INTRODUCTION
The two Afrotropical species of freshwater crabs, Potamonautes berardi (Audouin, 1826) and Potamonautes niloticus (H. Milne Edwards, 1837) belong to Family Potamonautidae are well known in the River Nile and its tributaries. These species are in close to the Palaearctic species, Potamon potamios Olivier, 1804 (Family: Potamidae), an eastern Mediterranean taxon whose range extends into the Sinai Peninsula (Brandis et al., 2000). Potamonautes niloticus and P. berardi are common and widespread throughout the River Nile basin from the delta in Egypt to Lake Victoria and its tributaries in equatorial Africa. The most common species in the northern limit is P. niloticus which has a very wide distribution; from Egypt, Sudan, Ethiopia, Uganda, Kenya and Rwanda. Fishermen have the crabs caught in their fishing nets while targeting mainly crayfish and then they discard the undesired caught crabs because these populations of crabs are to the best of my knowledge not yet commercially evaluated.
In fact, freshwater crabs were considered as an important source of chitin as the other crustaceans (Bolat et al., 2010). Chitosan is traditionally obtained from crustacean shells (crabs, shrimps and crayfish) with natural cationic polysaccharide composed of 2-acetamido-2deoxy-b-D-glucopyranose and 2-amino-2-deoxy-b-D-glucopyranose (De Oliviera et al., 2014).
Depending on the species and season, crustacean shells or exoskeletons are composed of 30%–40% protein, 30%–50% calcium carbonate and 20%–30% chitin. A rate of 1010–1011 tons per year is the amount of chitin produced from crustaceans, insect exoskeletons and cell wall of fungi, which are unusable wastes (Gopalan and Dufresne, 2003). It was determined that the shrimp (Pandalus borealis) contains about 17%–32.2% chitin (Shahidi and Synowiecki, 1991) while the chitin isolated from the crab, Chionoecetes opilio, contains 26.6% chitin (Hong and Mun, 1995).
Because of the outstanding biological features of chitosan, including its biodegradability, biocompatibility and nontoxicity; it is used in the food, agriculture, textile, water treatment, cosmetics and other industries with or without other natural polymers (alginates, starch and gelatin) additives (Kammoun et al., 2013). Since chitosan was an applicable compound to use in medical and commercial industries, it considers as biodegradable, non-toxic, anti-fungal and antibacterial biomaterial compound (Ahmed and Sastry, 2011; Baldrick, 2010; Daniela and Camelia, 2008; Gokarneshan, 2017; Shi et al., 2009).
The effect of chitin and its derivatives on fibroblasts and production of cytokines were reported by Mori et al. (1997); while Usami et al. (1994a, 1994b) found that chitin and chitosan have enhanced the activation of bovine and canine polymorph nuclear cells. Moreover, chitosan is of biomedical importance, for example, wound healing processes (Shakeel and Ikram, 2016). Moreover, Patrulea et al. (2015) concluded from their review that the chitosan is an efficient compound in wound healing application; also, it is a good accelerator material for wound healing (Yilmaz, 2004).
Wound repair or healing in which tissue repair itself after injury (Cordoso et al., 2010; Nilani et al., 2011) one of the applications that can be used to test the effect of chitosan on various tissues. The healing process begins with blood clotting and can be generally classified to: inflammation, granular tissue formation, re-epithelialization, matrix production and remodeling (Janis et al., 2010). While Barbul and Regan (1993) classified this process to three phases comprised: inflammation, proliferation and maturation. Archana et al. (2013) stated that the wound healing is a series of complex steps starting with inflammation followed by cell migration, angiogenesis provisional matrix synthesis, collagen deposition and re-epithelization.
Generally, there are two models to test wound healing; incisional and excisional (Dorsett-Martin, 2004), but excisional model is more appropriate to determine histological and morphological changes during the healing process. Azuma et al. (2015) studied the excisional model on rat’s wounds and found that wounds healed in group treated with chitosan are 99% higher than control groups 82%.
Hence, the present study aims to test an applicable material (chitosan) extracted from the non-economic freshwater crab P. niloticus on wound healing process caused by circular excision of skin in normal healthy rats.
MATERIALS AND METHODS
Extraction of chitosan
Forty six individuals of freshwater crab P. niloticus were collected from River Nile Tributaries, then transported to the laboratory, Faculty of Science, Al-Azhar University during August/2018. Crab exoskeletons were separated after dissecting all individuals in pre-iced jars. All crab exoskeleton wastes were washed with tap water then boiled and dried in room temperature for 24 hours. The extraction of chitosan was carried out according to Hossain and Iqbal (2014) with modification in demineralization step, using glacial acetic acid instead of hydrochloric acid. 1) Demineralization, by glacial acetic acid 5% for 1 hour at room temp. 2) Deproteinization by NaOH 4% for 2 hours at 65C° and 3) Deacetylation by NaOH 50% for 20 hours at 65C°.
Physical characterization of chitosan
Chitosan was characterized by using fourier transformer infrared spectroscopy (FTIR spectra) in a range of 400 to 3,600 cm−1.
Wound healing experiment
A total of 25 male albino rats were obtained from Vacsera Farm, Helwan branch by delivery mediator and transported to the Animal House, Faculty of Science, to perform this experiment during October/2018. The animals were acclimated for 2 weeks before the experiment, all cages containing the experimental animals were cleaned, sterilized and changed the bedding (sawdust) every 2 days. Animals were separated into five groups (five in each) comprised: (1) −ve CG (negative control group without any treatment), (2) +ve CG (positive control group treated with commercial ointment of 25% w/w β-sitosterol), (3) Ch 1% (chitosan film 1% treated group), (4) Ch 2% (chitosan film 2% treated group) and (5) Ch 3% (chitosan film 3% treated group). Chitosan concentrations were determined based on studies carried out by Bujang et al. (2013) and No et al. (2002). Diethyl ether was used as anesthetic before wound injury. A small sterilized puncher used for performing two round wounds, ~ 1 cm in diameter, on the back of each rat (Fig. 1).
Morphological evaluation
Wound closure was measured by Vernier Caliper at each: 1, 4, 8, 12 and 16 day’s intervals after surgery (Fig. 1).
Histological evaluation
It was performed by excision the rounded wounds of sacrificed rats (sacrificed by diethyl ether inhalation before excision). Then, the excised wounds preserved in formalin 10% and followed the basic histological preparation (fixation, dehydration, embedding and cutting sections). Sections were stained with hematoxylin-eosin to assess the phases of wound healing.
Total leucocytes count was estimated by automated Complete Blood Count veterinary Counter.
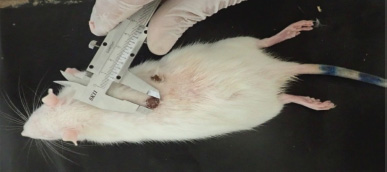 | Figure 1. Measuring of wound closure using Vernier Caliper during healing process after treatment. [Click here to view] |
Morphometric measurements
The stained sections were examined under light microscope (Leica DM –LB2) provided with a digital camera (Sanyo vcc-6580PE) for photography. For each section, prepared samples from each rat was subjected to the following measurements: i) Thickness of epidermal layer (μm), ii) Thickness of dermal layer (μm), iii) Number of hair follicles and Sebaceous glands /field.
Statistical analyses
Statistical analyses were carried out using Microsoft Office 365 Excel program, ANOVA (Single factor).
RESULTS AND DISCUSSION
Chitosan yield
A total of 57.6 g chitosan was extracted from 46 individual’s exoskeleton of the freshwater crab P. niloticus. This amount represents 30.64% out of 188 g of dried crab exoskeleton after final step of deacetylation, which in the range of those reported in some studied crustaceans (Cho et al., 1999) and being higher than that determined in crab C. opilio and shrimp P. borealis (Hong and Mun, 1995; Shahidi and Synowiecki, 1991).
Physical characterization
Fig. 2 represents FTIR transmittance spectra of chitosan powder isolated and prepared from P. niloticus. Stretching vibrations of primary and secondary hydroxyl groups are represented by the principal spectral broad band between 3,431 cm−1 to 3,009 cm−1. The stretching vibrations of C=O and C-O of primary alcohols were located at 1,413 and 1,052 cm−1, respectively. The band at 1,338 cm−1 was assigned to amide III and the spectral band at 1,243 cm−1 was attributed to complex vibration of NHCO group. The C-O-C glycoside linkage vibration was observed at 1,198 cm−1. The bands at 1,052 and 1,022 were ascribed to stretching of C-O bonds of the primary alcohols. An angular deformation of C-H bonds of methyl group were observed at the band of 1,475 cm−1. The existence of a band at 3,282 cm−1 is attributed to the hydrogen bonded NH groups of the secondary amide. The ring vibrations in C-C were represented by several bands at 677, 806 and 957 cm−1. The presence of spectral peak at 1,563 corresponds to the protonation of NH3+ groups. The bands at 2,931, 2,847, 957 cm−1 were ascribed to C-H bonds stretching. The stretching vibrations in amide II group was located at 1,643 cm−1. All bands that are observed in FTIR spectrum are detailed in Table 1.
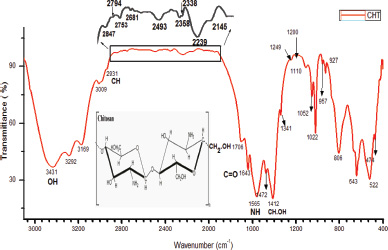 | Figure 2. FTIR transmittance spectra of chitosan powder isolated from P. niloticus (the graph range between 2,850 and 2,000 has been enlarged due to lower intensity of peak existence). [Click here to view] |
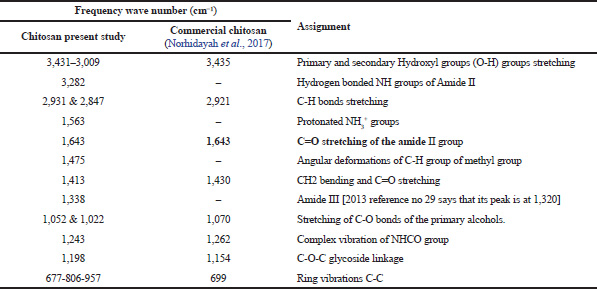 | Table 1. Characterization of chitosan (present study) using IR in comparison with commercial chitosan. [Click here to view] |
Noteworthy, the band of 1,643 cm−1, that is assigned to C=O of the primary amide group of the extracted chitosan shows similarity with the reported peak by Norhidayah et al. (2017) for commercial chitosan. The appearance of bands at 1,022 and 1,052 cm−1 is contributed by the stretching of C=O bonds of the primary alcohols. However, few bands of the chitosan prepared in the present study are absent in the commercial chitosan presented by Norhidayah et al. (2017).
Morphological and histological evaluation of wound healing
The uses of morphological and histological examination of healing rat wounds using the present extracted chitosan as a treatment are more appropriate methods to assess the wound healing factors as previously proposed (Gal et al., 2008; Vidinsky et al., 2006).
To evaluate the effectiveness of chitosan extracted from the crab exoskeleton waste as a wound dressing material, an in vivo macroscopic wound closure study was performed. During this study, the macroscopic observation of wound closure in control groups, including (−ve) untreated and (+ve) treated with commercial wound healing material versus treated groups with chitosan film (1%, 2% and 3%) was examined at 4, 8, 12 and 16 days after surgery (Fig. 3a and b).
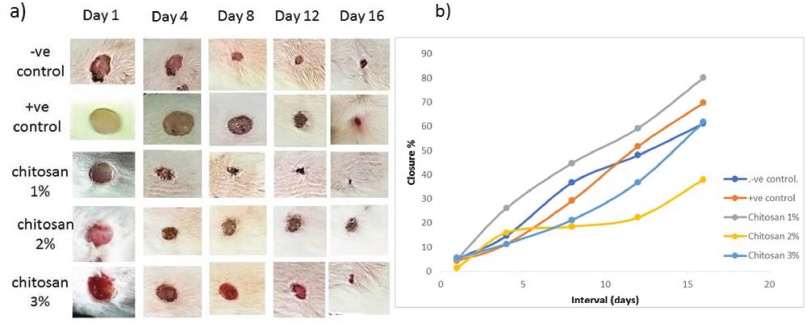 | Figure 3. (a) Morphological assessment of wound healing closure treated with different doses of chitosan compared with control. (b) Closure rate of treated groups versus control groups. [Click here to view] |
In −ve control group, the closure of wound not completely healed at the end of the experiment at Day 16, but the +ve control showed more healing performance at Days 12 and 16. Moreover, scar formation weakly observed in both previous groups. On the other hand, the Ch 1% treated group showed the best performance of wound closure compared with control groups started at Day 4, in addition to the formation of scar at Days 4, 8, 12, with closure rate, 44.7%, 59.1% and 80%, respectively and almost completed healing at Day 16, this was less than reported complete healing range period of 3 weeks (Dreifke et al., 2015). However, the macroscopic observation for treated groups, Ch 2% and 3% showed less effectiveness of wound closure during the period of the experiment despite of the formation of the scar, with lower closure rate. The present results showed that the most effective treatment on wound closure rate was chitosan treated group (1%) followed by positive control group (commercial product), indicating that the present prepared chitosan is more effective at concentration 1% comparable with commercial products and other concentrations and the statistical analyses confirmed the difference in closure rate, it was significant (p < 0.05) between groups.
Histological evaluation of wound healing
Microscopic examinations for control and treated groups sectioned samples of rat wounds stained with H&E at 4, 8 and 16 days intervals were carried out to evaluate the recovery of skin tissue (Fig. 4). After Day 4, the H&E stained sections showed no scar formation in −ve and +ve control groups but was clearly observed in Ch 1% treatment group and hardly indicated in the treatment groups of Ch 2% and 3%. On the other hand, re-epithelization was showed only in +ve CG and Ch 1% treatment groups. These results are in well agreement with Azuma et al. (2015) on the assessment of chitosan derivatives after 4 and 8 days and the re-epithelization was completed after 16 days indicating that chitosan prompts good re-epithelization with no scar formation which agree with No et al. (2002).
Moreover, sections of the treated groups were highly filled with newly formed blood vessels. In −ve and +ve control groups, new blood vessel formation delayed and observed at Day 8, while the granulation in the epidermis recorded first in the treatment groups at Day 8.
At Day 16, sebaceous glands observed in all groups except –ve CG, while hair follicles observed only in treatment groups; remarkably in Ch 1% and 2% groups. On the other hand, the thickness of the epidermis in Ch 1% & 2% groups were higher than Ch 3%, but the complete wound healing observed only in Ch 1% and 2% treatment groups at the end of the experiment.
By contrast, regeneration of tissues in wounds of treated groups were achieved at Days 4 and 8 but, delayed in control groups till Day 8. Moreover, the thickness of epidermis was increased significantly in Ch 1% and 2% treated groups at Days 4 and 8 comparable to control groups.
Total leucocyte count
The total averages of leucocyte count in the treatment groups versus control groups after 4, 8, 12, 16 days are shown in Table 2. Despite of there was no significance difference (p > 0.05) in the total leucocytes count between groups; the present results exhibit remarkable gradual decreasing in leucocyte number with an increasing period of treatment with chitosan. The maximum total leucocyte count estimated at Day 4 for all treated groups. The highest averaged values were 8.3, 6.1 and 11.6 for 1%, 2% and 3% chitosan treated groups, respectively. However, these values had decreased gradually at Days 8, 12 and reached the lowest values at Day 16 in the treated groups. On the other hand, control groups showed variable pattern at Days 4–16 with relatively lower values (Table 2). This indicates that chitosan promotes the inflammatory phase faster than control groups, as mentioned by both of Muzzarelli et al. (1990) and Seyfarth et al. (2008).
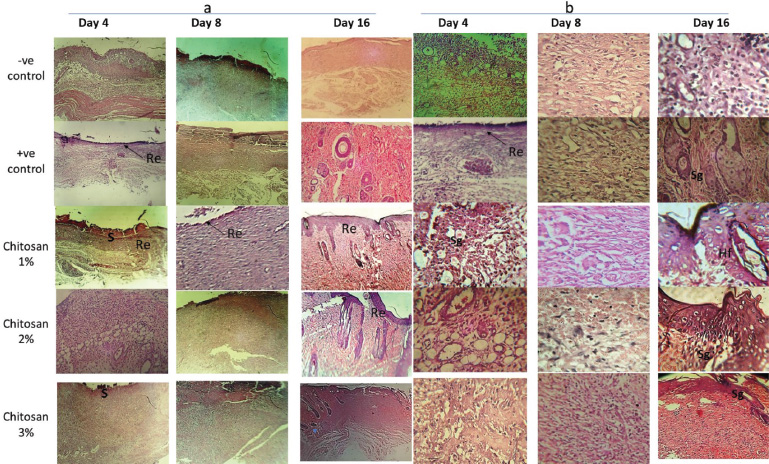 | Figure 4. Histological examination of rat skin wounds H&E staining for control (-ve & +ve) versus treated groups (ch. 1%, 2% and 3%) after 4, 8 and 16 days of treatment; re-epithelization indicated by arrows, S (scar formation), Sg; sebaceous glands and Hf; hair follicle. (a) magnification 50×. (b) magnification 100×. [Click here to view] |
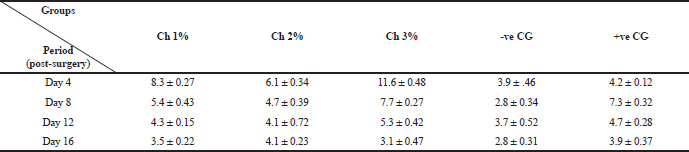 | Table 2. Showing average ±standard deviation total count of leucocytes in the chitosan treated groups versus control groups during this study. [Click here to view] |
CONCLUSION
The present study provides a commercial importance of freshwater crab P. niloticus which considers as a good source of chitosan extracted from its exoskeleton that evaluated in present study for wound healing application. The present results ensure the efficiency of chitosan in accelerating the wound healing process with less inflammatory phase.
ACKNOWLEDGMENTS
The first author is very grateful to Professors Omer Elmenshawy and Awaad M. El-Sayed, Faculty of Science, Al-Azhar University in reviewing this manuscript. Also, the author would like to thank Dr. Saad Moghannem and Dr. Ahmed Ali Radwan for their help in preparation of chitosan films, Faculty of Science, Al-Azhar University.
CONFLICT OF INTERESTS
The authors declare that there are no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The present studied animals were treated according to “Ethical guidelines for the care and use of animals in education & scientific Research” and approved through the Ethical Committee of Faculty of Pharmacy, Ain Shams University. (Reference Number: ENREC-ASU.2019-99)
FUNDING
No funding was obtained for this study.
AUTHORS’ CONTRIBUTIONS
M.A. collected the crab specimens and extracted the chitosan material; the wound healing experiment was investigated also by M.A. E.A. involved in the physical characterization of the chitosan material. M.A. and E.A. contributed in the preparing and editing the whole manuscript. All authors have read and approved the manuscript.
REFERENCES
Ahmed NMI, Sastry TP. Wound dressing application of chitosan based bioactive compounds. Inter J Phar Life Sci, 2011; 2(8):991–6.
Archana D, Dutta J, Dutta P. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Inter J Biol Macromolec, 2013; 57:193–203. CrossRef
Azuma K, Izumi R, Osaki T, Ifuku S, Morimoto M, Saimoto H, Minami S, Okamoto Y. Chitin, chitosan, and its derivatives for wound healing: old and new materials. J Funl Biomat, 2015; 6(1):104–42. doi: 10.3390/jfb6010104. CrossRef
Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol, 2010; 56:290–9. CrossRef
Barbul A, Regan MC. Biology of wound healing. In: Fisher JA (ed). Surgical basic science. Mosby-Yearbook, St. Louis, pp 68–88, 1993.
Bolat Y, Bilgin Åž, Günlü A, Izci L, Koca SB, Çetinkaya S, Koca HU. Chitin-Chitosan yield of freshwater crab (Potamon potamios, Olivier 1804) shell. Pak Vet J, 2010; 30(4):227–31.
Brandis D, Storch V, Turkey M. Taxonomic and zoogeography of the freshwater crabs of Europe, North Africa and the Middle East (Crustacea, Decapoda, Potamidae). Senckenberg. Biologica, 2000; 80:5–56.
Bujang A, Nur ‘Adila S, Suyatma NE. Physical properties of chitosan films as affected by concentration of lactic acid and glycerol. In fourth International Conference on Biology, Environment and Chemistry IACSIT Press, Singapore, 2013; 58. doi: 10.7763/IPCBEE. 2013. V58. 6 CrossRef
Cho YW, Cho YN, Chung SH, Yoo G, Ko SW. Water-soluble chitin as a wound healing accelerator. Biomaterials, 1999; 20:2139–45. doi: 10.1016/S0142-9612(99)00116-7. CrossRef
Cordoso CA, Favoreto S, Oliveira LL, Vancim JO, Barban GB, Ferraz DB, Silvia JS. Oleic acid modulation of the immune response in wound healing a new approach for skin repair. J Immunol, 2010; 216(3):409–15. CrossRef
Daniela E, Camelia EO. Functionalized Chitosan and its use in pharmaceutical, biomedical and biotechnological research. Chem Engg Comm, 2008; 195:1269–91. CrossRef
De Oliviera CE, Magnani M, Sales CV, Potes ALS, Campos-Takaki GM, Stamford TCM, Souza EL. Effects of chitosan from Cunninghamella elegans on virulence of post harvest pathogenic fungi in table grapes (vitis Labrusca l.) Int J Food Microbiol, 2014; 171:54–61. doi: 10.10161j.ijfoodmicro.2013.11.006. CrossRef
Dorsett-Martin WA. Rat models of skin wound healing: a review. Wound Rep Reg, 2004; 12:91–599. CrossRef
Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng, 2015; C(48):651–62. CrossRef
Gal P, Kilik R, Mokry M, Vidinsky B, Vasilenko T, Mozes S, Bobrov N, Tomori Z, Bober J, Lenhardt L. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: standardization of semi-quantitative and quantitative histological assessments. Veter Medic, 2008; 53(12):652–9. CrossRef
Gokarneshan N. Review article- role of chitosan in wound healing - a review of the recent advances. Glob J Addic Rehab Med, 2017; 4(3):555–636. doi: 002 10.19080/GJARM.2017.04.555637. CrossRef
Gopalan NK, Dufresne A. Crab shells chitin whiskers reinforced natural rubber nanocomposites. Processing and swelling behavior. Biomacromolecules, 2003; 4(3):657–65. CrossRef
Hong KN, Mun YL. Isolation of chitin from crab shell waste. J Korean Soc Food Nutr, 1995; 24:105–13.
Hossain MS, Iqbal A. Production and characterization of chitosan from shrimp waste. J Bangladesh Agril Univ, 2014; 12(1):153–60. CrossRef
Janis JE, Kwon RK, Lalonde DH. A practical guide to wound healing. Plast Reconstr Surg, 2010; 125:230–44. CrossRef
Kammoun M, Haddar M, Kallel TK, Dammak M, Sayari A. Biological properties and biodegradation studies of chitosan biofilms plasticized with PEG and glycerol. Int J Biol Macromol, 2013; 62:433–8. doi: 10.1016/j.ijbiomac.2013.09.025. CrossRef
Mori T, Okumura M, Matsuura M, Ueno K, Tokura S, Okamoto Y, Minami S, Fujinaga T. Effects of chitin and its derivatives on the proliferation and cytokine production of fibroblasts in vitro. Biomaterials, 1997; 18:947–51. doi: 10.1016/S0142-9612(97)00017-3. CrossRef
Muzzarelli R, Tarsi R, Filippini O, Giovanetti E, Biagini G, Varaldo PE. Antimicrobial properties of N-carboxybutyl chitosan. Anti Agents Chemother, 1990; 10:2019–23. doi: 10.1128/AAC.34.10.2019. CrossRef
Nilani P, Pranavi A, Duraisamy B, Damodaran P, Subhashini V, Elango K. Formulation and evaluation of wound healing dermal patch. Afr J Pharm Pharmacol, 2011; 5(9):1252–7. CrossRef
No HK, Park NY, Lee SH, Meyers SP. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol, 2002; 74:65–72. doi: 10.1016/S0168-1605(01)00717-6. CrossRef
Norhidayah MA, Faridah M, Azrilawani A, Alyza AA. Chitin and chitosan extraction from Portunus pelagicus. Malaysian J Analyt Sci, 2017; 21(4):770–7. doi.: 10.17576/mjas-2017-2104-02 CrossRef
Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm, 2015; 97:417–26. CrossRef
Seyfarth F, Schliemann S, Elsner P, Hipler UC. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int J Pharmaceut, 2008; 353:139–48. CrossRef
Shahidi F, Synowiecki J. Isolation and characterization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem, 1991; 39:1527–32. CrossRef
Shakeel A, Ikram S. Chitosan based scaffolds and their applications in wound healing. Achievem Life Sci, 2016; 10:27. CrossRef
Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of Chitosan and its derivatives in regenerative medicine. J Surg Res, 2009; 133:185–92. CrossRef
Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S, Shigemasa Y. Chitin and chitosan induce migration of bovine polymorphonuclear cells. J Vet Med Sci, 1994a; 56:761–2. doi: 10.1292/jvms.56.761. CrossRef
Usami Y, Okamoto Y, Minami S, Matsuhashi A, Kumazawa NH, Tanioka S. Shigemasa Y. Migration of canine neutrophils to chitin and chitosan. J Vet Med Sci, 1994b; 56:1215–6. doi: 10.1292/jvms.56.1215. CrossRef
Vidinsky B, Gal P, Toporcer T, Longauer F, Lenhardt L, Bobrov N, Sabo J. Histological study of the first seven days of skin wound healing in rats. Acta Vet Brno, 2006; 75:197–202. CrossRef
Yilmaz E. Chitosan: a versatile biomaterial, Adv Exp Med Biol, 2004; 553:59–68. CrossRef