representative chromatograms of spiked plasma at 145 ng/ml and blank of alpelisib (lower limit of quantification) and IS were presented in Figure 2. The retaining timings of alpelisib and IS were 2.50 and 1.18 minutes, respectively (Patel et al., 2017).
Sensitivity and linearity
Linearity graphs were executed for each lot, over concentration between 145 and 5,800 ng/ml for alpelisib (Table 1) in plasma sample. The average regression line equation gained for alpelisib was 0.0018 + 0.0056 x = y (n = 6) for alpelisib, where x is the plasma concentrations and y is meant for fractions of analytes to dapagliflozin. The Lower Limit of Quantification (LLOQ) standard of alpelisib was set to 145 ng/ml with less than 3.54% accuracy and precision values and the S/N findings were more than 10.0 (Shah et al., 2017).
Matrix effects
Table 4 represents findings of matrix effect processed at LQC and HQC levels (Titier et al., 2007). The peak response ratios of drug/IS, which were liquefied with plasma blank extractions to those liquefied with movable phase ranges between 93.87% and 102.85% for alpelisib at LQC level and 94.21% to 102.89% at HQC level. These findings recommended that matrix effect of alpelisib were nil under current LC–MS/MS circumstances.
Recovery, precision, and accuracy
Table 2 and Figure 3 were represents findings of intraday and inter-day accuracy and precision. Intraday precision values were ranged between 1.75% and 3.19% relative standard deviation (RSD) for drug component, while the accuracy values were present in between −0.71% and 3.25% of relative error. Likewise, for inter-day experimentations, accuracy values were present in between 0.67% and 3.45% of relative error, while the precision differ in between 1.67% and 3.17% (RSD) for alpelisib. The findings were evidenced that the technique was accurate and precise (Patel et al., 2011). The alpelisib average recovery values were varied in between 94.85% and 102.35% at three levels of QC standards (Table 3).
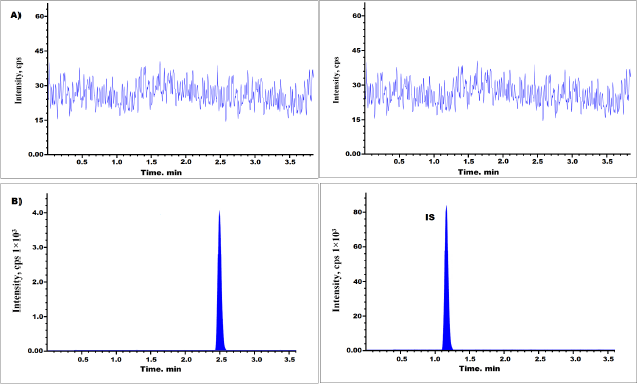 | Figure 2. Representative chromatograms of drug A) Blank and B) LLOQ samples. [Click here to view] |
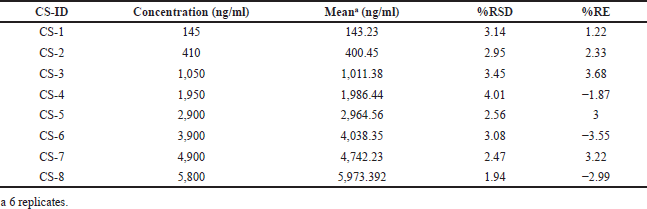 | Table 1. Calibration standards for alpelisib. [Click here to view] |
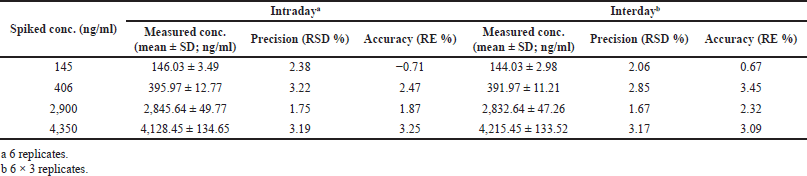 | Table 2. Intraday and inter-day accuracy and precision of alpelisib. [Click here to view] |
Stability analysis
Alpelisib stability studies were processed by exposing the QC standards to variable storage environments. The subjected environments comprise short term stability at long-term stability after storage at −20.0°C for 30 days, room temperature for 8.0 hours, the prepared extract sample stability after 24.0 hours at 4.0°C and three complete freeze thaw cycle (freeze temperature at −20.0°C for 12.0 hours) (Titier et al., 2007). The findings of stability studies at QC levels at processed and plasma sample were represented in Table 5. The evaluated accuracy findings for alpelisib were present in between 92.34%–103.65% of the nominal concentrations which were within the satisfactory limits. As an end result, alpelisib was considered to be more stable under dissimilar storage environments.
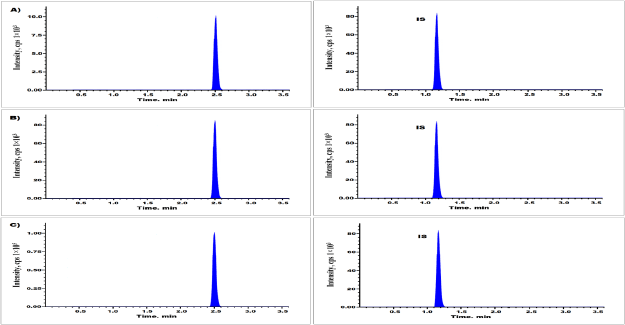 | Figure 3. QC standards of alpelisib at A) LQC, B) MQC, and C) HQC levels. [Click here to view] |
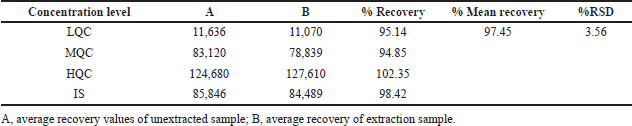 | Table 3. Extraction recovery rates of analytes. [Click here to view] |
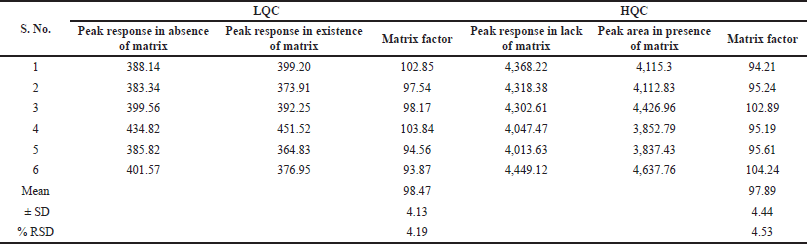 | Table 4. Matrix effect for alpelisib at HQC and LQC level. [Click here to view] |
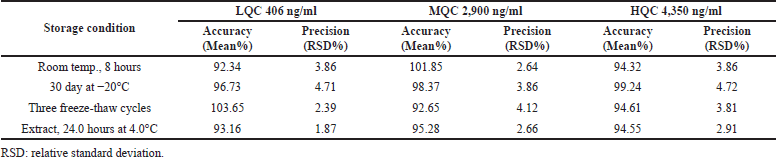 | Table 5. Stability findings of alpelisib (n = 3). [Click here to view] |
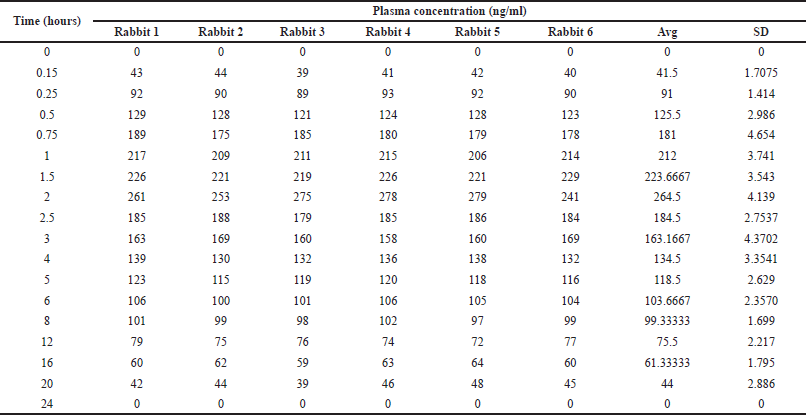 | Table 6. Measured plasma concentration values in healthy rabbits. [Click here to view] |
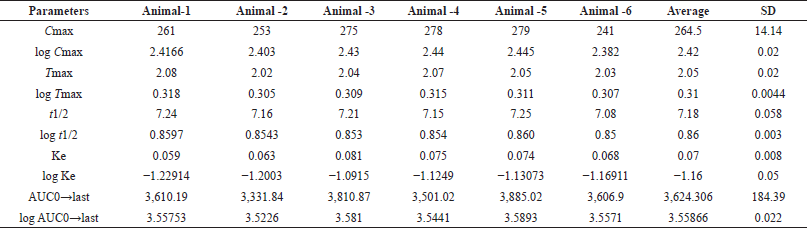 | Table 7. pk parameter values of test animals. [Click here to view] |
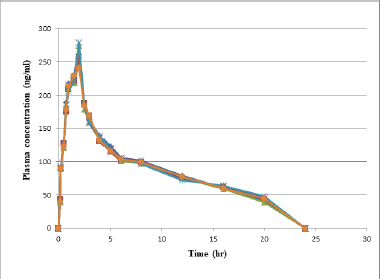 | Figure 4. Profile for test rabbits’ plasma concentrations and time. [Click here to view] |
Pharmacokinetics
The Pharmacokinetic parameters of alpelisib were estimated from the plot drawn by taking time on X-axis and plasma concentration on Y-axis by utilizing Pk-solver software. In the present work trapezoidal rule has been elected for the computing of an area beneath the plot from 0.0 to 24.0 hours(AUC0–24). Alpelisib has an average Tmax of 2.0 ± 0.21 and AUC0→α, AUC0→t and mean Cmax, for test formulation is 3,624.21 ± 315, 3,075.20 ± 316, and 269 ± 12.17, respectively. The resultant finding were represented in Tables 6 and 7, Figures 4 and 5.
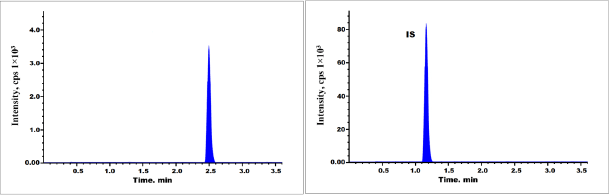 | Figure 5. Chromatogram of rabbit plasma sample. [Click here to view] |
CONCLUSION
A specific, reliable, and validated LC-MS/MS technique was developed for the quantification of alpelisib drug component in human plasma. The subjected validation parameters in obedience to FDA procedures were fully satisfied. Developed technique was specific and sensitive with 145 ng/ml as LLOQ and the total run time of 3.5 minutes. The intra-day and inter-day accuracy results existed in between −0.71% and 3.45% of relative error and the RSD findings related to precision were below 3.25%. Alpelisib was effectively stable beneath variable analytical environments. liquid liquid extraction (LLE) process was finalized for alpelisib extraction from plasma with average percentage recoveries of 97.45% by utilizing the dapagliflozin as an IS. Alpelisib has an average Tmax of 2.0 ± 0.21 and average Cmax, AUC0t and AUC0→α for drug was 269 ± 12.17, 3,075.20 ± 316, and 3,624.21 ± 315, respectively. The specificity, accuracy, and higher percentage recovery from plasma of the current technique considers it suitable for pharmacokinetic and bioequivalence studies.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
The study was approved by Institutional animal ethical committee with No: 1447/PO/Re/8/11/CPCSEA/18/A.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Pápai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D; SOLAR-1 Study Group. PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med, 2019; 380(20):1929–40. CrossRef
De Buck SS, Jakab A, Boehm M, Bootle D, Juric D, Quadt C, Goggin TK. Population pharmacokinetics and pharmacodynamics of BYL719, a phosphoinositide 3-kinase antagonist, in adult patients with advanced solid malignancies. Br J Clin Pharmacol, 2014; 78(3):543–55. CrossRef
EMA. European Medicines Agency, guideline on bioanalytical method validation 2011. ICH guidelines for validation of analytical procedures: text and methodology. Q2(R1) ICH, Geneva, Switzerland, pp 1–14, 2005.
Shankar CH, Bhikshapathi DVRN. Validation of a specific LC-MS/MS method for the quantification of anti-cancer alectinib in biological matrices. In. J Phrm. Res., 2021;13(1): 6513–21. CrossRef
James A, Blumenstein L, Glaenzel U, Jin Y, Demailly A, Jakab A, Hansen R, Hazell K, Mehta A, Trandafir L, Swart P. Absorption, distribution, metabolism, and excretion of [(14)C]BYL719 (alpelisib) in healthy male volunteers. Cancer Chemother Pharmacol, 2015; 76(4):751–60. CrossRef
James AD, Marvalin C, Luneau A, Meissner A, Camenisch G. Comparison of (19)F NMR and (14)C measurements for the assessment of ADME of BYL719 (Alpelisib) in humans. Drug Metab Dispos, 2017; 45(8):900–7. CrossRef
Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, Mayer EL, O’Cearbhaill RE, Coleman RL, Kochupurakkal B, Whalen C, Curtis J, Farooq S, Luo W, Eismann J, Buss MK, Aghajanian C, Mills GB, Palakurthi S, Kirschmeier P, Liu J, Cantley LC, Kaufmann SH, Swisher EM, D’Andrea AD, Winer E, Wulf GM, Matulonis UA. Olaparib and alpha-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol, 2019; 20(4):570–80. CrossRef
Patel NP, Sanyal M, Sharma N, Patel DS, Shrivastav PS, Patel BN. Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study Highly sensitive LC–MS/MS method to estimate doxepin and its metabolite nordoxepin in human plasma for a bioequivalence study. J Pharm Anal, 2017; 6:145–50. CrossRef
Patel DS, Sharma N, Patel MC, Patel BN, Shrivastav PS, Sanyal M. Development and validation of a selective and sensitive LC–MS/MS method for determination of cycloserine in human plasma: application to bioequivalence study. J Chromatogr B Analyt Technol Biomed Life Sci, 2011; 879:2265–73. CrossRef
Ravi Y, Shankar CH, Bhikshapathi DVRN, Rajkamal B. Development of fast and simple LC-ESI-MS/MS technique for the quantification of regorafenib; application to pharmacokinetics in healthy rabbits. Curr. Phrm. Anal., 2021;17:554-563. CrossRef
Rodon J, Curigliano G, Delord JP, Harb W, Azaro A, Han Y, Wilke C, Donnet V, Sellami D, Beck T. A phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget, 2018; 9(60):31709–18. CrossRef
Seo SW, Kim JM, Han DG, Geum D, Yun H, Yoon IS. A sensitive HPLC-FLD method for the quantification of alpelisib, a novel phosphatidylinositol 3-kinase inhibitor, in rat plasma: Drug metabolism and pharmacokinetic evaluation in vitro and in vivo. Journal of Chromatography B Analyt Technol Biomed Life Sci, 2021; 1163:122508. CrossRef
Shah JV, Shah PA, Shah PV, Sanyal M, Shrivastav PS. Fast and sensitive LC–MS/MS method for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma. J Pharm Anal, 2017; 7:163–9. CrossRef
Titier K, Castaing N, Le-Déodic M, Le-Bars D, Moore N, Molimard M. Quantification of tricyclic antidepressants and monoamine oxidase inhibitors by high-performance liquid chromatography-tandem mass spectrometry in whole blood. J Anal Toxicol, 2007; 21:200–7. CrossRef
Yang X, Zhang X, Huang M, Song K, Li X, Huang M, Meng L, Zhang J. New insights into PI3K inhibitor design using X-ray Structures of PI3Kalpha complexed with a potent lead compound. Sci Rep, 2017; 7(1):14572. CrossRef