INTRODUCTION
Amino acids are organic compounds consisting of nitrogen, carbon, hydrogen, and oxygen, together with the groups of variable side chains. Amino acids and proteins are the building blocks of life cycle. Essential amino acids (EAA) cannot be generated by the body; therefore, these shall be supplied through the diet (Essential Amino Acids, 2019).
About 10 essential amino acids include histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and valine which must be taken in the daily diet. Tyrosine is derived from phenylalanine by hydroxylation in the para position.
Therefore, tyrosine is not included in the list of the EAA of the literature. However, this is also an essential amino acid if phenylalanine is deficient in the diet.
Failure to obtain enough of even one of the 10 EAA (Lewis and Cole, 1976) causes detrimental health effects. Unlike fat and starch, the human body does not store excess amino acids for later use; therefore, the EAA must be in the everyday food or be taken as supplements.
Essential amino acid supplements have the following benefits (10 Essential amino acids, 2019):
- These supplements may help in improving mood and sleep with tryptophan by generating serotonin.
- The branched-chain amino acids (Leu, Ile, and Val) can boost the exercise performance by energy production.
- These can prevent muscle loss by increasing muscle function during bed rest (Ferrando et al., 2010).
- Branched chain EAA can be effective in promoting weight loss.
These EAA can be classified into three groups based on the side chain (Table 1). These side chains play an important role in the separation of amino acids by chromatography.
In general, two different methods are used for the analysis of the amino acid. One is postcolumn derivatization, in which the separation of amino acids is performed on an ion exchange column and their quantification using ninhydrin (Samejima et al., 1971), Fluorescamine (Undenfriend et al., 1972) or orthophthalaldehyde (OPA) (Roth, 1971). The alternative approach was to derivatize the amino acids before separation on a reverse-phase High Performance Liquid Chromatography (HPLC) column. The precolumn derivatization reagents in practice for amino acid analysis are dansyl (Tapuhi et al., 1981), phenyl isothiocyanate (Bidlingmeyer et al., 1984), 9-fluorenylmethyl chloroformate (FMOC) (Haynes et al., 1991), and 6-aminoquinolyl-N-hydroxysuccinimyl carbamate (AQC) (Wang et al., 2013).
 | Table 1. Category of EAA based on side chain R group. [Click here to view] |
Furthermore, OPA (Dorrestein et al., 1996) is being used as a precolumn derivatization reagent. Recently, the usage of o-phthalaldehyde, AQC, and FMOC has increased as precolumn derivatization reagent taking into their advantages over others. In the view of the sensitivity of the method, FMOC derivatives can be detected by ultraviolet (UV) light at the picomole level. Further, FMOC derivatives are found to be stable compared to OPA (Molnar-Perl, 2011). In this study, FMOC was used as a derivatization reagent for the Ultraperformance Liquid Chromatography (UPLC) determination of 10 EAA derivatives. In the pharmaceutical industry, validations of the analytical method are performed according to the ICH Q2R1 guidelines. A new approach has been introduced, based on the tolerance range, as well as on the total error and accuracy profile (Hubert et al., 2004). This type of validation method will give more confidence in quantifying the drugs accurately during routine use. Indeed, the classical approach concludes about the validity of an analytical procedure by comparing the already fixed acceptance limits, whereas this new approach deals with systematic and random errors. Another advantage is to include the expected risk in the obtained assay results, which will be included within acceptance limits fixed according to the requirements. It is also one of the growing regulatory requirements for risk management associated with the use of these methods in the routine analysis (OPS Process Analytical Technology (PAT) Initiative, Fda.Gov, 2019, and ICH Q9, 2005). This study deals with the same approach by the combination of systematic (accuracy) and random (precision) errors, and this approach is called a total error concept. Indeed, the ISO.ORG (2019) guidelines provide a suitable definition for determining the accuracy of quantitative methods, defined as the sum of trueness (ICH-Part II interpretation of accuracy) and precision.
In the past, this approach was used successfully for analytical method validation for several times (Aasodi et al., 2018; Gibelin et al., 2009; Mbinze et al., 2015; Rozet et al., 2007a, 2007b, 2007c). An accuracy profile is a decision tool for the interpretation of the performance of the analytical method. The goal of this study is to:
- Develop an analytical method to test EAA in the formulation.
- Validate the analytical method using total error concept.
- Present the generated accuracy profiles of each amino acid for easy interpretation of method performance.
In this study, e-Noval (Enoval 4.1b, 2019) web-based software was used to perform the statistical calculations. An extensive literature survey was conducted, and the comparison of the method parameters against the current method is shown in Table 2.
MATERIALS AND METHODS
Chemicals and reagents
Histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and hydrochloric acid of American Chemical Society (ACS) grade were obtained from Sigma-Aldrich (Saint Louis, USA). Boric acid was obtained from Spectrum (India). Fluorenylmethyloxycarbonyl chloride (FMOC-Cl) was purchased from Apollo Scientific Limited (India). Acetonitrile of UPLC grade was obtained from Biosolve (Chimie, France). Trifluoroacetic acid (TFA) of HPLC grade was purchased from Sigma-Aldrich (Germany). The chromatography water of HPLC grade was obtained from Merck (India). The essential amino acid tablets (Auximin® produced by Alniche LifeSciences Pvt. Ltd.) were obtained from the Netmeds pharmacy, India.
Instruments
Acquity UPLC TUV detector (Water Corporation, MA, United States) was used with the Waters Empower 3 software. Other instruments used in this study were Ultra Sonicator (Anna Matrix, Bangalore, India), electronic scale (Metrohm), and Vortex Shaker (IKA, Malaysia)
Parameters
The separation was obtained using a gradient flow rate of 0.25 ml/min containing 0.1% (v/v) trifluoroacetic acid in water as mobile phase A, and a mixture of acetonitrile and water (90:10 v/v) was used as mobile phase B. UPLC CORTECS C18+ column (internal diameter 2.1 × 100 mm, 1.6 μm), Catalogue no. 186007117 (Waters Corporation, MA, United States), was used for separation; detection wavelength: 265 nm; injection volume: 1 μl; column temperature: 30°C; The gradient elution program is shown in Table 3. Auto sample temperature was 5°C.
Method development
Selection and preparation of the mobile phase
Amino acids are zwitterion in nature with different functional groups, of which one has positive and second has a negative electrical charge, but the net charge of the molecule is zero. Due to the presence of such a charge, molecules exhibit high polar nature with different pH values. Therefore, an acidic modifier (0.1% TFA) buffer and acetonitrile were selected for separation in the C18 column. An amount of 0.1% TFA and acetonitrile in different ratios was tried with different flow rates. A good separation of amino acid derivatives and the symmetrical peaks was found with the mobile phase composition as shown in Table 3.
Mobile phase A was prepared by transferring 1.0 ml of TFA acid into a volumetric flask of 1 L, diluted to the volume with water for chromatography, and mixed well. Mobile phase B was prepared by mixing 900 ml of acetonitrile with 100 ml of water for chromatography. These mobile phases were sonicated for 5 minutes to degas.
 | Table 2. Comparison of published FMOC pre-column derivatization methods with the current method. [Click here to view] |
Preparation of boric acid solution of pH 6.2
FMOC reaction with amino acids is carried out in basic medium. Therefore, borate buffer is selected in various studies in the range from 0.01 to 0.325 M from pH 6 to 11.4. In this study, 0.2 M boric acid solution was selected for derivatization at pH of 6.2.
Preparation of Derivatization reagent solution
Acetonitrile was selected as a solvent to prepare this reagent since it generates fewer impurities compared to acetone. Therefore, 4 mg/ml of FMOC-Cl was prepared in acetonitrile.
Preparation of standard stock solutions
Each amino acid standard stock solution was prepared at 25 mg/ml solution in the diluted HCl and stored in the refrigerator.
Preparation of calibration curve
The calibration curve was constructed using five-level working standard (WS) solutions prepared from the standard stock solution containing 25 mg/ml concentration of each EAA as shown in Table 4. Calibration curves were calculated from peak area versus the concentrations of respective amino acids. The system suitability was assessed in every sample set from the calibration curve by calculating the resolution, precision, accuracy, and correlation coefficient of standards.
Derivatization
About 50 μl of blank/standard/sample was transferred to a test tube. A 450 μl unit of 0.2 M boric acid solution and 500 μl of FMOC-Cl were added and vortexed for 30 seconds. In addition, 30 μl of concentrated HCl was added and thoroughly mixed. Finally, 4.0 ml of n-hexane was added and vortexed. After 10 minutes of permanence, the bottom layer of the solution was pipetted into a UPLC vial for analysis.
Method validation
Validation of the analytical method to demonstrate suitability for the intended use is a vital part, in particular, for quantitative methods from the regulatory point of view. In this study, the concept of systematic and random errors (total error) was chosen to test the performance of the procedure. Three individual series were made. The accuracy profile of ±7% and the risk profile of 5% are considered acceptance criteria for each analyte.
Specificity
To demonstrate the specificity of the method, the blank, the mobile phase A and B derivatives were injected once into the chromatographic system together with the standard solutions.
 | Table 3. Mobile phase gradient elution program. [Click here to view] |
 | Table 4. Preparation of working standard solutions. [Click here to view] |
Accuracy, precision, linearity and trueness
- Truthfulness refers to the closeness of the agreement between a conventionally accepted value and an experimental one. The amount of trueness is a systematic error.
- Precision is the closeness of the agreement between multiple sampling measurements of a homogeneous sample under the recommended circumstances. Precision measurement is a random error.
- Accuracy is the closeness of the agreement between the generated result and the accepted reference value. Accuracy measurement is a combination of systematic and random errors. Accuracy considers the total error.
- The linearity of an analytical method is its ability to obtain the test results that are directly, or through a well-defined mathematical transformation, proportional to the concentration of the analyte in the sample within a certain range
Accuracy, precision, trueness, and linearity samples (marketed formulation) were ascertained at three levels around the target concentration level as described below.
Label claim of each amino acid and the average weight of the formulation were recorded. Validation solutions (VS) were prepared in three replicates at three levels from powered tablets on 3 different days.
The replicates, such as 796 mg (level-1), 995 mg (level-2), and 1194 mg (level-3), were transferred into the separate 100-ml volumetric flasks and dissolved and diluted with the diluted HCl. About 20 ml of each solution was diluted to 50 ml with water. Concentrations of each amino acid at each level are shown in Table 5.
 | Table 5. Composition and concentration of validation samples. [Click here to view] |
Accuracy, precision, trueness, and linearity were demonstrated from the validation samples prepared in triplicate (as per Table 5) at three different levels in three series. The reagents and standard solutions were prepared independently in three series for 3 days. To obtain the intermediate precision results, which are represented as much as possible to the analytical procedure variability during its application for routine use, the analysis was performed independently with different combinations of the available factors. The linearity graph was plotted for the theoretical concentrations (introduced concentration) against the experimental concentrations (results).
Robustness
The robustness of the method was studied in the VS2 validation sample prepared from the formulation. The experiment was carried out by intentionally changing the temperature and flow of the column. The same standard solutions and the VS2 solution have been analyzed under different varied conditions and nominal condition to avoid distortions. The mobile phase has been kept the same in varied and nominal experiments. The robustness was calculated by calculating the percentage of agreement between the experimental concentration values obtained between nominal and various conditions.
RESULTS
Method development and optimization
To develop an efficient method for the quantification of EAA in tablets, FMOC reaction time and volume of concentrated HCl required (to stop the reaction) were studied to confirm the effect on amino acid response and additional peak generation. The derivatization procedure presented in this study was found to be satisfactory. Several UPLC chromatographic systems were used to separate the amino acids, and the chromatographic parameters presented in this study were found to be satisfactory for the better separation of amino acids.
System suitability
System suitability test is an essential parameter to ensure the quality of the method for correct measurements. As a part of system suitability, precision (%RSD < 2), tailing factor (t = 0.8–1.2), resolution between amino acids (r> 1.8) in middle standard, calibration linearity (correlation coefficient > 0.99), and accuracy (% deviation between experimental and theoretical concentration < 2) were studied. System suitability assessment was performed independently for each series, and all the results were found to be satisfactory.
Method validation
Specificity
No interference at amino acid retention time (RT) was detected from the blank, mobile phase A and B (MP-A and MP-B) derivatives. The comparison between blank, MP-A, MP-B, and WS1 chromatograms is shown in Figure 1. Furthermore, no interference peaks at the EAA retention time were not eluted in any of the sample injections. From these experimental results, the method has proven to be specific with respect to any interference peak.
Accuracy, precision, trueness, and linearity
To assess the three series data, e-Noval application was used to generate the results using the experimental concentrations and theoretical concentrations. Accuracy and trueness results are shown in Table 6. The accuracy profiles of validation solutions are shown in Figure 2, the precision results are presented in Table 7, and linearity results are presented in Table 8. The relative beta-expected tolerance results are presented in Table 9. A chromatogram of sample injection and graphic abstract is shown in Figure 3.
Robustness
The solidity of the procedure was verified by deliberately varying the column temperature (30 ± 2°C) and the flow rate (0.25 ± 0.02 ml/min). The system suitability results produced under nominal and varied conditions have been found satisfactory. The percentage of the agreement results calculated between the conditions is presented in Table 10.
 | Figure 1. Overlay chromatogram of derivatized blanks and sample injection. [Click here to view] |
DISCUSSION
In this UPLC precolumn derivatization method, the linearity of the amino acids is in the range of 0.10–1.00 mg/ml. The method was successfully validated under optimized conditions using the e-Noval statistical tool with acceptance criteria of the 95% confidence interval (5% risk) with an accuracy profile of ± 7%. This provided a greater confidence in the performance of methods. The results of the validation were considered satisfactory; therefore, it can be concluded that the accuracy profile results in ± 7% (limit) for all EAA were −5.272–5.827, as shown in Table 9. The results of the predictive interval (%) at a risk level of 5% in the EAA range were between 0.0001 and 1.7 (Table 6). The average recovery results were within 98.73%–102.7%, as shown in Table 6. The precision results were also satisfactory: the results of maximum repeatability (% RSDRe) = 1.4%, and therefore, the maximum intermediate precision (% RSDIP) = 1.7%. The linearity correlation coefficient (r) was found to be greater than 0.99 for all amino acids. No interference peaks were observed at the retention time of any amino acid. Figure 1 shows that the method is specific. The method was robust because the percentage agreement results were within 100 ± 3% (Table 10).
 | Table 6. Accuracy and trueness results. [Click here to view] |
 | Figure 2. Accuracy profile of EAA in Validation solution: The plain red line is the relative bias; the dashed blue lines are the β-expectation tolerance limits and the dashed black lines represent the acceptance limits. The dots represent the relative error of the results and are plotted with respect to their targeted concentration; X-axis = concentration (mg/ml) and Y-axis = relative error (%). [Click here to view] |
 | Table 7. Precision results. [Click here to view] |
 | Table 8. Linearity results. [Click here to view] |
 | Table 9. Accuracy relative beta expected tolerance limit (%) results (limit ± 7%). [Click here to view] |
 | Figure 3. Graphical abstract and sample chromatogram. [Click here to view] |
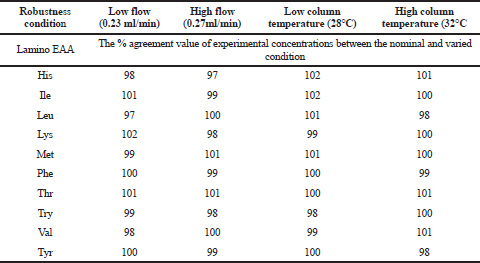 | Table 10. Robustness results at column temperature and flow rate. [Click here to view] |
CONCLUSION
To implement a method for daily routine analysis, the validation of the analytical method is very important to demonstrate that the results are reliable. However, the decision on method performance and reliability of the validation results on traditional validation are challenged due to difficulty in the assessment of results and unexpected bias in regular day-to-day use. Therefore, it has been shown that the usage of the accuracy profile built on β-expected tolerance intervals shows a predicted region where a predefined proportion of future results will be observed. The developed and validated UPLC precolumn derivatization method is not only simple, sophisticated, and reliable but also specific for the quantification of EAA in pharmaceutical solid oral dosage formulations. Compared to the previous mythologies, the current method does not generate double derivatization peaks, and the separation and performance results are good. Therefore, this method could be used for regular EAA tests in pharmaceutical formulations.
ACKNOWLEDGMENT
The authors are grateful to the Head of the Department of Baxter Global Research Centre, Bangalore, India, for providing the facilities for working.
REFERENCES
10 Essential amino acids. 2019. Available via https://biochemist01.wordpress.com/tag/10-essential-amino-acids/ (Accessed 2 April 2019).
Aasodi RR, Murugan V, Kumari P, Shree V. Development of analytical method for separation and quantification of cysteine hydrochloride monohydrate, followed by validation with total error concept by using ultra performance liquid chromatography with pre-column derivatization. J Chromatogr Sep Tech, 2018; 9(4):406.
Acquaviva A, Romero LM, Castells CB. Analysis of citrulline and metabolic-related amino acids in plasma by derivatization and RPLC. Application of the extrapolative internal standard calibration method. Microchem J, 2016; 129:29–35. CrossRef
Bidlingmeyer BA, Cohen SA, Tarvin TL. Rapid analysis of amino acids using pre-column derivatization, J Chromatogr B, 1984; 336(1):93–104. CrossRef
Dorrestein RC, Berwald LG, Zomer G, De Gooijer CD, Weiten G, Beuvery EC. Determination of amino acids using o-phthalaldehyde-2-mercaptoethanol derivatization effect of reaction conditions. J Chromatogr A, 1996; 724(1–2):159–67. CrossRef
Enoval 4.1b. Pharmalex, Arlenda.Com. 2019. Available via https://www.arlenda.com/enoval4.1 (Accessed 3 April 2019).
Essential Amino Acids. Definition, Benefits and Food Sources, Healthline. 2019. Available via https://www.healthline.com/nutrition/essential-amino-acids (Accessed 3 April 2019).
Fabiani A, Versari A, Parpinello GP, Castellari M, Galassi S. High-performance liquid chromatographic analysis of free amino acids in fruit juices using derivatization with 9-Fluorenylmethyl-Chloroformate. J Chromatogr Sci, 2002; 40(1):14–8. CrossRef
Ferrando AA, Jones DP, Hays NP, Kortebein P, Ronsen O, Williams RH, McComb A, Symons TB, Wolfe RR, Evans W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin Nutr, 2010; 29(1):18–23. CrossRef
Gibelin N, Dupont D, Imbert S, Rozet E. Use of total error concept in the validation of viral activity in cell cultures. J Chromatogr B, 2009; 877(23):2407–11. CrossRef
Haynes PA, Sheumack D, Kibby J, Redmond WJ. Amino acid analysis using derivatistion with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr A, 1991; 540:177–85. CrossRef
Hubert P, Nguyen-Huu JJ, Boulanger B, Chapuzet E, Chiap P, Cohen N, Compagnon PA, Dewe D, Feinberg M, Lallier M, Laurenite M, Mercier N, Muzard G, Nivet C, Valat L. Harmonization of strategies for the validation of quantitative analytical procedures ASFSTP proposal-part I. J Pharm Biomed Anal, 2004; 36(3):579–86. CrossRef
ISO.ORG. (2019). Available via https://www.iso.org/obp/ui/#iso:std:iso:5725:-1:ed-1:v1:en (Accessed 3 April 2019).
Jambor A, Molnar-Perl I. Amino acid analysis by high-performance liquid chromatography after derivatization with 9-fluorenylmethyloxycarbonyl chloride. J Chromatogr A, 2009a; 1216(15):3064–77. CrossRef
Jambor A, Molnar-Perl I. Quantitation of amino acids in plasma by high-performance liquid chromatography: Simultaneous deproteinization and derivatization with 9-fluorenylmethyloxy carbonyl chloride. J Chromatogr A, 2009b; 1216(34):6218–23. CrossRef
Lewis D, Cole DJA. Amino acid requirements. Proc Nutr Soc, 1976; 35(1):87–91. CrossRef
Lozanov V, Benkova B, Mateva L, Petrov S, Popov E, Slavov C, Mitev V. Liquid chromatography method for simultaneous analysis of amino acids and biogenic amines in biological fluids with simultaneous gradient of pH and acetonitrile. J Chromatogr B, 2007; 860(1):92–7. CrossRef
Lozanov V, Petrov S, Mitev V. Simultaneous analysis of amino acid and biogenic polyamines by high-performance liquid chromatography after pre-column derivatization with N-(9-fluorenylmethoxycarbonyloxy) succinimide. J Chromatogr A, 2004; 1025(2):201–8. CrossRef
Mbinze JK, Mpasi JN, Maghe E, Kobo S, Mwanda R, Mulumba G, Bolande JB, Bayebila TM, Amani MB, Hubert P, Marini Djang’Eing’A R. Application of total error strategy in validation of affordable and accessible UV-visible spectrophotometric methods for quality control of poor medicines. Am J Anal Chem, 2015; 6(2):106–17. CrossRef
Molnar-Perl I. Advancement in the derivatizations of the amino groups with the O-phthaldehyde-thiol and with the 9-fluorenylmethyloxycarbonyl chloride reagents. J Chromatogr B, 2011; 879(17–18):1241–69. CrossRef
OPS Process Analytical Technology (PAT) Initiative, Fda.Gov. (2019). Available via https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm088828.htm (Accessed 3 April 2019).
Quality Risk Management. ICH Q9, ICH.ORG. (2005). Available via https://www.ich.org/products/guidelines/quality/quality-single/article/quality-risk-management.html (Accessed 3 April 2019).
Roth M. Fluorescence reaction for amino acids. Anal Chem, 1971; 43(7):880–2. CrossRef
Rozet E, Ceccato A, Hubert C, Ziemons E, Oprean R, Rudaz S, Boulanger B, Hubert P. Analysis of recent pharmaceutical regulatory documents on analytical method validation. J Chromatogr A, 2007b; 1158(1–2):111–25. CrossRef
Rozet E, Hubert C, Ceccato A, Dewé W, Ziemons E, Moonen F, Michail K, Wintersteiger R, Streel B, Boulanger B, Hubert P. Using tolerance intervals in pre-study validation of analytical methods to predict in-study results, J Chromatogr A, 2007a; 1158(1–2):126–37. CrossRef
Rozet E, Wascotte V, Lecouturier N, Preat V, Dewe W, Boulanger B, Hubert P. Improvement of the decision efficiency of the accuracy profile by means of a desirability function for analytical methods validation. Application to a diacetyl-monoxime colorimetric assay used for the determination of urea in transdermal iontophoretic extracts. Anal Chim Acta, 2007c; 591(2):239–47. CrossRef
Samejima K, Dairman W, Undenfriend S. Condensation of ninhydrin with aldehydes and primary amines to yield highly fluorescent ternary products: I. Studies on the Mechanism of the reaction and some characteristics of the condensation product. Anal Biochem, 1971; 42(1):222–36. CrossRef
Shi Z, Li H, Li Z, Hu J, Zhang H. Pre-column derivatization RP-HPLC determination of Amino Acids in Asparagi Radix before and after Heating process. IERI Proc, 2013; 5:351–6. CrossRef
Tapuhi Y, Schmidt DE, Lindner W, Karger BL. Dansylation of amino acids for high-performance liquid chromatography analysis. Anal Biochem, 1981; 115(1):123–9. CrossRef
Undenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigle M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science, 1972; 178(4063):871–2. CrossRef
Wang H, McNeil YR, Yeo TW, Anstey NM. Simultaneous determination of multiple amino acids in plasma in critical illness by high performance liquid chromatography with UV and fluorescence detection. J Chromatogr B: Analyt Technol Biomed Life Sci, 2013; 940:53–8. CrossRef