INTRODUCTION
Asciminib is a tyrosine kinase inhibitor that is utilized in the treatment of chronic-phase Ph+ chronic myeloid leukemia (CML). Specifically, it inhibits the ABL1 kinase functioning of BCR-ABL 1 fused protein, which drives CML amplification in the majority of diseased patients. It has demonstrated efficacy in Ph+ CML with a mutation of T315I, which generates resistance to treatment mutant BCR-ABL 1 compared to wild-type BCR-ABL 1. The progression of CML is predominantly driven by the Philadelphia chromosome translocation, which generates a fusion oncogenic gene [1], BCR ABL 1, between the ABL and BCR genes [2]. The combination of this gene results in the production of a fusion protein, BCR-ABL1, with transforming activities and increased tyrosine kinases that contribute to the proliferation of CML [3].
Asciminib allosteric inhibitor of the BCR ABL 1 tyrosine kinase. Because it attaches to the myristoyl pockets of an ABL 1 part of the fusion proteins, it may block the oncogenic activity that would otherwise be caused by the fusion proteins by trapping it into an inactive conformation [4]. The steady-state AUCtau and Cmax at a dose of 40 mg twice daily were 5,262 ng.hour/ml and 793 ng/ml, respectively. It is chemically named as N-4-[chloro (difluoro) methoxy] phenyl]-6-[(3R)-3-hydroxy pyrrolidin-1-yl]-5-(1H-pyrazo l-5-yl) pyridine-3-carboxamide; hydrochloride, with chemical formula (Figure 1) and weight [5,6] of C20H18ClF2N5O3 and 449.84 g·mol−1.
Literature on asciminib reveals that no analytical approaches were reported for the quantification of asciminib by liquid chromatographic tandem mass spectrometric (LC-MS/MS). Therefore, there was a need for an analytical method for the quantification of asciminib in the biosamples with a high degree of sensitivity. The current study directed the development of an LC-MS/MS method for the determination of asciminib in samples of plasma.
MATERIAL AND METHOS
Reagent chemicals
Asciminib was gifted by Novartis India Ltd., Mumbai. India. HCOOH of analytical quality and methyl alcohol and can (acetonitrile) of LC grade were acquired from JT Bakers in Hyderabad, India. The Milli-Q®RO system’s built-in water supply was used for the preparation of the moveable and washing solvent systems.
LC-MS/MS instrument
Analytes have been extracted out of plasma samples with standard C-18 Sep-Pak tubes made by Waters Corporation, MA, USA. Chromatographic elution was done on an LC-MS/MS instrument with a Waters 2695 Alliances separating model (Waters Corporation, USA) for sample introduction and delivery of solvent, Micro mass Quattromicro API triple quadruple mass spectrometric system connected to an Electro spray ionization (ESI) (Z-spray) source as detection system (Micromass, Manchester, UK). At room temperature, an analysis was done with RPAtlantis dC18 (2.1 × 100 mm, 3 μm) column that was covered by a guard column (3.9 × 20 mm, 5 μm). Mass Lynx software (Version 4.0) running on Microsoft Windows XP professionals was employed to handle the device, get the data, measure the signals-to-noise fraction, and integrate and smoothen peaks.
LC and mass system conditions
Chromatographic isolation was processed with Atlantis/dC18 (2.1 × 100 mm, 3 μm) stationary phase and mobile solvent system of formic acid (0.1%) and methyl alcohol (20:80, v:v), conveyed at 0.7 ml/minute flow rate. The ESI interface has functioned in ionization of positive mode. The following mass constraints were utilized: 400°C: desolvation temperature, 150°C: source temperature, and 3.1 kV: capillary voltage. Cone and desolvation gas of N2 was employed at a flow of 50 and 800 l hour−1, correspondingly. Collisional gas of argon was processed at 0.17 ml/minute flow in a collision cell. Collisional energy and voltages of the cone for asciminib and voriconazole were optimized as 20/34 and 16/24 V, respectively. Analytes were quantified by ionization in a positive approach with electro-spray ionization at the mass transitions of (m/z): asciminib, 450.11/239.09, and voriconazole [internal standard (IS)], 350.3/281.1.
Quality control (QC) and standard sample solutions
Asciminib and IS stock solutions (1,000 μg/ml) were executed in the mobile phase separately. They were dissolved even more with plasma to make workable solutions with a concentration of 10 g/ml. Human plasma was used to make eight calibration standards with concentration levels from 39 to 1,586 ng/ml and four QC sample solutions with concentrations of 39, 111, 793, and 1,189 ng/ml [7]. QCs and calibration standards were stirred for 1 minute, and then 1 ml aliquots were poured into borosilicate glass (13 × 100 mm) culture tubing and preserved at −20?C till they were needed.
Sample processing
Allow 1 ml samples of QC, calibration curve, and blank plasma samples to attain room temperatures. The end concentration of each tube was 85 ng/ml, so 250 μl of the IS solution was incorporated and each of the tubes was shaken for 20 seconds. Before samples were put on the C18 Sep-Pak cartridges, they were treated with one milliliter of methyl alcohol and then 2 ml water. Then 1 ml was combined with water (1 ml) and sample solutions were isolated with 1 ml of 0.1% HCOOH in methyl alcohol [8]. The samples were then dried out under light streams of N2 gas in a block of heat at 45°C. The leftovers were diluted with100 μl of movable solvent system, placed in an auto-sampling container, and 10 μl was loaded into LC-MS/MS equipment.
Stability study
By keeping the processed QC samples in an auto sampler that was kept at a temperature of 5°C ± 3°C for a time of 2 days, 20 hours, and 27 minutes, it was possible to assess their auto sampler stability. By screening at −20°C for 2 months, long-term stabilities for the analyte and IS were processed at Low quality control (LQC) and High quality control (HQC). By holding at 2°C–8°C for 10 days, stock solution stability for the analyte was processed at LQC and HQC levels. Three cycles of freeze and thaw stability processing were performed at −20°C and room temperature [9]. The drug and IS were treated for stability of short-term by placing them at room conditions for 8 hours. A 17 hours, 28 minutes storage duration at room temperature was used to test the benchtop condition of the QC spiked sample solutions. The QC spiked samples for dry extract stability were assessed for the duration of 2 days by placing those samples at −28 C ± 5 C.
Validation of the analytical method
The technique was validated in accordance with the accepted practices outlined in the US FDA guidelines [10] on the validation of bioanalytical methods. Specificity, accuracy, linearity, precision, stability, and recovery were among the validation criteria [11,12].
RESULT AND DISCUSSION
Mass and LC conditions optimization
The precursor and product ions of asciminib and voriconazole were examined by introducing the standard methanolic solution (1.0 μg/ml) into a mass system utilizing a syringe pump with 20 μl flow. Analytes were quantified by ionization in a positive approach with electro-spray ionization in Multiple reaction monitoring (MRM) mode at the mass transitions of (m/z): asciminib, 450.11/239.09 and voriconazole (IS), 350.3/281.1. The following mass constraints were utilized: 400°C: desolvation temperature, 150°C: source temperature, and 3.1 kV: capillary voltage. Cone and desolvation gas of N2 was employed at a flow of 50 and 800 l hour−1, correspondingly. Collisional gas of argon was processed at 0.17 ml/minute flow in a collision cell. Collisional energy and voltages of the cone for asciminib and voriconazole were optimized as 20/34 and 16/24 V, respectively.
Specificity
We analyzed six samples of blank plasma and IS, which stands for asciminib. Asciminib, or IS, did not co-elute with any other drugs or endogenous components. The chromatogram shown in Figure 2A is an example of a typical chromatogram of drug-free human plasma (blank) [13,14], which was used in the creation of standards and QC samples. Figure 2B shows blank plasma that has been spiked with IS at a concentration of 85 ng/ml.
 | Figure 1. Asciminib chemical structure. [Click here to view] |
Calibration curve
The linearity of the test was determined by conducting an analysis on a series of standard blends that included asciminib and IS in human plasma at eight different concentrations ranging from 39 to 1,586 ng/ml. A regression analysis was carried out on the peak height ratios that corresponded to the IS as well as the concentrations [15,16]. For asciminib, the mean equations that were found were y = 0.0012x − 0.0022, and the r2 value was 0.9994 (n = 6). It was determined that the calibration curves were appropriate for use by doing a back calculation to determine the concentration of asciminib in human plasma using the calibration curves (Table 1). Every computed concentration was found to be substantially below the maximum permissible level. Figure 3 represents the mean calibration curve of asciminib. The Lower limit of quantification quality control (LLOQQC) of asciminib was 39.0 ng/ml (S/N ratio > 10) and was sufficient for accurate quantification of asciminib in the analysis of the plasma sample.
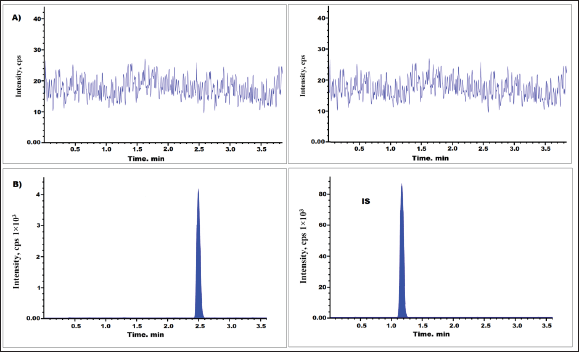 | Figure 2. Asciminib chromatogram of (A) blank plasma and (B) LLOQQC levels. [Click here to view] |
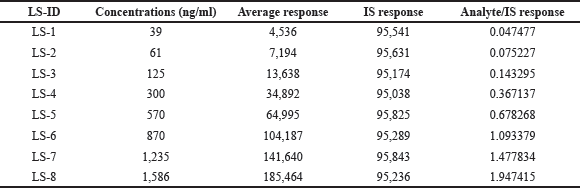 | Table 1. Calibration standards for asciminib. [Click here to view] |
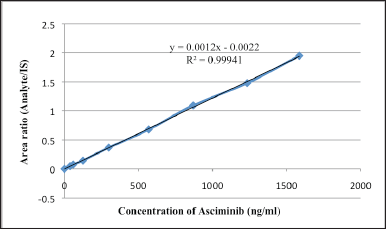 | Figure 3. Linearity of asciminib. [Click here to view] |
Accuracy and precision
Accuracy and precision were assessed for four QC levels (39, 111, 793, and 1,189 ng/ml). Intraday precision (n = 10) was ≤4.65% and inter-day precision (n = 20, throughout the course of 3 days) was ≤04.79% for asciminib (Table 2). Interday bias was found to be −4.28% to 5.76% and intraday bias was −3.75% to 4.53 [13]. The LC-MS/MS chromatograms of low QC (111 g/ml) and high QC (1,189 g/ml) spiked with IS (85 ng/ml) were shown in Figure 4.
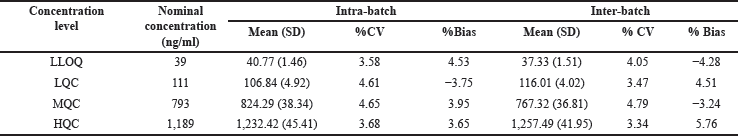 | Table 2. Asciminib precision and accuracy for inter-batch and intra-batch. [Click here to view] |
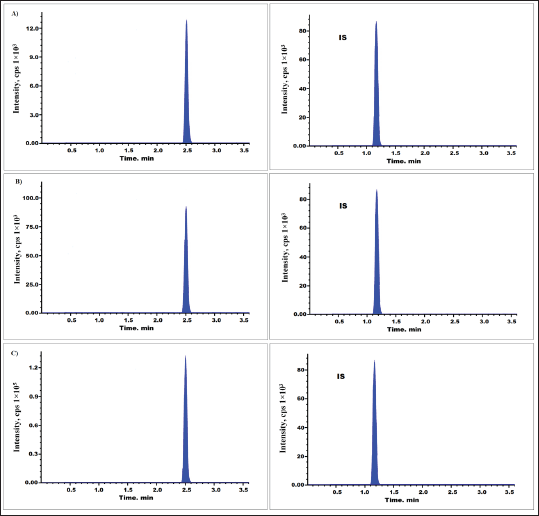 | Figure 4. Asciminib chromatograms for (A) LQC, (B) MQC, and (C) HQC level. [Click here to view] |
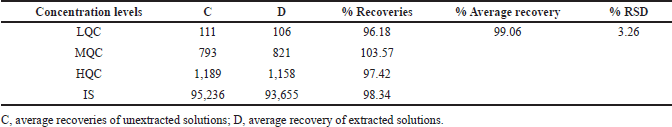 | Table 3. Recoveries of analytes after the extraction. [Click here to view] |
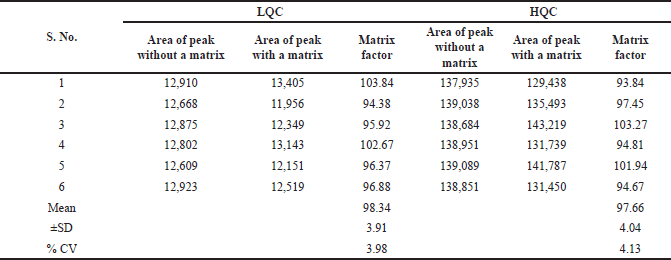 | Table 4. Matrix effect of asciminib. [Click here to view] |
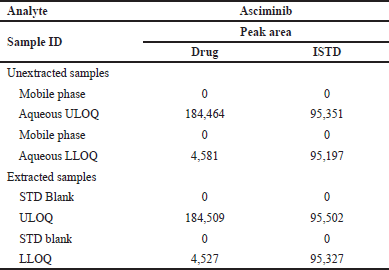 | Table 5. Carry over effect. [Click here to view] |
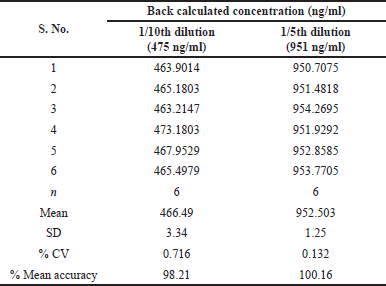 | Table 6. Dilution integrity. [Click here to view] |
Recovery
Extraction recovery of asciminib at three concentrations (111, 793, and 1,189 ng/ml) and IS at one concentration level (85 ng/ml) were assessed by equating peak responses of spiked solutions before the extraction process and spiked solutions after the extraction process (six sets) [15]. Averagely determined extraction recoveries of asciminib were 99.06% (Fig. 4 and Table 3). Recovery of IS was 98.34%.
Matrix effects
The matrix effects were quantified by comparing asciminib peak intensity in the absence and presence of matrix constituents. It was measured at low and high QC levels by infusing the six sample solutions. The %CV findings at the LQC and HQC levels were 3.98 and 4.13 (Table 4), respectively.
Carryover effect
The auto sampler’s carryover impact was investigated by injecting mobile phase, aqueous Upper limit of quantification (ULOQ), Lower limit of quantification (LLOQ), and standard (STD) blank samples. This experiment showed no carryover. The results are summarized in Table 5.
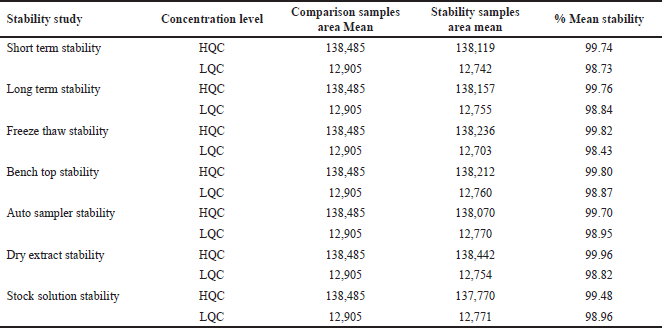 | Table 7. Stability data of asciminib. [Click here to view] |
Dilution integrity
The method’s dilution integrity was tested by spiking the plasma with a Dilution quality control (DIQC) concentration of 4,758 ng/ml from a Dilution integrity (DI) spiking solution that was three times the ULOQ. DIQC sample is diluted 1/5 (951 ng/ml) and 1/10 (475 ng/ml). Analyzing samples against calibration curve standards established the precision and accuracy of 1/5 and 1/10 dilution integrity standards. The results are summarized in Table 6.
Stability
The stability of asciminib and the IS in unprocessed and processed plasma samples at LQC and HQC levels were examined. Table 7 summarizes the findings of the stability investigations.
CONCLUSION
The concentration of asciminib in human plasma was determined using a sensitive, accurate, and linear LC-MS/MS approach. C18-cartridges were used to separate plasma samples for the presence of voriconazole, IS, and asciminib. An Atlantis/dC18 (2.1 100 mm, 3 m) stationary phase was used for chromatographic separation. The mobile solvent system was made up of formic acid (0.1%) and methyl alcohol (20:80, v/v). Ionization in the positive method using electrospray ionization allowed for the quantification of the following analytes: asciminib (m/z 450.11/239.09) and voriconazole (IS) (m/z 350.3/281.1). No plasma blank or outside component was found to be interfering. The relationship between asciminib concentration levels and their respective peak response fractions to voriconazole was rectilinear between 39.0 and 1,586.0 ng/ml. Asciminib had a precision of 4.79% both throughout the day and between days. The daily bias ranged from −4.28% to −5.76%, and the hourly bias ranged from −3.75% to 4.53%. Asciminib was recovered at a mean rate of 99.06% during extraction. The recovery of IS was 98.34%. Asciminib in plasma samples can now be routinely quantified using the established method, making it useful for a wide range of applications in industry, forensics, and clinical research. The high sensitivity using the Liquid liquid extraction (LLE) approach was achieved by the development of a straightforward and specific validated LC-MS/MS method for the quantification of infigratinib. The metrics of specificity, sensitivity, carry-over, recovery, precision, accuracy, stability, and the impact of the matrix were used to verify the devised analytical method. Within 6.5 minutes, the drug and IS were eluted on a PhenomenexSB-C18 column (250 × 4.6 mm × 5 m) using acetonitrile and 0.1% V/V formic acid in water at a flow rate of 0.9 ml/minute. Infigratinib’s Retention time (RT) was 5.12 minutes, whereas IS’s RT was 3.31 minutes. Infigratinib required a total of 6.5 minutes to elute. With a r2 value of 0.999, the equation for the linear regression line was determined to be y = 0.994x + 2.662. The calibration graph %CV measurements for infigratinib came in at 3.73 or below. High QC sample %CV was determined to be 1.64%, and low QC sample %CV was determined to be 0.70%; these numbers represent the matrix effect.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Breccia M, Colafigli G, Scalzulli E, Martelli M. Asciminib: an investigational agent for the treatment of chronic myeloid leukemia. Expert Opin Investig Drugs. 2021;30(8):803–11. CrossRef
2. Jones Jill K, Thompson Eric M. Allosteric inhibition of ABL kinases: therapeutic potential in cancer. Mol Cancer Ther. 2020;19(9):1763–9. CrossRef
3. Schoepfer J, Jahnke W, Berellini G, Buonamici S, Cotesta S, Cowan-Jacob SW, et al. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J Med Chem. 2018;61(18):8120–35. CrossRef
4. Tran P, Hanna I, Eggimann FK, Schoepfer J, Ray T, Zhu B, et al. Disposition of asciminib, a potent BCR-ABL1 tyrosine kinase inhibitor, in healthy male subjects. Xenobiotica. 2020;50(2):150–69. CrossRef
5. Hou JZ, Ye JC, Pu JJ, Liu H, Ding W, Zheng H, et al. Novel agents and regimens for hematological malignancies: recent updates from 2020 ASH annual meeting. J Hematol Oncol. 2021;14(1):66. CrossRef
6. Khatri A, Wang J, Pendergast AM. Multifunctional Abl kinases in health and disease. J Cell Sci. 2016;129(1):9–16. CrossRef
7. Saraner N, Karagoz A, Guney B, Saglam O. Determination of dasatinib in human plasma by using liquid chromatography-tandem mass spectrometry. Int J Anal Bioanal Methods. 2019;1:2. CrossRef
8. Guodong He, Liping Mai, Xipei Wang. Development and validation of an HPLC-MS/MS method for rapid simultaneous determination of cefprozil diastereomers in human plasma. Int J Anal Chem. 2018;6959761:1–9. CrossRef
9. Darshan Bhatt, Rajkamal B. A UPLC-MS/MS method development and validation for the estimation of sofosbuvir from human plasma. Int J Appl Pharm. 2017;9(1):30–6. CrossRef
10. US FDA. Guidance for industry bioanalytical method validation. Rockville, MD: Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2001.
11. ICH. ICH guidelines for validation of analytical procedures: text and methodology. Geneva, Switzerland: Q2(R1) ICH; 2005.
12. Zhou C, Tian J, Lin P, Liu T, He A, Fang L, et al. Quantitation of fostemsavir, a mesenchymal-epithelial transition factor inhibitor by UPLC-MS/MS in rat plasma and its application to a pharmacokinetic study. Bioanalysis. 2020;12(5):285–93. CrossRef
13. Shankar CH, Bhikshapathi D, Medipalli V, Arjuna RN, Sadasivam RK. Bioanalytical method development and validation for the quantitation of larotrectinib in human plasma: application to pharmacokinetics in healthy rabbits. J Appl Pharm Sci. 2023;13(11):111–8.
14. Glaenzel U, Jin Y, Hansen R, Schroer K, Rahmanzadeh G, Pfaar U, et al. Absorption, distribution, metabolism, and excretion of fostemsavir (INC280) in healthy male volunteers and in vitro aldehyde oxidase phenotyping of the major metabolite. Drug Metab Dispos. 2020;48(10):873–85. CrossRef
15. Shah JV, Shah PA, Shah PV, Sanyal M, Shrivastav PS. Fast and sensitive LC–MS/MS method for the simultaneous determination of lisinopril and hydrochlorothiazide in human plasma. J Pharm Anal. 2017;7:163–9. CrossRef
16. Lolla S, Gubbiyappa KS, Cheruku S, Bhikshapathi DVRN. Validation of an LC–MS/MS method for quantitation of fostemsavir in plasma. J Pharm Toxicol Methods. 2023;120:107254. CrossRef