INTRODUCTION
A novel, advanced, fifth-generation, broad-spectrum cephalosporin parental antibiotic, namely Ceftaroline Fosamil (Fig.1) ((6R,7R)-7-[[(2Z)-2-ethoxyimino-2-[5-(phosphonoamino)-1,2,4-thiadiazol-3yl]acetyl]amino]-3-[[4-(1-methylpyridin-1-ium-4-yl)-1,3-thiazol-2-yl]sulfanyl ]-8-oxo-5-thia-1azabicyclo[4.2.0]oct-2-ene-2-carboxylate), is a potent bactericidal. It has potent activity against a broad range of bacteria, especially methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumonia (Gram-positive), Moraxella catarrhalis (Gram-negative), including positive lactamase strains, such as Haemophilus influenza, and bacteria with various resistance phenotypes (Kaushik et al., 2011).
A hyphenated methodology is a valuable tool for assessing drugs in various biological samples (Junza et al., 2011; Junza et al., 2014; Van den Meersche et al., 2016). The hyphenated technique, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS), couples a chromatographic system to a spectroscopic system with the appropriate interface. It is well known that the performance features of ultra-performance liquid chromatography (UPLC) benefit detection significantly. By optimizing sample pretreatment and minimizing analysis time, LC-MS/MS can detect over 300 compounds of different classes with a minimal injection volume. On the other hand, MS/MS measures the compound’s m/z with its intermediates (by-products) (Glish and Burinsky, 2008).
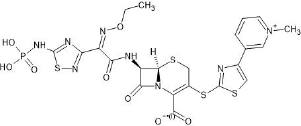 | Figure 1. Chemical structure of Ceftaroline Fosamil. [Click here to view] |
Various chromatography-based assays were published to estimate Ceftaroline Fosamil (Alarcia lacalle et al., 2021; Gregoire et al., 2016; Izabela et al., 2017; Reddy Govardhan et al., 2018; Suneetha and Venkanna, 2013). But none of the current analytical methods have reported measurements of Ceftaroline Fosamil on hyphenated techniques, such as UPLC-MS/MS. The proposed methodology is simple, with easy sample pretreatment and fast analysis by optimizing all chromatographic and mass detection parameters at various stages of drug analysis. The purpose is to develop a sensitive, selective, and accurate method for analyzing Ceftaroline Fosamil on the UPLC column coupled to MS/MS by using triple quadrupole (QQQ) mass detection. Acetonitrile (ACN) and 0.1% formic acid in water, in a proportion of 70:30 v/v, were used as the mobile phase. The proposed UPLC-MS/MS method was validated, and the forced degradation studies were conducted for Ceftaroline Fosamil.
MATERIALS AND METHODS
Chemicals and reagents
All the chemicals were analytical grade with 99.0% purity. Ceftaroline Fosamil standard (99.8%) was obtained from Clear Synth (Hyderabad). The LC-grade water, ACN, and methanol were procured from Merck (Mumbai, India). Formic acid and all other reagents were purchased from SD Fine Chemicals Ltd. The Zinforo injection (Ceftaroline Fosamil 600 mg) was purchased from Apollo Pharmacy (Hyderabad).
Instrumentation and chromatographic conditions
All chromatographic separations were achieved on a hyphenated UPLC system coupled to MS/MS (UPLC-MS/MS-All Waters, USA). Acquity C18 UPLC BEH (50 × 2.1 mm, 1.7 µm) was used as the analytical column with a QQQ mass detector using MassLynx software. ACN and 0.1% formic acid in water, in a proportion of 70:30 v/v, were used as the mobile phase. The mobile phase flow rate was 0.35 ml/minute with isocratic elution mode. The column temperature was maintained at 30°C with an injection volume of 5 μl and the runtime was 3 minutes. The mass spectrophotometer operated in MRM mode with positive electrospray ionization (+ESI). The typical optimized MS/MS parameters included are capillary voltages of 3.00 and 2.94 kV, cone voltages of 40 and 43 V, source temperatures of 120°C and 119°C, desolvation gas flow of 850 l/hour, cone gas flow of 102 l/hour, nebulizer gas flow of 7 bars, ion energy of 2 1.0, syringe pump flow of 100 l/minute, pressure of 6.54e-3, collision gas flow of 0.15 ml/minute, collision energy of 40–41 eV, and with dwell seconds of 0.20.
Preparation of diluent solution
The diluent used was a 50:50 v/v mixture of methanol and water. The diluent was used to obtain blank mass spectra.
Preparations of standard stock solution
A standard stock solution (stock solution A) of Ceftaroline Fosamil was prepared using a diluent solvent with a concentration of 1,000 µg/ml. Stock solution A was diluted to get standard solutions of Ceftaroline Fosamil with concentrations of 1,000 and 100 ng/ml, respectively.
Preparation of sample solution (marketed formulation)
Each ampoule has 600 mg of Ceftaroline Fosamil (Zinforo) and 10.66 mg of injection powder was dissolved in 10 ml of diluent to give a 1,000 µg/ml solution. It was diluted further to a concentration of 1,000 ng/ml.
Preparation of the mobile phase
ACN and 0.1% formic acid in water, in a 70:30 v/v ratio, were prepared and used as the mobile phase. Mixed solvents were sonicated and employed as the mobile phase.
VALIDATION PROCEDURE
Parameters like precision, linearity, specificity, accuracy, LOQ, system suitability, and LOD were determined to evaluate the validation for the proposed Ceftaroline Fosamil UPLC-MS/MS method.
System suitability
Many analytical methods, include system suitability testing as an intrinsic and essential part of the procedure, analytical procedures with tailing factor < 2, Relative Standard Deviation (RSD) < 1%, and theoretical plates > 2,000, will show the appropriateness of the method (Shabir, 2003). The system compatibility of the proposed analytical method was determined by analyzing six samples of Ceftaroline Fosamil of 1,000 ng/ml.
Specificity
To evaluate the specificity, a blank sample (methanol: water of 50:50 v/v) and working standard (1,000 ng/ml) were prepared as per the developed method and injected into the UPLC-MS/MS system.
Linearity and range
Seven linear concentration solutions were prepared from the standard stock solution by dilution to obtain calibration standards ranging from 100 to 1,500 ng/ml and to evaluate the linearity of the method proposed. The calibration curve is plotted against the area using the above range of solutions. Slope, intercept, and correlation coefficient (r2) was used to evaluate linearity.
Sensitivity
By injecting the range of diluted solutions of known concentration, the LOD and LOQ were determined using signal-to-noise (S/N) ratios of 3:1 and 10:1, respectively.
Precision (%repeatability)
Precision was measured by injecting (n = 6) 100 ng/ml of Ceftaroline Fosamil. Intraday and interday precisions were measured using Ceftaroline Fosamil standard solutions with concentrations of 1,000, 500, and 100 ng/ml, six times on the same day and six times on other days, respectively. The results were expressed as %RSD.
Accuracy
Accuracy was measured at three concentration levels, 50%, 100%, and 150%, of the standard solutions. A preanalyzed sample solution (1,000 ng/ml) was spiked with 50%, 100%, and 150% standard solutions prior to analysis, and the results were reported as a percentage recovery.
Robustness
Robustness was measured by altering the experimental parameters, such as injection volume, column temperature, and flow rate. Flow rate was modified to 0.32 ml/minute and 0.37 ml/minute, respectively. The effect of temperature on the column was studied at 27°C and 32°C. The mobile phase composition was not altered in any of these experiments.
Solution stability
A solution containing 1,000 ng/ml Ceftaroline Fosamil standard was kept at room temperature over 24 hours to check its stability. Ceftaroline Fosamil (1,000 ng/ml) was injected thrice to evaluate solution stability. The percentage RSD was used to express the results.
Assay procedure
5 µl standard and sample solutions (1,000 ng/ml) were injected separately into the chromatographic system. The assay of the sample solution was determined using the peak area.
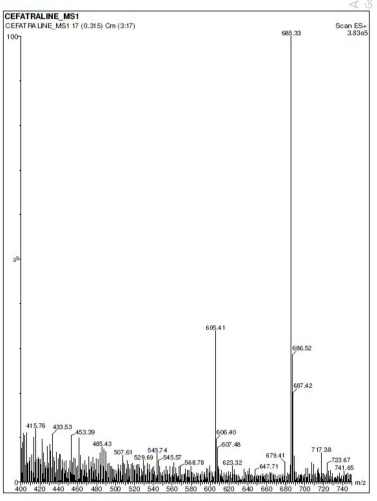 | Figure 2. Mass spectrum of the parent ion of Ceftaroline Fosamil. [Click here to view] |
Forced degradation studies of Ceftaroline Fosamil
UPLC-MS/MS was used to conduct the forced degradation studies for Ceftaroline Fosamil. The forced degradation of Ceftaroline Fosamil was accelerated by using 0.5 ml of 1N HCl, 0.5 ml of 1N NaOH, 0.5 ml of water, and 0.5 ml of 3% peroxide solution for forced acidic, basic, neutral, and oxidation stress studies, respectively. The standard solution was kept in a hot air oven at 70°C for 60 minutes to accelerate thermal degradation. Accurately 0.5 ml of standard Ceftaroline Fosamil (10 µg/ml) was added to the stress-specific reagent, and the final volume was made to 5 ml by adding the diluent. The final solution injected into the UPLC-MS/MS system of Ceftaroline Fosamil was 1,000 ng/ml. All the solutions were analyzed at regular intervals for 1 week, every 6, 12, and 24 hours.
RESULTS AND DISCUSSION
Chromatographic conditions
MS analysis confirmed that the molecule is Ceftaroline Fosamil and found that the mass spectrum of [M+H]+ peak (parent ion peak) is at m/z 685.33. [M+H]+ fragmented peak (daughter ion peak) after ionization was found at m/z 208.1. Ceftaroline Fosamil was quantified using +ESI in positive MRM mode at the transition pair m/z 685.30 → 208.10. The compound Ceftaroline Fosamil showed good resolution with a retention time of 0.91 ± 0.04 minute when a C18 column (Aquity UPLC BEH) was used with 0.1% formic acid and ACN (30:70 v/v) as the mobile phase at 0.35 ml/minute flow rate and 100 ng/ml concentration. The [M+H]+ spectra of Ceftaroline Fosamil parent ion and fragmented daughter ion are shown in Figures 2 and 3, respectively.
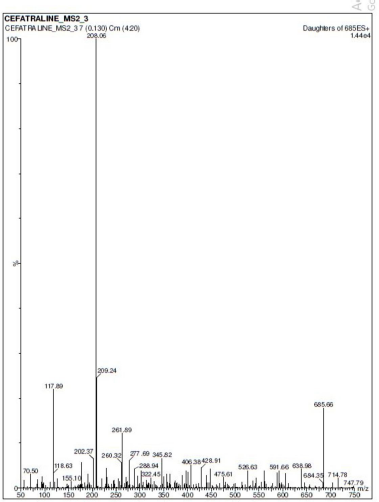 | Figure 3. Mass spectrum of the daughter ion of Ceftaroline Fosamil. [Click here to view] |
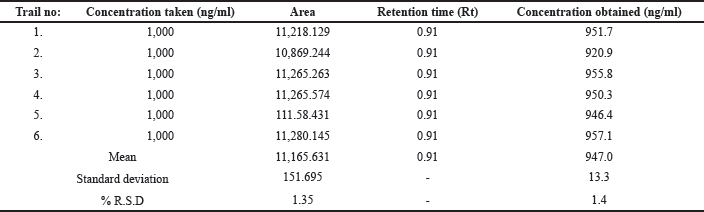 | Table 1. System suitable parameters for Ceftaroline Fosamil by UPLC-MS/MS. [Click here to view] |
Validation
For system suitability parameters, peak area and concentration were determined with %RSD of 1.35 and 1.4, respectively. The tailing factor was equal to 1.16. Table 1 presents the results. The retention time, peak area, and peak symmetry are all within acceptable limits, indicating the suitability of the proposed method. The specificity was evaluated using a blank sample (methanol: water of 50:50 v/v) and working standard (1,000 ng/ml). The result found was that Ceftaroline Fosamil [M+H]+ had a retention time of 0.91 minute with a 685.3 m/e ratio with respect to the blank sample. Figure 4 shows specific chromatograms of blank and standard Ceftaroline Fosamil peaks. The linearity of the proposed method was measured by constructing a calibration curve in a range of 100–1,500 ng/ml. The findings reveal that the peak area and analyte concentration have an excellent correlation with linearity in the investigated concentration range with Y = 11.3263 * × + 439.292 (R2 = 0.99) as the linear regression equation. Figure 5 shows the calibration curve. Table 2 presents the linearity results. The sensitivity was measured for LOD at 30 ng/ml and LOQ at 100 ng/ml, using signal-to-noise (S/N) ratios of 3:1 and 10:1, respectively. Figure 6 shows the chromatograms at LOD and LOQ levels. The precision was measured as intraday and interday precisions at three concentrations. The %RSD for intraday results at 1,000, 500, and 100 ng/ml was found to be 1.40, 1.09, and 2.01, respectively. The %RSD for interday results at 1,000, 500, and 100 ng/ml were found to be 1.1, 1.3, and 2.5 respectively. Table 3 presents the precision results. Percentage recovery at 50%, 100%, and 150% were 106.98%, 95.48%, and 95.48%, respectively. Table 4 shows the accuracy results for the proposed method. Method robustness was measured by modifying flow rate, column temperature, and %RSD of the same was within 2%. Table 5 presents the method robustness results. %RSD of solutions stability was <2%. Table 6 shows solution stability results.
The percentage assay of marketed Zinforo (600 mg) was found to be 99.63 ± 0.58 with the amount obtained as 598.53 mg.
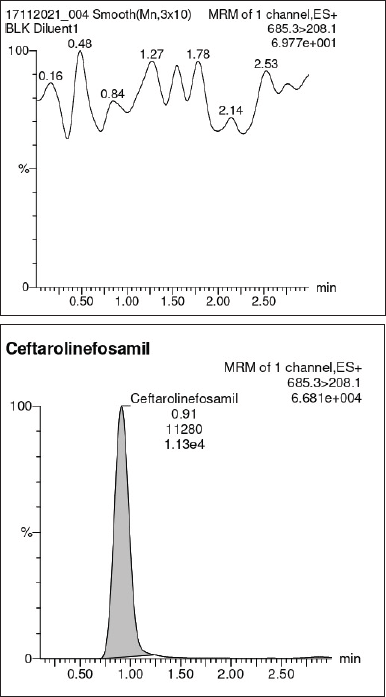 | Figure 4. Specificity. (A) Blank. (B) Working standard of Ceftaroline Fosamil. [Click here to view] |
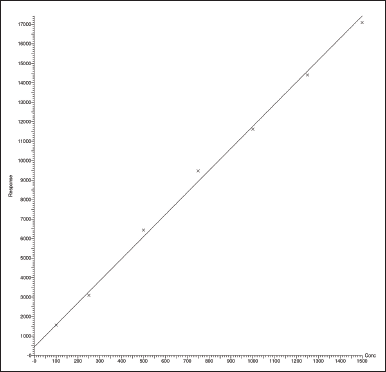 | Figure 5. Linear calibration curve for Ceftaroline Fosamil. [Click here to view] |
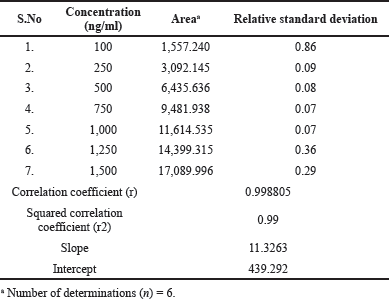 | Table 2. Linearity of Ceftaroline Fosamil by UPLC-MS/MS. [Click here to view] |
Forced degradation studies
In acidic conditions, the retention time of forced degradation acidic solution was found to be 0.87 minute with no degradation of Ceftaroline Fosamil, indicating its stability in acidic solution. In basic conditions, the retention time of forced basic solution of Ceftaroline Fosamil was at 1.39 minutes with a degradant fragment at an m/z ratio of 263.08, indicating its instability in basic medium and that it has degraded completely with no Ceftaroline Fosamil parent peak (Niessen, 2010). Figure 7 shows the degradant fragmentation of Ceftaroline Fosamil in the basic medium. Figure 8 shows the MS spectra of Ceftaroline Fosamil in forced basic condition. In neutral oxidation and thermal conditions, the retention time of forced degradation solutions was found to be 0.87 minute with no degradation of Ceftaroline Fosamil, indicating its stability in water, 3% hydrogen peroxide solution, and heat. In thermal conditions, it was observed that the peak area obtained has slightly deviated. Table 7 shows a brief summary of the Stability Indicating Assay Method (SIAM) method for Ceftaroline Fosamil using UPLC-MS/MS.
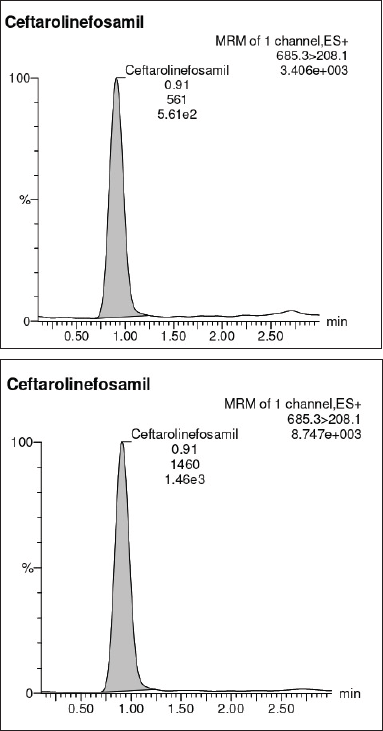 | Figure 6. Sensitivity. (A) LOD. (B) LOQ. [Click here to view] |
DISCUSSION
Although many methods for estimating Ceftaroline Fosamil using various chromatography techniques, such as HPLC or LC-MS are available, the current method was found to be more accurate, precise, and faster by utilizing an advanced hyphenated UPLC-MS/MS technology. A comparison of the published LC-MS/MS (Izabela et al., 2017) and the current proposed work is shown in Table 8. The main focus of the proposed work was to develop a UPLC-MS/MS method and apply this method to study the stability studies of Ceftaroline Fosamil. On the contrary, this method enables analysts to estimate Ceftaroline Fosamil samples using simple and uncomplicated methods involving no complex samples preparation or mobile phase, or use of buffer solution, resulting in an eco-friendly greenness method.
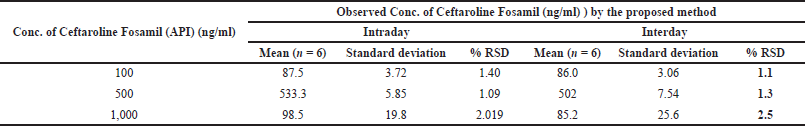 | Table 3. Intraday and interday precision of Ceftaroline Fosamil by UPLC-MS/MS. [Click here to view] |
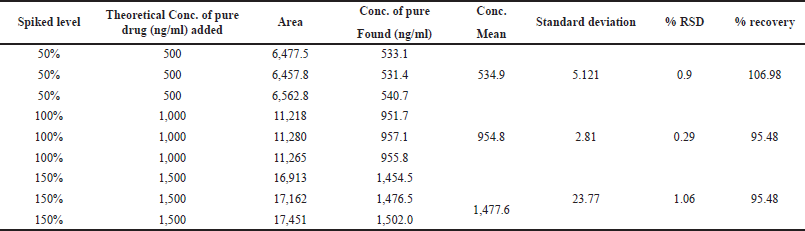 | Table 4. Accuracy and %recovery of the spiked samples of Ceftaroline Fosamil by UPLC-MS/MS. [Click here to view] |
 | Table 5. Robustness of the proposed method by UPLC-MS/MS for Ceftaroline Fosamil. [Click here to view] |
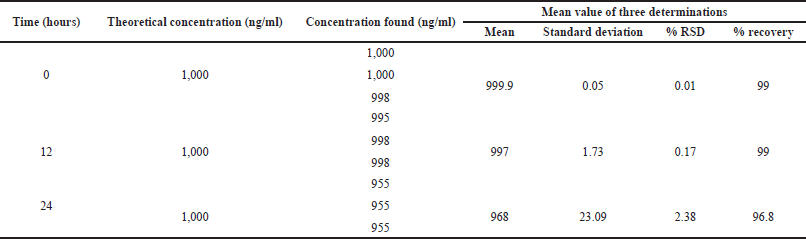 | Table 6. Solution stability of Ceftaroline Fosamil. [Click here to view] |
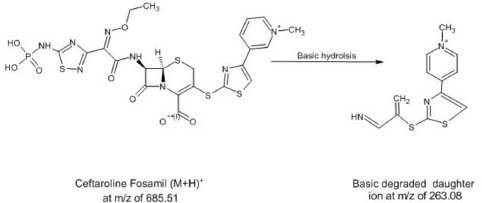 | Figure 7. Fragmentation of Ceftaroline Fosamil in basic medium. [Click here to view] |
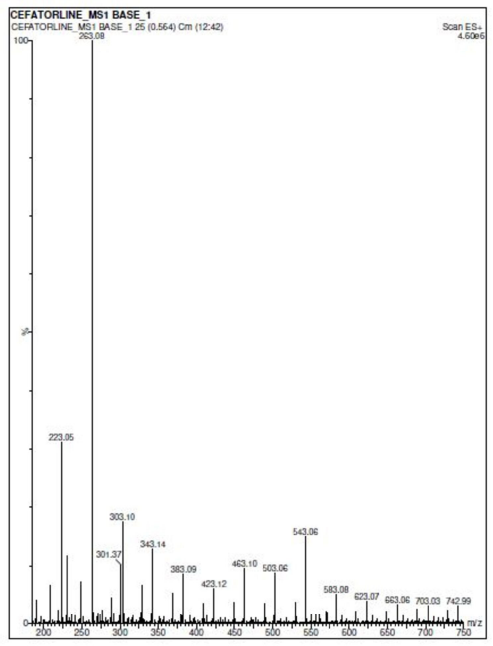 | Figure 8. Mass spectra of Ceftaroline Fosamil in a forced basic condition. [Click here to view] |
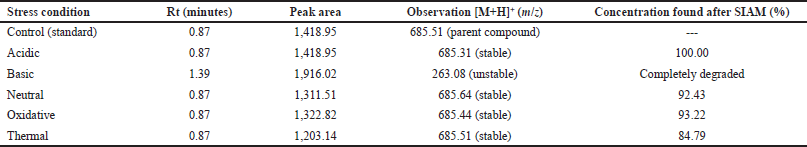 | Table 7. Summary of the SIAM method for Ceftaroline Fosamil. [Click here to view] |
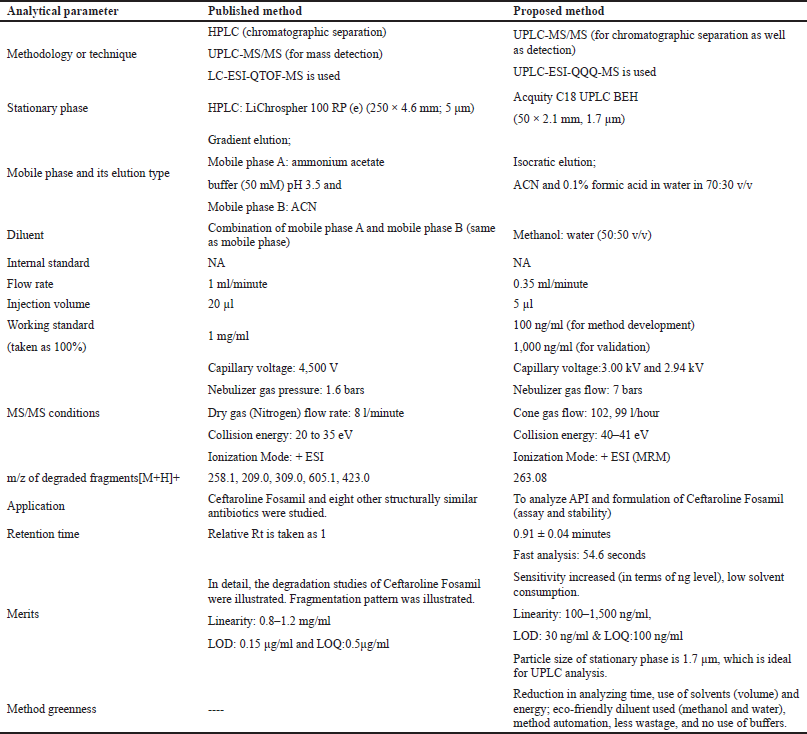 | Table 8. Comparative study of the published LC-MS/MS work (Izabela et al., 2017) and proposed UPLC-MS/MS work. [Click here to view] |
CONCLUSION
A novel, stability-indicating, hyphenated tandem MS coupled to the UPLC method to determine stability in various stress conditions for Ceftaroline Fosamil has been developed. The proposed method is fast, precise, economical, and advanced to achieve higher and better specificity, and also allows for the isolation of API from its excipients and other impurities if they are present in the formulation. The proposed method can be used to analyze Ceftaroline Fosamil parental powder regularly. The development of a fast and simpler MS/MS detection method was prioritized to obtain a retention time within 1 minute, i.e., 54.6 seconds. The parent ion has been protonated to get a daughter ion, therefore increasing the sensitivity toward detection and quantification. Forced degradation studies and all the validation parameters were conducted according to ICH guidelines. The proposed UPLC-MS/MS process is simple (with easy sample preparation), accurate, consistent, linear, and precise, evidenced by high recoveries and an appropriate RSD. The assay findings show that the method can be used to analyze Ceftaroline Fosamil in formulations. All of the parameters are within acceptable ranges, so it can be concluded that the method was created and validated as per the ICH requirements (European Medicines Agency, 1995; Harron, 1994).
ACKNOWLEDGMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
FUTURE PLAN OF WORK
The developed method can be applied to determine Ceftaroline Fosamil in blood, CSF, and urine samples by using this automated UPLC-MS/MS technique.
REFERENCES
Alarcia lacalle A, Barrasa H, Maynar J, Canut blasco A, Gómez gonzález C, Solinís M, Isla A, Rodríguez-Gascón A. Quantification of ceftaroline in human plasma using high performance liquid chromatography with ultraviolet detection: Application to pharmacokinetic studies. Pharmaceutics, 2021; 13(7):959. CrossRef
European Medicines Agency. ICH Topic Q 2 (R1) validation of analytical procedures: text and methodology, note for guidance on validation of analytical procedures: text and methodology. European Medicines Agency, London, UK, 1995.
Glish GL, Burinsky DJ. Hybrid mass spectrometers for tandem mass spectrometry. J Am Soc Mass Spect, 2008; 19(2):161–72. CrossRef
Gregoire M, Leroy AG, Bouquie R, Malandain D, Dailly E, Boutoille D, Renauda C, Jollietad P, Caillonbc J, Deslandesa G. Simultaneous determination of ceftaroline, daptomycin, linezolid and rifampicin concentrations in human plasma by on-line solid phase extraction coupled to high-performance liquid chromatography-tandem mass spectrometry. J Pharma Biomed Anal, 2016; (118):17–26. CrossRef
Harron DWG.1994.Technical requirements for registration of pharmaceuticals for human use: the ICH Process. In: Griffin JP (ed.7). The textbook of pharmaceutical medicine. West Sussex, UK: Wiley-Blackwell - A John Wiley & Sons, Ltd. Publication, pp.447–60. CrossRef
Izabela K, Katarzyna M, Katarzyna B, Monika K, Stefan T. Identification and determination of related substances of Ceftaroline Fosamil in medicinal product by high performance liquid chromatography with diode array detection and tandem mass spectrometry. J Pharma Biomed Anal, 2017; (145):651–60. CrossRef
Junza A, Amatya R, Barrón D, Barbosa J. Comparative study of the LC-MS/MS and UPLC-MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J Chromatograph B Anal Technol Biomed Life Sci, 2011; 879(25):2601–10. CrossRef
Junza A, Dorival-Garcia N, Zafra-Gomez A, Barron D, Ballesteros O, Barbosa J, Navalonc A. Multiclass method for the determination of quinolones and β-lactams, in raw cow milk using dispersive liquid-liquid microextraction and ultra high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A, 2014; (1356):10–22. CrossRef
Kaushik D, Rathi S, Jain A. Ceftaroline: a comprehensive update. Int J Antimicrob Agents, 2011; 37(5):389–95. CrossRef
Reddy Govardhan P, Kumar Kiran V, Raju Appala VVSS, Ram Raghu J, Raju Appala N. The Estimation of Ceftaroline Fosamil in lyophillized powder for injection by RP-HPLC. Res J Pharm Technol, 2018; 11(2):455–8. CrossRef
Shabir GA. Validation of high-performance liquid chromatography methods for pharmaceutical analysis: understanding the differences and similarities between validation requirements of the US Food and Drug Administration, the US Pharmacopeia and the International Conference on Harmonization. J Chromatogr A, 2003; 987:57e66. CrossRef
Suneetha A, Venkanna KC. Development and validation of stability indicating RP-HPLC method for estimation of Ceftaroline Fosamil in bulk and its parenteral dosage forms. Hindawi Publishing Corporation, London, UK, 5 p, 2013. CrossRef
Van den Meersche T, Pamel E, Van Poucke C, Van Herman L, Heyndrickx M, Rasschaert G, Daeseleirea E. Development, validation and application of an ultra high performance liquid chromatographic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of veterinary antibiotics in swine manure. J Chromatogr A, 2016; 1429:248–57. CrossRef
Wilfried M.A. Niessen. 2010. Liquid Chromatography – Mass Chromatography, Third edition, Boca Raton London New York: Taylor & Francis, pp. 385 – 386.