INTRODUCTION
Progestin (Wiegratz and Kuhl, 2004) medications, like drospirenone, are used in birth control pills to prevent pregnancies and are included in hormone replacement therapy (Langer et al., 2021; Stuenkel et al., 2015) for menopausal women, among other applications (Kuhl, 2005). It is available solely under the brand name Slynd, but it is also available in combination with estrogen (or other hormones) under the brand name Yasmin, as well as many other brands. The medication is administered orally. The following are the most common side effects: acne (Scott et al., 2019), headache, breast tenderness (Salzman et al., 2012), weight gain, and menstrual changes (Atteson and Zaluski, 2019; Catalini and Fedder, 2020). Other less common side effects may include a dangerously high level of potassium and blood clots, to name a few (Han and Jensen, 2015). While progestins, or synthetic progestogen, are biologically active as agonists of the progesterone receptor, drospirenone is a progestin (Nath and Sitruk-Ware, 2009) and acts as such by binding to the progesterone receptor. In addition to the amino corticoid (Kolkhof and Bärfacker, 2017; Kosaka et al., 2010) and anti-androgenic (Gillatt, 2006; Iversen et al., 2001) activity, it also has antimineralocorticoid (Kolkhof and Bärfacker, 2017; Kosaka H et al., 2010) and antiandrogenic (Gillatt, 2006; Iversen et al., 2001) activities. The drospirenone molecule is thought to closely resemble bioidentical progesterone in terms of its antimineralocorticoid activity and lack of undesirable off-target activity (Oelkers, 2000, 2002). Drospirenone is used as a progestogen-only birth control pill on its own, in combination with estrogen, ethinyl estradiol, or estetrol (E4), with or without supplemental folic acid (vitamin B9), and for use in menopausal hormone therapy. For the treatment of moderate acne, premenstrual syndrome (Dickerson et al., 2003), premenstrual dysphoric disorder (Pearlstein, 2016; Reid and Soares, 2018), and dysmenorrheal (Gomathy et al., 2019), a birth control pill with low-dose ethinyl estradiol is also indicated (painful menstruation). Moderate to severe vasomotor symptoms (hot flashes) (Freedman, 2014), vaginal atrophy, and postmenopausal osteoporosis (Maclennan et al., 2004; Torgerson and Bell-Syer, 2001) are appropriate uses of this medication. To help reduce the risk of estrogen-induced endometrial hyperplasia (Whitehead, 2006), the drospirenone component has been included in this formulation. Drospirenone has also been used in the treatment of transgender women alongside estrogen as part of hormone therapy (Majumder and Sanyal, 2017; Majumder et al., 2020).
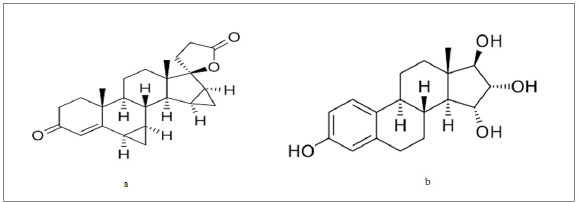 | Figure 1. Structural representations of (A) drospirenone and (B) esterol. [Click here to view] |
In humans, E4 is present in a detectable amount only during pregnancy (Holinka et al., 2008). Only fetal liver (Abdel-Misih and Bloomston, 2010) can make it. E4 is a very similar estrogen to estriol, which is a pregnancy-only hormone that is very prevalent during gestation (Elias and Bengelsdorf, 1952). Estetrol (E4) is a prominent estrogen only during pregnancy. Estetrol is an estrogen and in various tissues it has estrogenic effects. Estetrol interacts with ERα (which is also known as the nuclear ER) (Levin, 2005) in the same manner as other estrogens, but distinct from that found with SERMs (Abot et al., 2014; Foidart et al., 2019). To date, the physiological role of E4 has not been determined. Estetrol, a possible fetal wellness marker, has been studied extensively. Pregnancy appeared to be too difficult to control because of the large intra and inter-individual variation in maternal E4 plasma levels (Kundu and Grant, 1976). The chemical structures of drospirenone and E4 are shown in Figure 1.
The present study shows the simultaneous estimation of drospirenone and E4 using UPLC because UPLC allows for better separation and very fast analysis than the HPLC. So, we used the UPLC technique for the separation of drospirenone and E4.
EXPERIMENTAL STUDY
Chemicals and reagents
Acetonitrile (99.99% purity) and formic acid (99.99% purity) (UPLC marked) were obtained from Merck (India) Ltd, Worli, Mumbai, India. Spectrum Pharma Research Solutions Pvt Ltd, Hyderabad, is the source of the reference standards for drospirenone and E4 APIs.
Equipment
Waters Acquity UPLC system with a quaternary pump with a PDA detector (PDA lamp) was used. Empower 2.0 was used for data processing.
Chromatographic conditions
Isocratic chromatography was carried out using a Luna C18 column (100 × 2.6 mm, 1.6 µ) at 25°C temperature to separate the samples based on their eluent composition. A mixture of 70:30 v/v acetonitrile and 0.1% formic acid was used as the mobile phase with a flow rate of 1 ml/minute, injection volume of 10 µl, and total run time of 3 minutes. A chromatographic pressure of 900 psi was maintained in the separation of drospirenone and E4. A wavelength of 262 nm was selected because the two drugs were absorbed as maximum at the 262 nm wavelength.
Preparation of buffer
To make this solution, we dissolved 1 ml of formic acid in a liter of UPLC-grade water and then filter it through a 0.45-µ filter paper (observed pH = 2.3).
Diluent
The diluent phase was applied as a moving phase.
Standard preparation
Carefully we weighed and transferred 30 mg of drospirenone and 142 mg of E4 into a volumetric flask of 100 ml capacity and added approximately 70 ml of diluents, sonicated for 30 minutes to melt it, and then added diluents up to the mark. To make up for the previous solution that was diluted down to 50 ml, we diluted the above solution to a total of 5 ml with diluents.
Sample preparation
Approximately 30 mg of drospirenone and 142 mg of E4 sample were weighed and transferred into a flask of 100 ml with 70 ml of the diluent, which was diluted to the mark with a solution containing diluents. To do this, we took 5 ml of the solution and diluted it to 50 ml. We used a 0.45 µ nylon spin-pure disposable syringe filter to obtain a clean, filtered solution.
Method validation
System suitability
System suitability parameters were measured in order to confirm system performance. Within acceptable limits, the calculated values for the other two parameters (USP plate count, USP tailing, and RSD percent) were discovered. All the data were obtained from the chromatographic software Empower 2.0.
Specificity
The capacity to evaluate the analyte unambiguously in the presence of other constituents, while considering the fact that samples and standards are commonly found in the mixture, is referred to as specificity. Drospirenone and E4 were used to screen for contamination before the samples were analyzed by UPLC.
Accuracy
Accuracy is the closeness of the test findings to the true value. In vitro and in vivo tests were conducted at three different concentrations in order to assess how well the product works. To meet the minimum requirements, three injections were administered at each level and three different quantities of the drug were used. Afterward, the recovered percentage was calculated alongside the standard deviations for each level.
Precision
The degree of agreement among multiple independent tests is known as the precision of the analytical technique. The analysis of multiple sampling of homogeneous samples was employed to learn about it. The repeatability, intra-day, and inter-day variations of the current process were analyzed. Additionally, it was checked by analyzing the samples taken on the same day at various time intervals and on different days.
Linearity
In linear analytical methods, there is an agreed upon upper and lower limit for the concentrations of the analyte, and any measurement will result in a direct correlation to that level. Six series of standard solutions were selected to examine the linearity range. The calibration curve was plotted and the regression equations were measured using peak area as opposed to the concentration of the standard solution. Using least squares, the slope, intercept, and correlation coefficient were calculated.
Stress degradation
Under no circumstances should the peaks of the forced degradation solutions used in the chromatogram be interfered with. In accordance with the ICH guidelines, stress degradation studies were conducted. In order to ensure an even degradation across the peaks, the distance between them should be at least 1.0. Only when there is separation will the principle peak purity pass. Forced degradation tests were conducted under various stress conditions (Kulkarni et al., 2016) to evaluate the degradation of approximately 20%.
Robustness
The robustness of an analytical procedure shows how resilient it is to small differences in the methodology’s parameters and indicates how reliable it is in general use. A robustness study was carried out by using the UPLC injection and a range of chromatography settings (flow rate: ± 0.2 ml/minute, mobile phase: 10% organic content, and temperature: ± 5°C). The separation factor, retention time, and peak asymmetry were estimated once the effects of the modified parameters were established.
RESULTS AND DISCUSSION
Method development
The purpose of this study was to develop a simple, accurate, and rapid RP-UPLC method for simultaneous drospirenone and E4 estimation in bulk and pharmaceutical dosage form. To optimize the chromatographic conditions, different ratios of phosphate buffer and acetonitrile in the mobile phase with isocratic and gradient modes were tested. However, the mobile phase composition was modified at each trial to enhance the resolution and also to achieve acceptable retention times. Finally, 0.1% formic acid buffer and acetonitrile with isocractic elution was selected because it resulted in a greater response of active pharmacy ingredients. During the optimization of the method, various stationary phases such as C8, C18, and amino, phenyl columns were tested (Kulkarni et al., 2016). From these trials, peak shapes were relatively good with Luna C18 column of 100 × 2.6 mm, 1.6 µ, with a PDA detector. The mobile phase flow rate was evaluated at 262 nm in order to obtain enough sensitivity. The retention time for drospirenone and E4 was 0.989 minutes and 1.878 minutes, respectively. According to the ICH guidelines, all of the proposed methods were validated. The total runtime was 3 minutes. The optimised chromatographic conditions are presented in Table 1.
System suitability
The system suitability test was completed by injecting six replicates of a standard solution containing 30 µg/ml of drospirenone and 142 µg/ml of E4. These system suitability parameters (Table 2) appeared to be within acceptable limits. System suitability chromatogram is shown in Figure 2. All the data were obtained from the chromatographic software Empower 2.0.
Specificity
There was no blanketing of drospirenone and E4 at the retention time of the molecules. In Figure 3, we can see that the chromatogram is empty.
Linearity
The area of the linearity peak versus different concentrations has been evaluated for drospirenone and E4 as 10%, 25%, 50%, 75%, 100%, 125%, and 150%, respectively. It was found that the calibration curves were linear between 3.0 and 45.0 μg per ml (µg/ml) for drospirenone and 14.0–213.0 µg/ml for E4. The value of correlation coefficient for drospirenone was 0.9992 and the regression equation was y = 18,252.64x + 87.60; for E4, the correlation coefficient was 0.9999 and the regression equation was y = 16,149.69x + 351.33. The results (Table 3) clearly show the relationship between drospirenone and E4, as demonstrated by the calibration plots (Fig. 4).
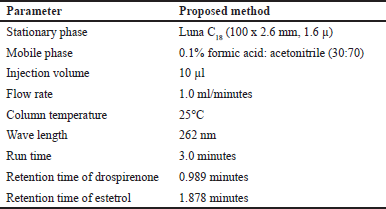 | Table 1. Optimized chromatographic conditions. [Click here to view] |
 | Table 2. Results of system suitability. [Click here to view] |
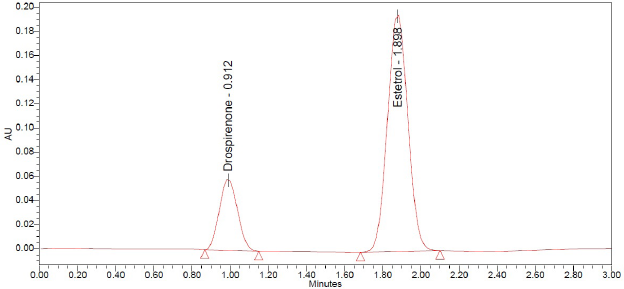 | Figure 2. Chromatogram of system suitability. [Click here to view] |
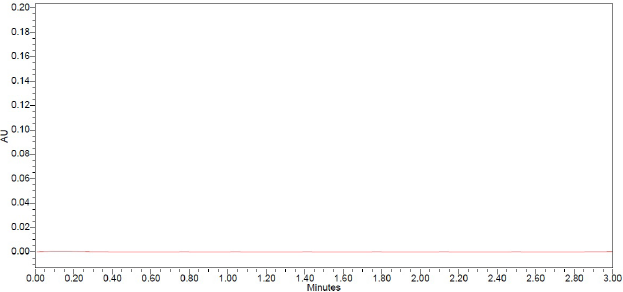 | Figure 3. Chromatogram of blank. [Click here to view] |
Precision
This method was assessed for intraday and intermediate precision variation accuracy. A sample solution of drospirenone and E4 was examined six times on the same day using six different analytical methods. These analyses were used to find the intraday studies. The research was carried out in the same laboratory by measuring the analytical precision with various analysts and with various analytical instruments. The method precision results (Table 4) show that the RSD percentage is well under 2%, which allows the methodology to be highly precise. Approximately perfect recoveries of the drug were seen at each added concentration, suggesting that the method was exact.
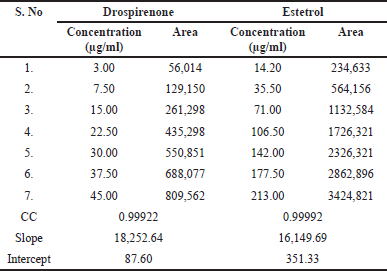 | Table 3. Results of linearity. [Click here to view] |
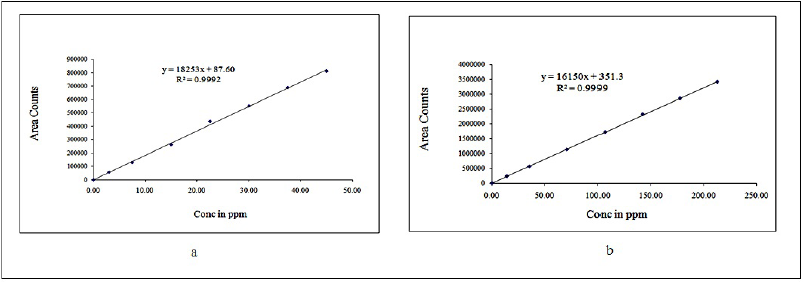 | Figure 4. Calibration plots of (a) drospirenone and (b) esterol. [Click here to view] |
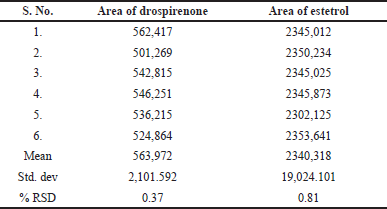 | Table 4. Results of method precision. [Click here to view] |
Intermediate precision (ruggedness)
Intermediate precision results are shown in Table 5.
Accuracy
The method’s accuracy was confirmed through the recovery experiments at three different levels (50%, 100%, and 150%). Drospirenone and E4 APIs were formulated to have 15, 30, and 45 µg/ml of drospirenone, and 71, 142, and 213 µg/ml of E4, respectively. To simulate an actual application of the test solution, three injections of the test solution were conducted and the assay was executed according to the test method. Over 90% of the patients achieved full recovery, with RSD values less than or equal to 2%. A percentage recovery calculation was carried out. The values of accuracy (Table 6) were close to what was expected, and the method was effective.
Robustness
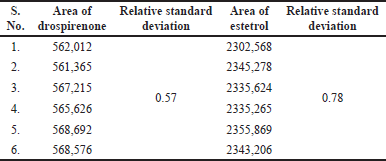 | Table 5. Results of intermediate precision. [Click here to view] |
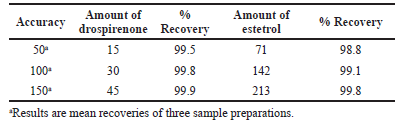 | Table 6. Results of accuracy. [Click here to view] |
The robustness of the chromatography technique was assessed by varying flow rate, composition of the mobile phase, and change in temperature. From the results (Table 7), we found that the percentage of RSD was within the acceptable range.
Forced degradation
This proposed method is effective for both release and stability studies, and as such, can be seen as a better technique for stability. Acid, base, oxidation, reduction, and thermal degradation are all part of the forced degradation study required by the ICH requirements. Dependent on the type of chromatography used, it is apparent that the drugs under consideration were stable during the stress testing, even though degraded peaks were observed. Results of forced degradation are presented in Table 8.
Acid degradation: Add 1 ml of the sample stock solution to a volumetric flask of 10 ml and add 1 ml of 1 N HCl and leave it for 15 minutes. After 15 minutes, add 1 ml of 1N NaOH and make up to the diluent mark. Filter the solution using a syringe filter and inject it into the UPLC system.
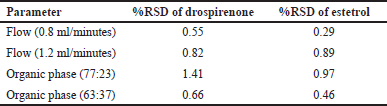 | Table 7. Results of robustness. [Click here to view] |
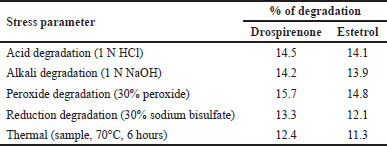 | Table 8. Results of forced degradation. [Click here to view] |
Alkali degradation: Add 1 ml of the sample stock solution to a volumetric flask of 10 ml and add 1 ml of 1N NaOH and leave it for 15 minutes. After 15 minutes, add 1 ml of 1 N HCl and make up to the mark. Filter the solution using a syringe filter and inject it into the UPLC system.
Peroxide degradation: Add 1 ml of sample stock solution to a volumetric flask of 10 ml and add 1 ml of 30% hydrogen peroxide solution and make up to the mark with diluents. Filter the solution using a syringe filter and inject it into the UPLC system.
Reduction degradation: Add 1 ml of sample stock solution to a volumetric flask of 10 ml and add 1 ml of 30% sodium bisulfate solution and make up to the mark with diluents. Filter the solution using a syringe filter and inject it into the UPLC system.
Thermal degradation: The sample solution was set in an oven at 105°C for 6 hours. The resultant solution was then injected into the UPLC system.
CONCLUSION
To quantify drospirenone and E4 in both the API and pharmaceutical dosage form, a novel, rapid, economical, sensitive, and easily available UPLC method was employed. An advantage of this technique is that no UPLC method appears to have been reported. Other merits include low operating costs, quick start-up time, low price, widespread accessibility, pinpoint sensitivity, high reliability, and easily duplicated results. When a larger number of samples must be analyzed, these properties are significant. All the parameters were tested and found to be within acceptable limits. According to the results obtained, the validity of the technique and the results gained are in fair agreement. It would be easy to implement this method for the routine use in quality control laboratories, as well as for the pharmaceutical formulations of drospirenone and E4, without a preliminary separation step.
ACKNOWLEDGMENTS
The authors thank Dr. Kantipudi Rambabu (Research guide) and Shree Icon Pharmaceutical Laboratories for all of the support they provided for this research, and they acknowledge their obligation to these two sponsors.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
None.
REFERENCES
Abdel-Misih SRZ, Bloomston M. Liver anatomy. Surg Clin North Am, 2010; 90(4):643–53. CrossRef
Abot A, Fontaine C, Buscato M, Solinhac R, Flouriot G, Fabre A, Drougard A, Rajan S, Laine M, Milon A, Muller I, Henrion D, Adlanmerini M, Valéra MC, Gompel A, Gerard C, Péqueux C, Mestdagt M, Raymond-Letron I, Knauf C, Ferriere F, Valet P, Gourdy P, Katzenellenbogen BS, Katzenellenbogen JA, Lenfant F, Greene GL, Foidart JM, Arnal JF. The uterine and vascular actions of Estetrol delineate a distinctive profile of Estrogen receptor α modulation, uncoupling nuclear and membrane activation. EMBO Mol Med, 2014; 6(10):1328–46. CrossRef
Matteson KA, Zaluski KM. Menstrual health as a part of preventive health care. Obstet Gynecol Clin North Am, 2019; 46(3):441–53. CrossRef
Catalini L, Fedder J. Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom. Biol Reprod, 2020; 102(6):1160–9. CrossRef
Dickerson LM, Mazyck P J, Hunter M H. Premenstrual Syndrome. Am Fam Physician, 2003; 67(8):1743–52.
Elias H, Bengelsdorf H. The Structure of the Liver of Vertebrates. Acta Anat, 1952; 14(4):297–337. CrossRef
Foidart JM, Jean Michel, Jean Francois Arnal. 30th annual meeting of the north America menopause society september 25–28, 2019, Chicago, IL. Menopause, 2019; 26(12):1445–81. CrossRef
Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol, 2014; 142:115–20. CrossRef
Gillatt D. Antiandrogen treatments in locally advanced prostate cancer: are they all the same?. J Cancer Res Clin Oncol, 2006; 1:S17–26. CrossRef
Gomathy N, Dhanasekar KR, Trayambak D, Amirtha R. Supportive therapy for dysmenorrhea: time to look beyond mefenamic acid in primary care. J Family Med Prim Care, 2019; 8(11):3487–91. CrossRef
Han L, Jensen JT. Does the Progestogen used in combined hormonal contraception affect venous thrombosis risk?. Obstet Gynecol Clin North Am, 2015; 42(4):683–98. CrossRef
Holinka CF, Diczfalusy E, Bennink HJTC. Estetrol: a unique steroid in human pregnancy. J Steroid Biochem Mol Biol, 2008; 110(1–2):138–43. CrossRef
Iversen P, Melezinek I, Schmidt A. Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function. BJU Int, 2001; 87(1):47–56. CrossRef
Kolkhof P, Bärfacker L. 30 years of the mineralocorticoid receptor: Mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol, 2017; 234(1):T125–40. CrossRef
Kosaka H, Hirayama K, Yoda N, Sasaki K, Kitayama T, Kusaka H, Matsubara M. The L, N-, and T-type triple calcium channel blocker benidipine acts as an antagonist of mineralocorticoid receptor, a member of nuclear receptor family. Eur J Pharmacol, 2010; 635(1–3):49–55. CrossRef
Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric, 2005; 8(Suppl. 1):3–63. CrossRef
Kundu N, Grant M, Radioimmunoassay of 15a-hydroxyestriol (estetrol) in pregnancy serum. Steroids, 1976; 27:785–96. CrossRef
Langer RD, Hodis HN, Lobo RA, Allison MA. Hormone replacement therapy - where are we now?. Climacteric, 2021; 24(1):3–10. CrossRef
Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol, 2005; 19(8):1951–9. CrossRef
Maclennan A H, Broadbent J L, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev, 2004;2004(4):CD002978. CrossRef
Majumder A, Chatterjee S, Maji D, Roychaudhuri S, Ghosh S, Selvan C, George B, Kalra P, Maisnam I, Sanyal D. IDEA group consensus statement on medical management of adult gender incongruent individuals seeking gender reaffirmation as female. Indian J Endocrinol Metab, 2020; 24(2):128–35. CrossRef
Majumder A, Sanyal D. Outcome and preferences in male-to-female subjects with gender dysphoria: experience from Eastern India. Indian J Endocrinol Metab, 2017; 21(1):21–5. CrossRef
Nath A, Sitruk-Ware R. Different cardiovascular effects of progestins according to structure and activity. Climacteric, 2009; 12(Suppl1):96–101. CrossRef
Oelkers W. Antimineralocorticoid activity of a novel oral contraceptive containing drospirenone, a unique progestogen resembling natural progesterone. Eur J Contracept Reprod Health Care, 2002; 7(Suppl3):19–26. discussion 42–3.
Oelkers W. Drospirenone a new progestogen with antimineralocorticoid activity, resembling natural progesterone. Eur J Contracept Reprod Health Care, 2000; 5(Suppl. 3):17–24.
Pearlstein T. Treatment of premenstrual dysphoric disorder: Therapeutic challenges. Expert Rev Clin Pharmacol, 2016; 9(4):493–6. CrossRef
Kulkarni RM, Bhamare VS, Santhakumari B. Mechanistic and spectroscopic investigations of Ru+3 catalyzed oxidative degradation of azidothymidine by heptavalent manganese at environmentally relavant pH. Desalin Water Treat, 2016; 57(58):28349–62. CrossRef
Kulkarni RM, Bhamare VS, Santhakumari B. Oxidative transformation of antiretroviral drug zidovudine during water treatment with permanganate: reaction kinetics and pathways. Desalin Water Treat, 2016; 57(52):24999–5010. CrossRef
Reid RL, Soares CN. Premenstrual dysphoric disorder: contemporary diagnosis and management. J Obstet Gynaecol Can, 2018; 40(2):215–23. CrossRef
Salzman B, Fleegle S, Tully AS. Common breast problems. Am Fam Physician, 2012; 86(4):343–9.
Scott AM, Stehlik P, Clark J, Zhang D, Yang Z, Hoffmann T, Mar CD, Glasziou P. Blue-light therapy for acne vulgaris: a systematic review and meta-analysis. Ann Fam Med, 2019; 17(6):545–53. CrossRef
Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab, 2015; 100(11):3975–4011. CrossRef
Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA, 2001; 285(22):2891–7. CrossRef
Whitehead M. Hormone replacement therapy with estradiol and Drospirenone: an overview of the clinical data. J Br Menopause Soc, 2006; 12(Suppl. 1):4–7. CrossRef
Wiegratz I, Kuhl H. Progestogen therapies: differences in clinical effects? Trends Endocrinol Metab, 2004; 15(6):277–85. CrossRef