UPLC method development and validation for Cefditoren Pivoxil in active pharmaceutical Ingredient
Ram Garg, Navneet Singh, Kona S. Srinivas, Binayak Deb, Ayaz Ahmed
Pages: 149-153

A Novel UPLC–MS/MS Method for Determination of γ-Amino Butyric acid Analogue in Human Plasma: Application to Pharmacokinetic Study
Anju Aji, Sarita Karthikeyan, Sarabjit Singh, Shivanand P. Puthli
DOI: 10.7324/JAPS.2013.3630Pages: 172-178

Development and validation of UPLC method for simultaneous estimation of Efavirenz and Lamivudine in pharmaceutical formulations
Madhusudhanareddy Induri, Bhagavan Raju Mantripragada, Rajendra Prasad Yejella
DOI: 10.7324/JAPS.2016.60305Pages: 029-033

Simultaneous determination of ciprofloxacin hydrochloride and metronidazole in spiked human plasma by ultra performance liquid chromatography-tandem mass spectroscopy
Ramzia El-bagary, Asmaa Ahmed El-Zaher, Ehab Elkady, Asmaa Abdelkerim Mandour
DOI: 10.7324/JAPS.2016.60307Pages: 041-047

A new rapid Stability indicating RP-PDA-UPLC method for the estimation of Assay of Pemetrexed disodium-An anti-Lung cancer drug from lyophilized parenteral formulation
Vamsi Krishna Galla, V. Archana, Rajeswari Jinka
DOI: 10.7324/JAPS.2017.71019Pages: 131-137

Development and Validation of the UPLC Method for the Simultaneous Assay of the Compounding Ointment Components
Lesia P. Savchenko, Liudas Ivanauskas, Victoriya A. Georgiyants
DOI: 10.7324/JAPS.2018.8508Pages: 061-067

A Sensitive, Stability indicating UPLC method for the identification and characterization of forced degradation products for Drometrizole Trisiloxane through MSn studies
M. Ajay Babu, G. V. Krishna Mohan, J. Satish, Pradipbhai D. Kalariya, CH. Krishnam Raju, Sharad D. Mankumare
DOI: 10.7324/JAPS.2018.8609Pages: 065-074

Efficient validated method of UPLC-MS/MS to determine curcumin in rat plasma and ovarium
Wenny Trias Ramadanty, Wawaimuli Arozal, Melva Louisa, Vivian Soetikno, Sigit Purbadi, Priyanto Priyanto
DOI: 10.7324/JAPS.2019.90109Pages: 058-065

Isolation of flavonol rhamnosides from Pometia pinnata leaves and investigation of α-glucosidase inhibitory activity of flavonol derivatives
Fadhila Utari, Afrizal Itam, Syafrizayanti Syafrizayanti, Widya Hasvini Putri, Masayuki Ninomiya, Mamoru Koketsu, Kaori Tanaka, Mai Efdi
DOI: 10.7324/JAPS.2019.90808Pages: 053-065

Development of stability indicating method for the simultaneous estimation of alogliptin and pioglitazone in bulk and tablet dosage form by reversed-phase ultra-performance liquid chromatography method
Ramesh Dhani, Harish Kumar Donthi Ramachandra Guptha, Deveswaran Rajamanickam
DOI: 10.7324/JAPS.2019.91208Pages: 051-056
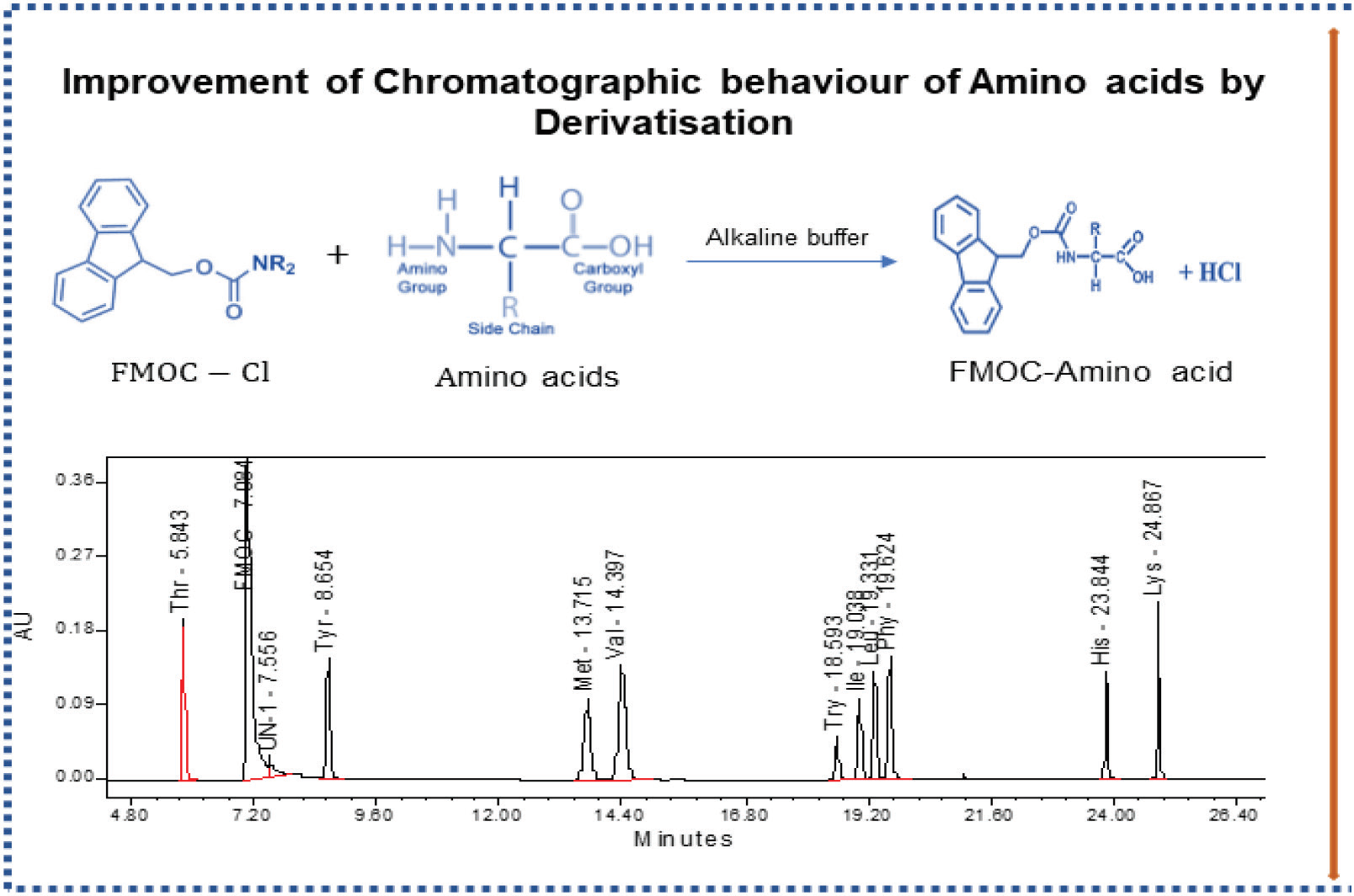
Application of total error concept in the analytical method validation for the assay of essential amino acids by precolumn derivatization
Ramachandra Reddy Aasodi, Murugan V, Premakumari KB
DOI: 10.7324/JAPS.2020.10505Pages: 031-042
Productivity of L-DOPA in in vitro shoots of Mucuna pruriens var. utilis enhanced by gamma radiation
Detmontree Wachisunthon, Sorrapetch Marsud, Subhadhcha Poonsatha, Suwimol Jetawattana, Worapan Sitthithaworn
DOI: 10.7324/JAPS.2021.110109Pages: 084-088

Development and validation of UPLC method for quantitative estimation of related impurities in tizanidine hydrochloride tablets
Sanjay Shesha Shetgar, Ramadevi Dharmasoth, Basavaiah Keloth, Bandlamudi Mallikarjuna Rao
DOI: 10.7324/JAPS.2021.110807Pages: 043-053

New validated Reverse Phase Ultra Performance Liquid Chromatography method for drospirenone and estetrol in Active Pharmaceutical Ingredient and tablet form and its stress studies
Rafi Syed, Rambabu Kantipudi
DOI: 10.7324/JAPS.2021.1101015Pages: 106-112
RP-UPLC method development and validation for simultaneous estimation of Etoricoxib and Thiocolchicoside in tablets
Sanjay Shesha Shetgar, Ramadevi Dharmasoth, Bandlamudi Mallikarjuna Rao, Basavaiah Keloth
DOI: 10.7324/JAPS.2021.120214Pages: 144-151

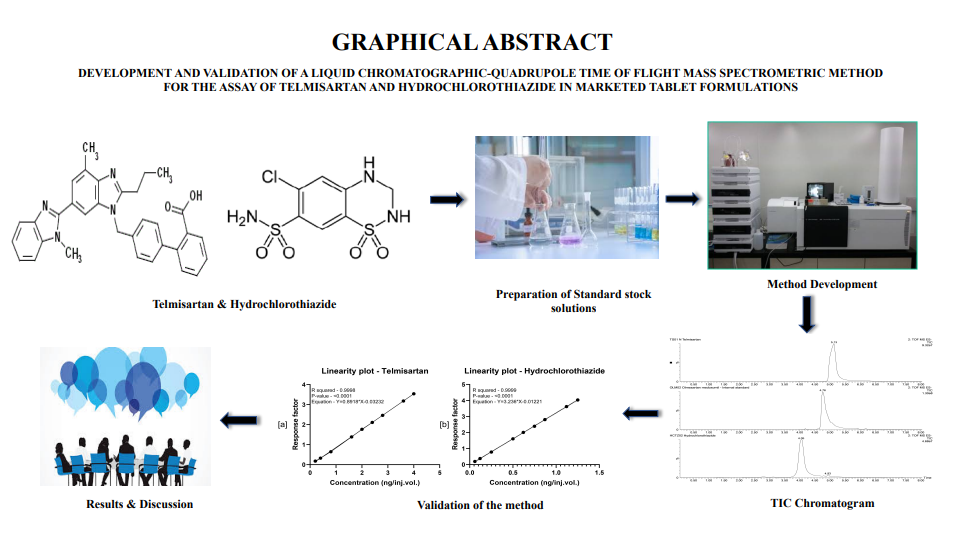
Development and validation of a liquid chromatographic-quadrupole time of flight mass spectrometric method for the assay of telmisartan and hydrochlorothiazide in marketed tablet formulations
Vignesh Madhavan, Kowmudi Gullapalli, Anoop Karthika, Ramalingam Peraman, Krishnaveni Nagappan
DOI: 10.7324/JAPS.2021.120207Pages: 066-074
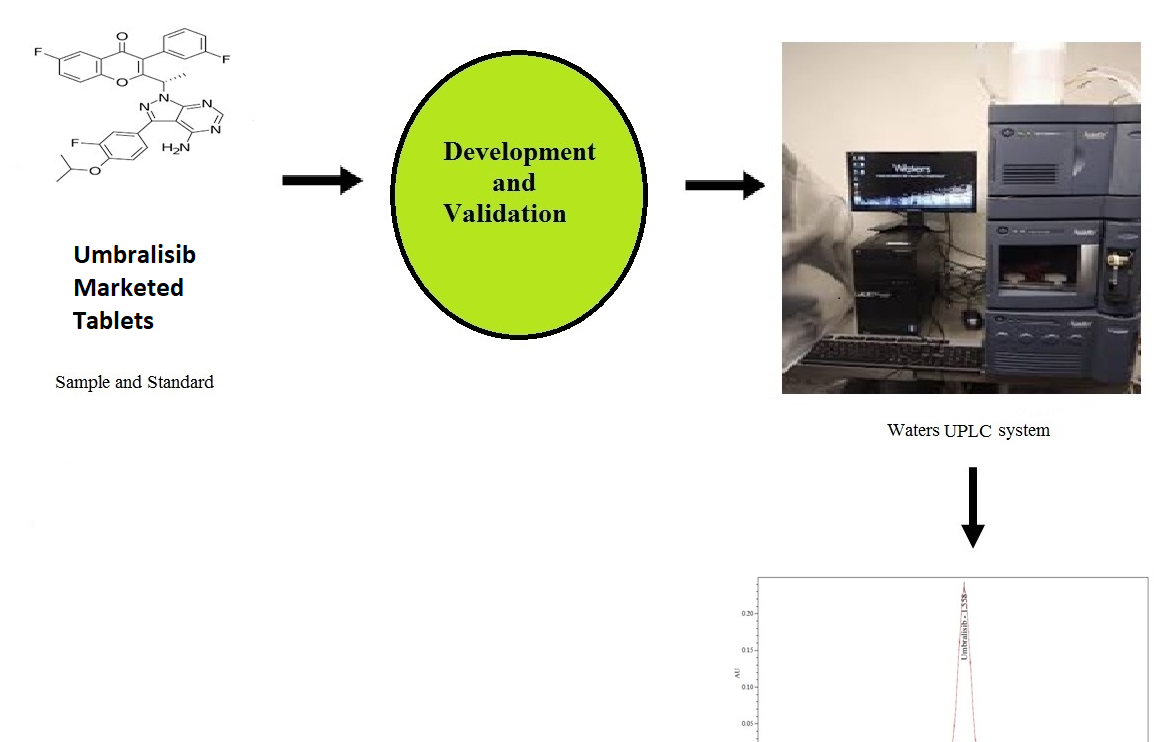
Determination of Umbralisib using reverse phase ultra performance liquid chromatography in bulk and pharmaceutical dosage form
Ramadevi Potturi, Rambabu Kantipudi
DOI: 10.7324/JAPS.2021.120218Pages: 172-178
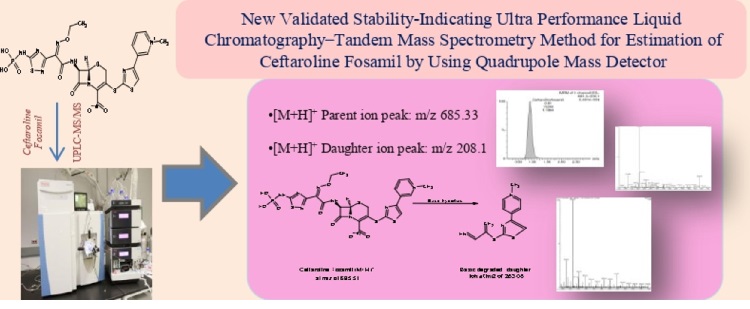
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
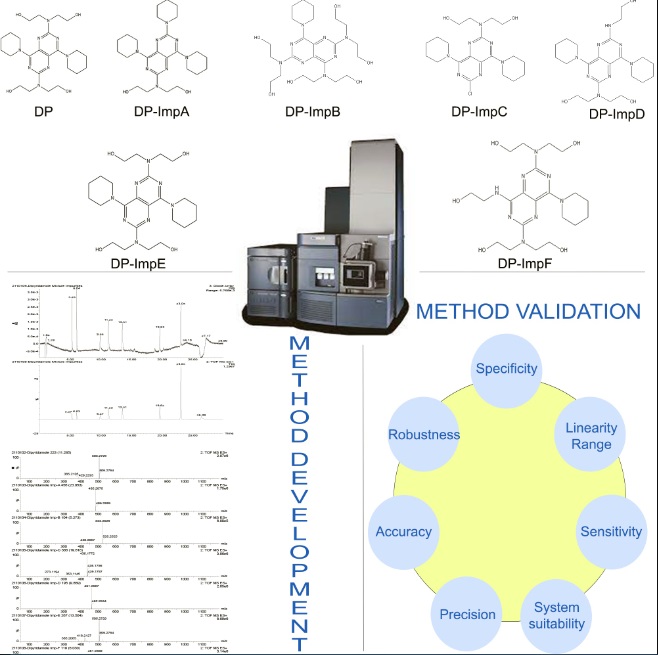
UPLC-Q-TOF-MS method development and validation for simultaneous analysis of dipyridamole and its related impurities
T. Menaka, Ramya Kuber
DOI: 10.7324/JAPS.2023.130120Pages: 201-211
Cytotoxicity and fragmentation pattern of Datura metel L. leaves using ultra-performance liquid chromatography-mass spectroscopy
Mohammad Adam Mustapa, Muhammad Taupik, A. Muthi Andi Suryadi, Mahdalena Sy Pakaya, Julyanty Akuba, Endah Nurrowinta Djuwarno, Warsono El Kiyat
DOI: 10.7324/JAPS.2023.87779Pages: 057-067

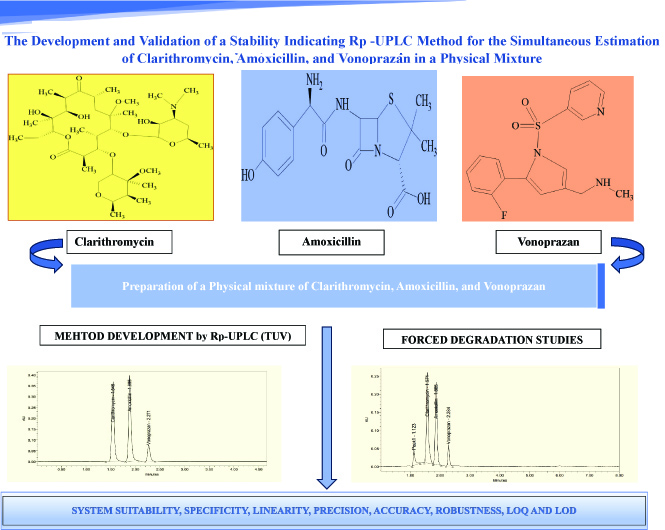
The development and validation of a stability indicating RP-UPLC method for the simultaneous estimation of clarithromycin, amoxicillin, and vonoprazan in a physical mixture
Charumathi Salva, Rajitha Galla
DOI: 10.7324/JAPS.2024.165836Pages: 193-202
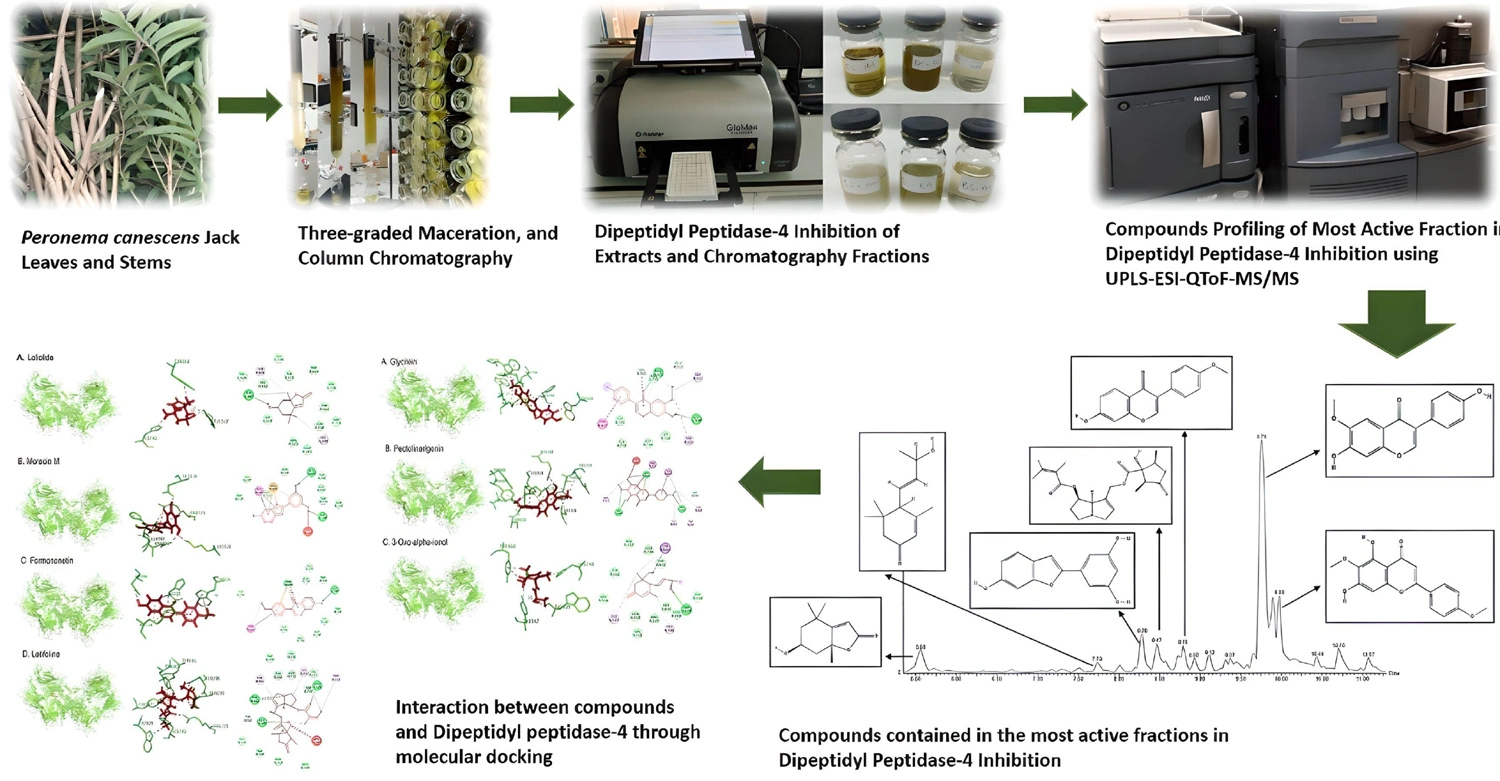
Dipeptidyl peptidase-4 inhibition of Peronema canescens Jack leaves and stems: Bioassay-guided fractionation, compound profiling by LC-MS/MS, and interaction mechanism
Berna Elya, Roshamur Cahyan Forestrania, Najihah Mohd Hashim, Nita Triadisti
DOI: 10.7324/JAPS.2024.161007Pages: 090-101
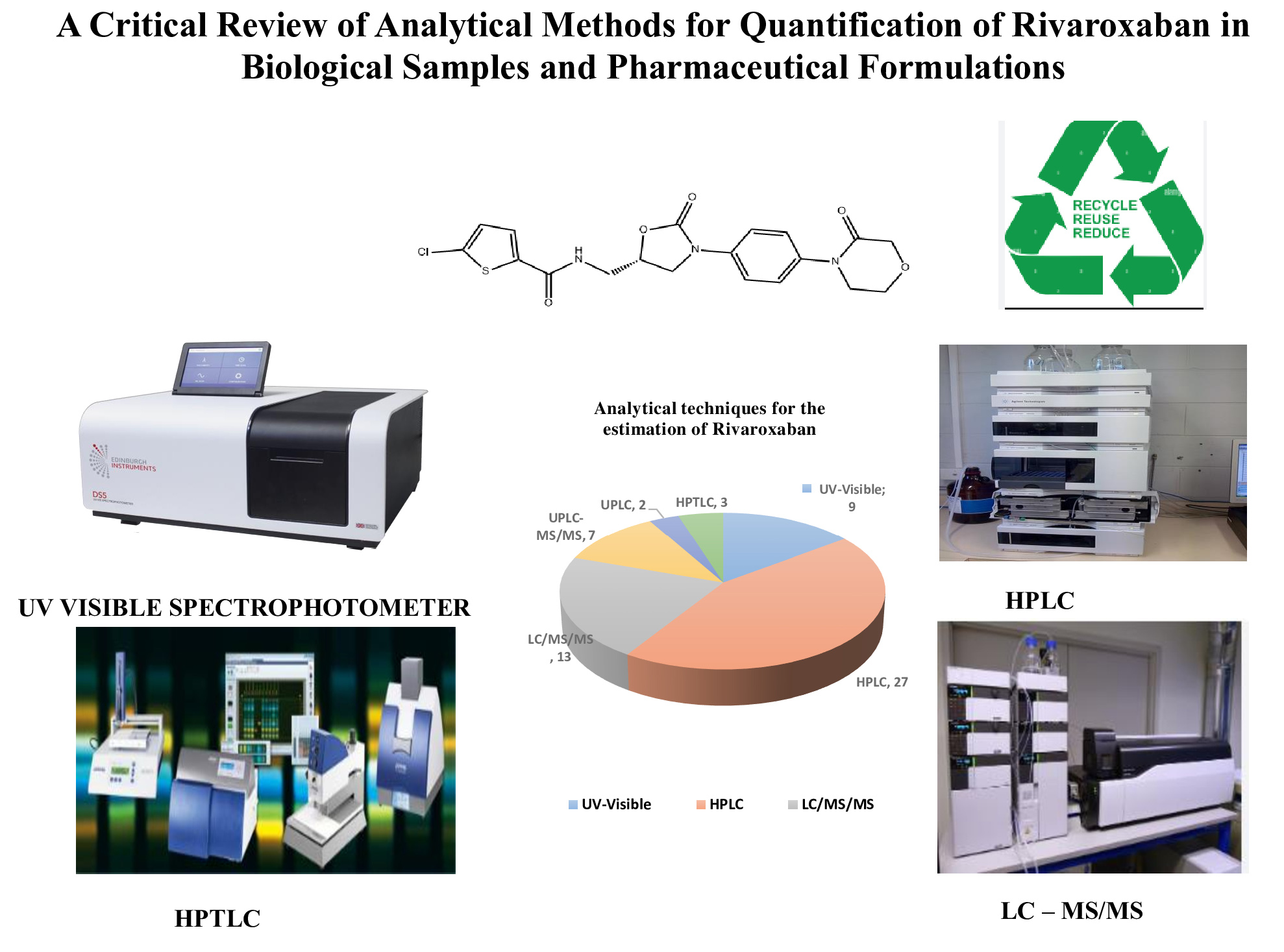
A critical review of analytical methods for quantification of rivaroxaban in biological samples and pharmaceutical formulations
Ajitha Azhakesan, Kishore Kumar Pasupuletti, Narendra Pentu
DOI: 10.7324/JAPS.2025.210368Pages: 067-085
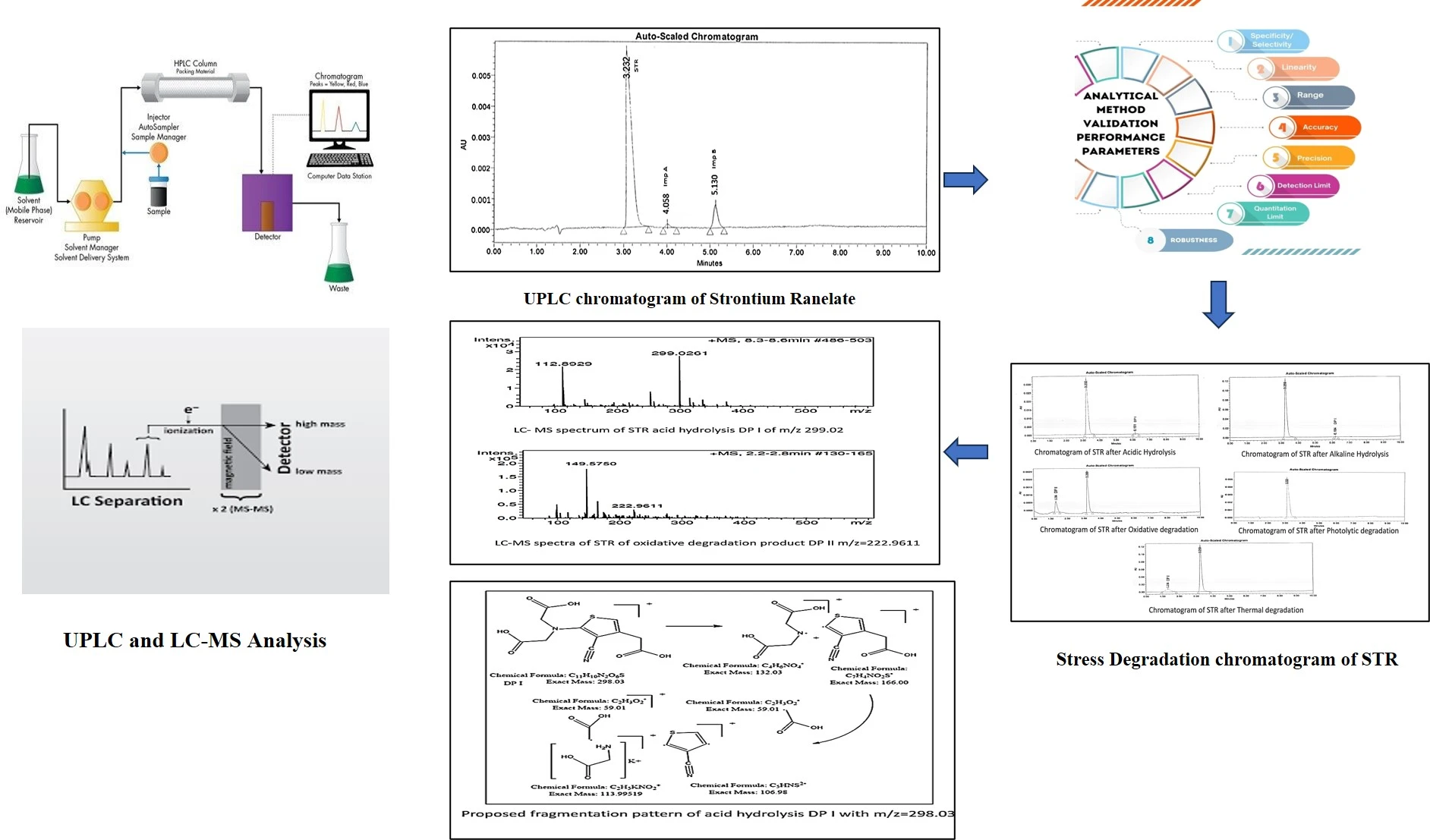
Impurity profiling of the strontium ranelate using stability indicating UPLC method and structural elucidation of degradants by LC-MS-TOF with the greenness assessment
Arti Swami, Aarti Shastri, Vishnu Choudhari
DOI: 10.7324/JAPS.2025.234119Pages: 132-141
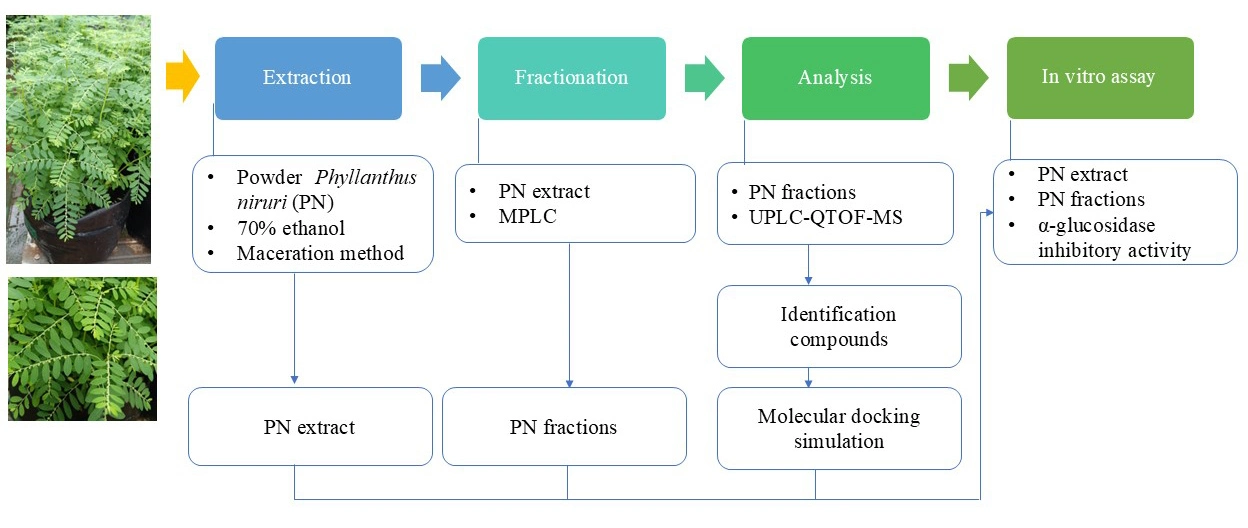
In vitro assessment of α-glucosidase inhibitory activity and compound prediction in Phyllanthus niruri from West Java Indonesia
Idah Rosidah, Alfan Danny Arbianto, Firdayani Firdayani, Agus Supriyono, Kurnia Agustini, Ngatinem Ngatinem, Sri Ningsih
DOI: 10.7324/JAPS.2025.v15.i12.19Pages: 206-218