INTRODUCTION
Ukoniq, also known as Umbralisib, is a drug (da Fonseca et al., 2017) used to combat marginal zone lymphoma (MZL) (Bron and Meuleman, 2019; Sriskandarajah and Dearden, 2017) and follicular lymphoma (FL) (Boughan and Caimi, 2019; Takata et al., 2018). The patient ingests it. The most frequently observed side effects are an increase in creatinine (McDonald et al., 2012; Samra and Abcar, 2012), diarrhea, colitis (Sun et al., 2015; Yang et al., 2015), fatigue (Marcora et al., 2009), nausea (Scorza et al., 2007), neutropenia (Donadieu et al., 2017; Hsieh et al., 2007), transaminase elevation (Giboney, 2005; Oh and Hustead, 2011), musculoskeletal pain (Kumaraveloo and Lunner Kolstrup, 2018; Mishra and Sarkar, 2021), anemia, thrombocytopenia (Ahmed et al., 2007), upper respiratory tract infection (Grande et al., 2015), vomiting, abdominal pain (Tytgat, 2007; Viniol et al., 2014), a rash and a decreased appetite Umbralisib is a class of kinase inhibitor that includes PI3K-delta (Sarker et al., 2015) and casein kinase CK1-epsilon. The phosphoinositide-3-kinases (PI3Ks) are a family of enzymes involved in many important cellular functions, including cell proliferation and survival, cell differentiation, intracellular trafficking, and immunity. There are four isoforms of PI3K (alpha, beta, delta, and gamma), of which the delta isoform is highly expressed in hematopoietic cells and malignant lymphoid diseases. Dysregulation of the PI3K pathway is among one of the most commonly mutated pathways across all of cancer biology. Umbralisib is highly selective for the delta isoform of PI3K and has limited to no impact on the other PI3K iso forms. CK1-epsilon (Burris et al., 2018; Lunning et al., 2019) is a major regulator of oncoprotein translation, which drives growth and survival of lymphoma cells, including c-Myc. For adults with MZL recurrence or refractory MZL who have received at least one prior anti-CD20 (Kuijpers et al., 2010) based regimen, and adults with relapsed or refractory FL who have received at least three prior lines of systemic therapy, Umbralisib is indicated. The drug’s prescribing information also alerts patients to the adverse reactions they should watch out for, including infections, neutropenia, diarrhoea, and non-infectious colitis, as well as hepatotoxicity (Mumoli et al., 2006; Riordan and Williams, 2002), and severe cutaneous reactions (Mumoli et al., 2006; Riordan and Williams, 2002). Its effects on chronic lymphocytic leukemia (Gribben, 2009; Kipps et al., 2017) have been tested in clinical trials. Mantle cell lymphoma (Rajabi and Sweetenham, 2015; Skarbnik and Goy, 2015) is just one of many cancers they are testing in combination trials and other lymphomas. For the MZL indication, priority review was given to the application for Umbralisib, and it was also designated as an orphan drug for the treatment of MZL and FL. Figure 1 represents the chemical representation of Umbralisib.
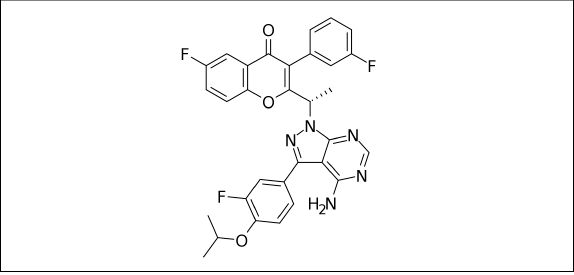 | Figure 1. Chemical structure of Umbralisib. [Click here to view] |
UPLC was used to quantify Umbralisib in the present research. Until now, there were no quantification methods for Umbralisib. Developing an UPLC strategy for the creation of Umbralisib has gained more interest than developing other strategies. UPLC offers better separation and can thus yield more information in a short period of time than High Performance Liquid Chromatography (HPLC). Thus, UPLC separation techniques were employed for the purpose of separating Umbralisib.
EXPERIMENTAL STUDY
Solutions and reagents
This research was supported by Glenmark Pharmaceutical Private Ltd., Andheri (E), Mumbai, India, which provided the pure Umbralisib standard and samples used in this research (99.7%–99.9% purity). Everything else, including acetonitrile, triethyl amine, and water, was obtained from Merck (India) Ltd. Worli in Mumbai and was of 99.99% purity for UPLC analysis.
Equipment
The researchers used a Waters Acquity chromatographic device equipped with a quaternary pump, variable UV, and Photo Diode Array (PDA) detectors to conduct the experiment. When it came to data collection and processing, we turned to the chromatographic programme Empower-2.0.
Step of mobility
In this study, the mobile step consisted of 0.1% formic acid buffer in a 40:60 (v/v) acetonitrile mixture that was degassed prior to review. The selected mobile phase produced a sharp peak with a low tailing factor (2.0), as well as a plate count of more than 2,000, which was satisfactory.
Diluent
Mobile phase was used as diluents.
Conditions of chromatography
Kinetex column (100 × 4.6 mm, 2.6 μm) was used in the UPLC experiments. In this example, the process is isocratic elution with acetonitrile, which delivers formic acid (0.1%) (60:40 v/v) as a mobile step at a stream of 1.0 ml/minute. To calculate the amount of the injection required, 10 μl of solution was injected into the injection channel and the experiment ran for 3 minutes, with the machine temperature kept at ambient. The sample was then taken out of the instrument and the absorbance was measured at 219 nm (because it gives the maximum absorbance of the Umbralisib). The proposed method was validated according to International Conference on Harmonisation (ICH) guidelines.
Standard solution preparation
Measure and carefully weigh out 20 mg of Umbralisib into a volumetric flask with a capacity of 100 ml, then mix in an additional 70 ml of diluents. Sonicate the solution for 30 minutes to thoroughly dissolve it. Finally, top off the flask with additional diluents. To dilute the solution, add 5 ml of the other solution to 50 ml of the original solution.
Preparation of the sample
Consider the measurements, and weigh out 27.8 mg of the powder from each tablet of the tablet form of Umbralisib (200 mg of the drug). Transfer that weight to a flask with a capacity of 100 ml, along with 70 ml of diluent. To achieve the desired consistency, it is sonicated first and then diluted. Add 50 ml of water to the solution and then filter it using a 0.45-micron syringe filter.
RESULTS AND DISCUSSION
The study’s goal is to develop a single isocratic UPLC method for the quantitative determination of Umbralisib in bulk and pharmaceutical dosage form that is accurate, precise, and economical. Developers tested acidic buffers, methanol, and acetonitrile with isocratic development procedures. Various stationary phases were used, including phenyl, biphenyl, amino, C4, and C8. Also, the mobile step plate count and retention time was changed at each trial to improve results. Kinetex column (100 × 4.6 mm, 2.6 μm) and a moving phase of 0.1%formic acid:acetonitrile (60:40 v/v) with a UV detector set to monitor at 219 nm were utilised. For the entire run of the show, it lasted 3 minutes. Chromatographic conditions optimized in Table 1 are shown.
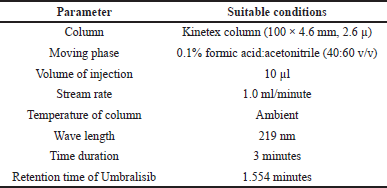 | Table 1. Method suitability conditions. [Click here to view] |
 | Table 2. Results of system suitability. [Click here to view] |
System suitability
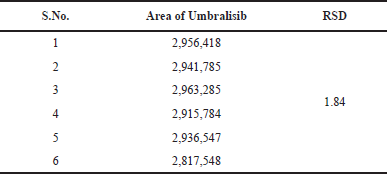
After six injections of a normal solution, device suitability parameters, such as theoretical plate number, time, peak area, tailing factor, and resolution, were collected from Empower software. Table 2 contains the system suitability results, while Figure 2 shows the chromatogram produced using the standard method.
Specificity
None of Umbralisib was found in the eluent during the time it was in contact with blank and placebo. This is shown in Figure 3.
 | Figure 2. Chromatogram of blank. [Click here to view] |
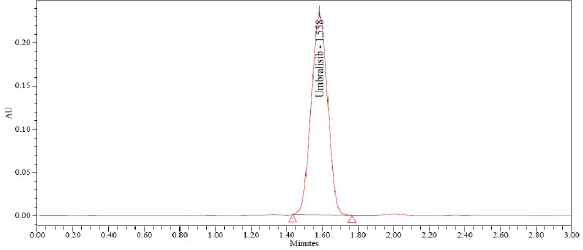 | Figure 3. Chromatogram of standard. [Click here to view] |
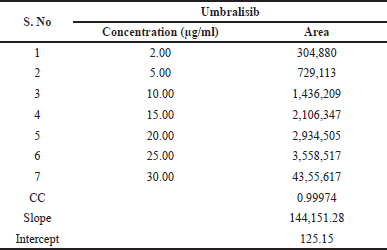 | Table 3. Results of linearity. [Click here to view] |
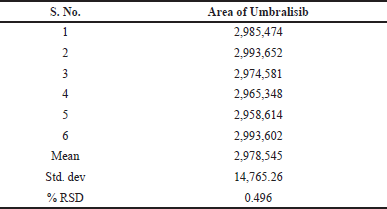 | Table 4. Outcomes of method precision. [Click here to view] |
Linearity
Linearity was discovered by drawing a calibration curve of the area of peak concentration against its corresponding concentration (10%, 25%, 50%, 75%, 100%, 125%, and 150%). It was possible to deduce from this calibration curve that the graph represented a straight line within the range of 2 to 30.0 μg/ml of Umbralisib. Y is calculated as 144,151X + 125 (R2−0.9997). In the study, the results were shown in Table 3, and in Figure 4, the calibration plot of Umbralisib was shown. From the linearity calculation sheet, the slope, intercept, and correlation coefficient values were found.
Precision
This procedure was studied to evaluate both intraday and intermediate intraday precision. Analyzing the sample solution of Umbralisib six times on the same day with the same conditions enabled the intraday studies to be done. This system was examined in the same laboratory by evaluating the data using various instruments and examiners to investigate the accuracy. It is exceptionally precise, with Relative standard deviation (RSD) percentage values of less than 2%. Meaningful recovery of usable drug occurred each time the process was conducted at a higher concentration, implying that the procedure was accurate. The Table 4 demonstrates the quality of the method precision results and the Figure 5 shows the method precision chromatogram.
Intermediate precision (ruggedness)
Intermediate precision results were shown in Table 5.
Accuracy
The precision was achieved through the three-stage measurement of the recovery experiments (50%, 100%, and 150%). Umbralisib levels of 10, 20 and 30 μg/ml were used in Active Pharmaceutical Ingredient (APIs). Triple injection of the test solution for each spike was performed and the test process was performed. The recovery rate was 100% similar, while the RSD rate was below 2%. The percentage of recovered data was determined, together with the average and relative standard variations. The approach was successful because the recuperation values were within the scope. Table 6 shows accuracy results.
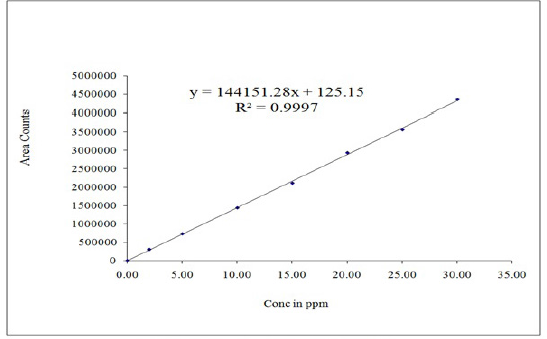 | Figure 4. Calibration plot of Umbralisib. [Click here to view] |
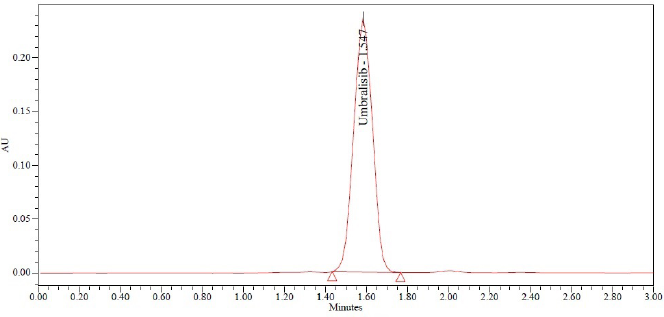 | Figure 5. Sample chromatogram. [Click here to view] |
 | Table 5. Results of intermediate precision. [Click here to view] |
 | Table 6. Results of accuracy. [Click here to view] |
 | Table 7. Outcomes of robustness. [Click here to view] |
Robustness
In the evaluation of chromatographic technique, the fluctuations in flow rate and the movement composition of phases were used. The RSD percentage was determined to be within reasonable limits. Table 7 shows robustness results.
Forced degradation
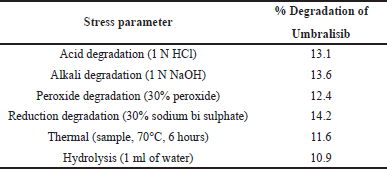
The proposed approach can be used for successful release and stability test evaluations and can be called a preferable stability technology. The required forced degradation analysis includes acid, alkali, oxidation, reduction, hydrolysis, and thermal degradation. The chromatograms show that, despite the presence of degraded peaks, the selected drugs remained stable under pressure conditions. The purity of the peak can be detected by using PDA detector. Table 8 shows the results of forced degradation.
Acid degradation
1 ml of sample stock solution was moved to a volumetric flask of 10 ml, add 1 ml of 1 N HCl and left it for 15 minutes. After 15 minutes, add 1 ml of 1 N NaOH and make up to the diluent mark. Filter the solution using syringe filter and injected into UPLC system.
 | Table 8. Outcomes of FD. [Click here to view] |
Alkali degradation
1 ml of sample stock solution was moved to a volumetric flask of 10 ml, add 1 ml of 1 N NaOH and left it for 15 minutes. After 15 minutes, add 1 ml of 1N HCl and make up to the mark. Filter the solution using syringe filter and injected into UPLC system.
Peroxide degradation
1 ml of sample stock solution was moved to a volumetric flask of 10 ml, add 1 ml of 30% hydrogen peroxide solution and make up to the mark with diluents. Filter the solution using syringe filter and injected into UPLC system.
Reduction degradation
1 ml of sample stock solution was moved to a volumetric flask of 10 ml and add 1 ml of 30% sodium bi sulphate solution and make up to the mark with diluents. Filter the solution using syringe filter and injected into UPLC system.
Thermal degradation
The sample solution was set in an oven at 105°C for 6 hours. The resultant solution was injected into UPLC system.
Reduction degradation
1 ml of sample stock solution was moved to a volumetric flask of 10 ml and add 1 ml of UPLC grade water and make up to the mark with diluents. Filter the solution using syringe filter and injected into UPLC system.
CONCLUSION
A novel, fast, low-cost, sensitive, and easy-to-access UPLC method was developed in this study to estimate Umbralisib in API and pharmaceutical dosage. The major advantage of this approach is that there are no UPLC methods. Low-cost, easier to use, more sensitive, reliable, and reproducible operations are all benefits. The analysis of many samples requires these characteristics to be critical. All parameters, including linearity, accuracy, specificity, robustness, and precision of process, were validated and found to be within reasonable limits. The RSD values for the parameters were <2%, indicating an accurate procedure and a reasonably consistent result obtained from that method. In the Quality Control (QC) laboratories, the current method can therefore be used for routine study and Umbralisib pharmaceutical formulation.
ACKNOWLEDGMENT
Shree Icon Pharmaceutical Laboratories expresses their gratitude for the provision of all necessary facilities to successfully complete this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J, 2007; 83(983):575–82. CrossRef
Boughan KM, Caimi PF. Follicular lymphoma: diagnostic and prognostic considerations in initial treatment approach. Curr Oncol Rep, 2019; 21(7):63. CrossRef
Bron D, Meuleman N. Marginal zone lymphomas: second most common lymphomas in older patients. Curr Opin Oncol, 2019; 31(5):386–93. CrossRef
Burris HA, Flinn IW, Patel MR, Patel MR, Fenske TS, Deng C, Brander DM, Gutierrez M, Essell JH, Kuhn JG, Miskin HP, Sportelli P. Umbralisib, a novel PI3Kδ and casein kinase-1ε inhibitor, in relapsed or refractory chronic lymphocytic leukaemia and lymphoma: an open-label, phase 1, dose-escalation, first-in-human study. Lancet Oncol, 2018; 19(4):486–96. CrossRef
Donadieu J, Beaupain B, Fenneteau O, Bellanné-Chantelot C. Congenital neutropenia in the era of genomics: classification, diagnosis, and natural history. Br J Haematol, 2017; 179(4):557–74. CrossRef
da Fonseca EM, Shadlen KC. Promoting and regulating generic medicines: Brazil in comparative perspective. Rev Panam Salud Publica, 2017; 41(e5):2. CrossRef
Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician, 2005; 71(6):1105–10. CrossRef
Grande AJ, Keogh J, Silva V, Scott AM. Exercise versus no exercise for the occurrence, severity and duration of acute respiratory infections. Cochrane Database Syst Rev, 2015; (6):CD010596. CrossRef
Gribben JG. Stem cell transplantation in chronic lymphocytic leukemia. Biol Blood Marrow Transplant, 2009; 15(1 Suppl):53–8. CrossRef
Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med, 2007; 146(7):486–92. CrossRef
Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, O’brien S, Gribben J, Rai K. Chronic lymphocytic leukaemia. Nat Rev Dis Prim, 2017; 3:16096. CrossRef
Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, Beaumont T, Tedder TF, van Noesel CJ, Eldering E, van Lier RA. CD20 deficiency in humans results in impaired T cell-independent antibody responses (PDF). J Clin Invest, 2010; 120(1):214–22. CrossRef
Kumaraveloo KS, Lunner Kolstrup C. Agriculture and musculoskeletal disorders in low- and middle-income countries. J Agromed, 2018; 23(3):227–48. CrossRef
Lunning M, Vose J, Nastoupil L, Fowler N, Burger JA, Wierda WG, Schreeder MT, Siddiqi T, Flowers CR, Cohen JB, Sportelli P. Ublituximab and umbralisib in relapsed/refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood, 2019; 134(21):1811–20. CrossRef
Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. J Appl Physiol, 2009; 106(3):857–64. CrossRef
McDonald T, Drescher KM, Weber A, Tracy S. Creatinine inhibits bacterial replication. J Antibiot, 2012; 65(3):153–6. CrossRef
Mishra SD, Sarkar K. Work-related musculoskeletal disorders and associated risk factors among urban metropolitan hairdressers in India. J Occup Health, 2021; 63(1):e12200. CrossRef
Mumoli N, Cei M, Cosimi A. Drug-related hepatotoxicity. N Engl J Med, 2006; 354(20):2191–3. CrossRef
Oh RC, Hustead TR. Causes and evaluation of mildly elevated liver transaminase levels. Am Fam Physician, 2011; 84(9):1003–8.
Rajabi B, Sweetenham JW. Mantle cell lymphoma: observation to transplantation. Ther Adv Hematol, 2015; 6(1):37–48. CrossRef
Riordan SM, Williams R. Alcohol exposure and paracetamol-induced hepatotoxicity. Addict Biol, 2002; 7(2):191–206. CrossRef
Samra M, Abcar AC. False estimates of elevated creatinine. Perm J, 2012; 16(2):51–2. CrossRef
Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, Clarke PA, Raynaud FI, Levy G, Ware JA, Mazina K. First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors. Clin Cancer Res, 2015; 21(1):77–86. CrossRef
Scorza K, Williams A, Phillips JD, Shaw J. Evaluation of nausea and vomiting. Am Fam Physician, 2007; 76(1):76–84.
Skarbnik AP, Goy AH. Mantle cell lymphoma: state of the art. Clin Adv Hematol Oncol, 2015; 13(1):44–55.
Sriskandarajah P, Dearden CE. Epidemiology and environmental aspects of marginal zone lymphomas. Best Pract Res Clin Haematol, 2017; 30(1–2):84–91. CrossRef
Sun J, Lin J, Parashette K, Zhang J, Fan R. Association of lymphocytic colitis and lactase deficiency in pediatric population. Pathol Res Pract, 2015; 211(2):138–44. CrossRef
Takata K, Miyata-Takata T, Sato Y, Iwamuro M, Okada H, Tari A, Yoshino T. Gastrointestinal follicular lymphoma: current knowledge and future challenges. Pathol Int, 2018; 68(1):1–6. CrossRef
Tytgat GN. Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs, 2007; 67(9):1343–57. CrossRef
Viniol A, Keunecke C, Biroga T, Stadje R, Dornieden K, Bösner S, Donner-Banzhoff N, Haasenritter J, Becker A. Studies of the symptom abdominal pain—a systematic review and meta-analysis. Fam Pract, 2014; 31(5):517–29. CrossRef
Yang M, Geng L, Chen P, Wang F, Xu Z, Liang C, Li H, Fang T, Friesen CA, Gong S, Li D. Effectiveness of dietary allergen exclusion therapy on eosinophilic colitis in chinese infants and young children ≤ 3 years of age. Nutrients, 2015; 7(3):1817–27. CrossRef