INTRODUCTION
Direct oral anticoagulants (DOACs) are widely prescribed in general practice to prevent clot formation. Rivaroxaban (RXN) an oxazolidine derivative is a novel DOAC usually referred to as a blood thinner agent which is extensively used to prevent deep vein thrombosis or pulmonary embolism. RXN is chemically 5-Chloro-N-({(5S)-2-oxo-3[4-(3-oxomorpholinyl)phenyl]-1,3oxazolidin-5-yl}methyl)2-thiophene carboxamide with an empirical formula of C19H18C1N3O5S and 435.881g/mol as the molecular weight (Fig. 1). It was developed by Bayer and marketed by Janseen Pharmaceuticals, initially approved by FDA on July 1, 2011 [1–3]. Film-coated tablets of RXN are sold under the trade name Xarelto (2.5 mg, 10 mg, 15 mg, and 20 mg). It competitively inhibits free clot bound factor Xa and decreases the clotting ability of blood and prevents the formation of blood clots. In 2019, USFDA has approved RXN as an add-on drug with aspirin for secondary prevention of peripheral arterial disease and acute coronary syndrome [1,2,4]. The development of new analytical methods is flourishing up day by day due to the advancement of hyphenated analytical instruments. The advancement of analytical instruments has led to cost-effective methods with improved precision and accuracy. An extensive review of various databases such as Science Direct, Springer, Pubmed, Scopus, Taylor & Francis, and Web of Science was accomplished. The published review lacks the detailed information required for the researchers with respect to the various published methods (Analytical, stability indicating, and bioanalytical methods), their merits and demerits, extraction techniques involved in the bioanalytical method, green assessment of the solvents used in various techniques [5]. Hence the present review surpasses the disadvantages of the existing review and embraces the future investigators with the overview of numerous simple and sophisticated analytical/bioanalytical methods for estimating RXN in different formulations (single and combined dosage forms) and biological matrixes. Also, since most of the methods [high-performance liquid chromatography (HPLC), HPLC/ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)] developed for the estimation of RXN is stability indicating, a special emphasis on the forced degradation conditions and their results are incorporated separately which in turn help to improve the formulation, manufacturing process and determine the storage conditions of RXN. Bioanalytical methods determine the concentration of RXN and its metabolites in biological fluids, i.e., blood, serum, plasma, and urine by HPLC, HPLC/UPLC-MS/MS. Challenges associated during method development include employing suitable extraction techniques such as solid phase extraction (SPE), liquid–liquid extraction (LLE), and protein precipitation to extract RXN from the complex composition of a biological sample (presence of many interfering substances). This review discusses the pros and cons of extraction techniques essential to removing biological matrices and the applicability of the method. In addition, prominence is sited on the sustainability of the reported methods and assessment of greenness profiles as per the National Environmental Method Index (NEMI). Last, a comprehensive view of all the published analytical methods, consisting of chromatographic conditions and the results obtained, merits/demerits, application of the bioanalytical methods along with the greenness profile, is outlined in tabular form for a quick summary. Figure 2 reveals the different analytical methods for the estimation of RXN in single and combined dosage forms from 2012 to 2023. The graphical representation of a number of articles published from 2012 to 2023 is provided in Figure 3.
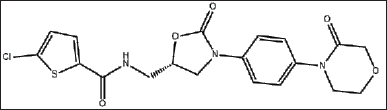 | Figure 1. Chemical structure of RXN. [Click here to view] |
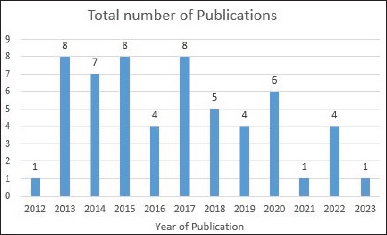 | Figure 2. Total number of analytical methods reported during the year 2012–2023. Databases: Science Direct, Springer, Pubmed, Scopus, Taylor & Francis and Web of Science. [Click here to view] |
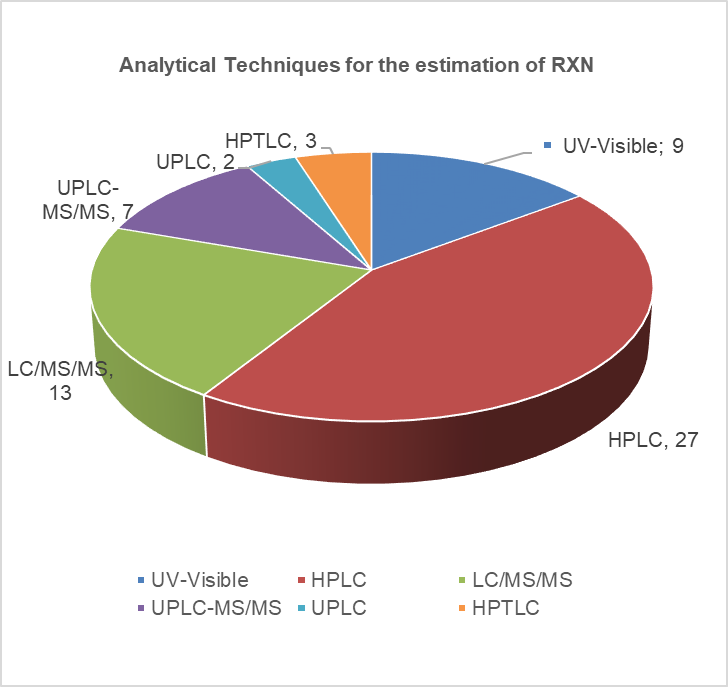 | Figure 3. Analytical methods for the estimation of RXN. [Click here to view |
ANALYTICAL METHODS FOR DETERMINATION OF RXN
Spectrophotometry
Ultra- violet (UV)-Visible spectrophotometry is a simple, non-destructive, cost-effective versatile technique used to analyse pharmaceutical substances. Because of its simplicity, precise, reliability, and minimum solvent usage, it is widely used for the estimation of RXN. Sekaran et al. [6] developed a novel method using dimethyl sulphoxide as the diluent and reported the apparent molar absorptivity (4.825 × 104 l/mol/cm) and Sandell’s sensitivity (2.262 × 10-3 µgcm−2) which represents the sensitiveness of the method (Fig. 4). Mustafa et al. [7] quantified RXN and also estimated the rate of drug release by in vitro study (Dissolution—USP Apparatus 2) medium. The drug release was 78% at 15 minutes using pH 4.5 acetate buffer containing 0.4% of sodium dodecyl sulfate as the dissolution medium. Muralikrishna et al. [8] reported a simple, sensitive, reproducible, and accurate method with methanol as the diluent. By using methanol as the diluent Kasad Pinaz et al. [9] reported a novel Area Under Curve method which would be better than zero-order UV spectrophotometric methods. Seshamamba et al. [10] reported derivative spectroscopy with two different reagents, 4-Chloro-7-nitrobenzo-2-oxa-1, 3-diazole (Method I) and p-Dimethylaminocinnamaldehyde (Method II) for the precise and accurate determination of RXN in bulk and in its tablet dosage forms. El bagary et al. [11] reported a thermodynamic simultaneous method for the estimation of cilostazol and RXN based on the oxidation of iron (III) in the presence of 1, 10-phenanthroline to form tris (1, 10-phenanthroline) iron (II) complex (ferroin). The authors evaluated the thermodynamic parameters (free energy, enthalpy, and entropy) of complexation, also estimated the molar absorptivity (53.02 × 103 and 1.28 × 103 l/mol/ cm) and sandell sensitivity (0.0069 and 0.28 μg/cm-2) for cilostazol and RXN, respectively. Simultaneous estimation of RXN and Clopidogrel (CPL) using three UV Spectrophotometric methods (First derivative spectrophotometric method, ratio derivative spectrophotometric method, and absorbance ratio method) with methanol as the diluent was reported by Sharaf et al. [12] The reported methods were novel and distinct from each other, whereas methanol is the solvent of choice in most of the methods. The different methods of analysis were Area under the Curve, Derivative spectrophotometry, and thermodynamic method which indicated the applications and importance of the UV spectrophotometric method for the Quality Control of RXN in pharmaceutical dosage forms [6–12]. The method and the spectrophotometric results were tabulated in Table 1.
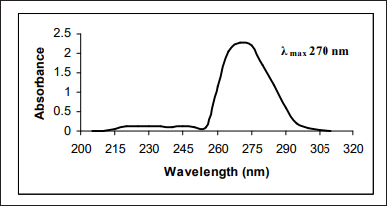 | Figure 4. UV spectrum for RXN (adapted from reference 7). [Click here to view] |
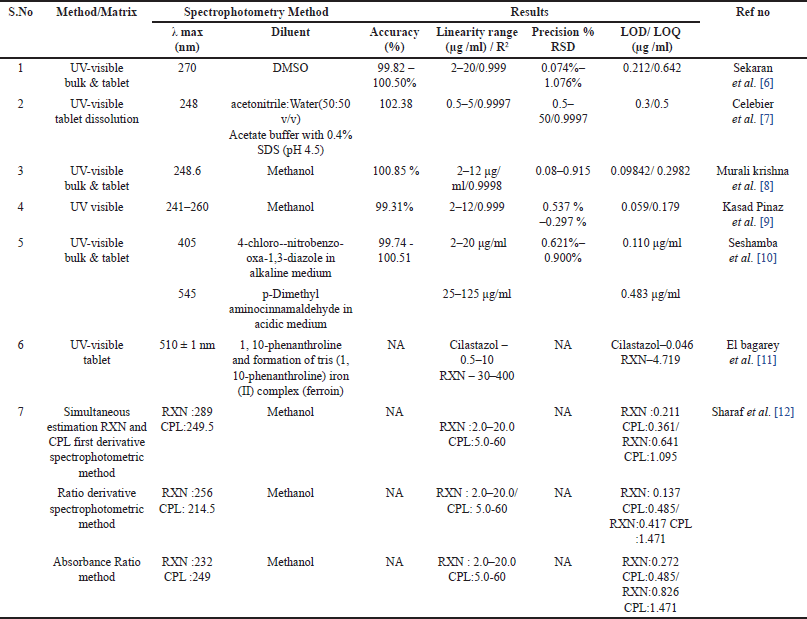 | Table 1. Spectrophotometry methods for determination of Rivaroxaban. [Click here to view] |
 | Figure 5. HPTLC densitogram of standard RXN (adapted from reference 15). [Click here to view] |
Chromatography methods
High-performance thin-layer chromatography (HPTLC)
HPTLC an effective tool in qualitative analysis, is rapid and economically friendly compared to the hyphenated techniques. Prawez et al. [13] developed an eco-friendly method using RP-18 Silica gel 60 F254S as the stationary phase and Ethanol: Water (7:3 v/v) as the mobile phase using a densitometric detection at 253 nm which resulted in an Rf of 0.71 minutes. The method was highly expedient with a shorter run time, minimal sample preparation, and excellent recovery of 99.20 % for the nano formulation (Fig. 5). Darshna et al. [14] developed a simple, specific, and cost-effective HPTLC method using Silica Gel 60 F254 under pure nitrogen stream as the stationary phase and Methanol: Toluene: Triethanolamine (7: 2.5:0.5 v/v) as the mobile phase. RXN was detected at 249 nm with an Rf of 0.60 minutes, linearity of 500–3,000 ng/spot, the limit of detection (LOD), and limit of quantification (LOQ) of 127.56 ng/spot and 386.57 ng/spot, respectively. The additives in the pharmaceutical formulations of the assayed analyte did not interfere with the determination of RXN. Both methods used an exclusive novel stationary phase, though the method showed a good recovery without compromising the usage of green solvents [13,14].
HPLC
HPLC, the most prevalent chromatographic method due to its simplicity, versatility, and broad scope. RP-HPLC uses a monolithic stationary phase and mixtures of organic solvents with buffers containing acidic/basic additives as mobile phases to separate moderately hydrophilic/hydrophobic compounds. Amelia et al. [15] Sahoo and Mekap et al. [16] and Eswarudu et al. [17] developed and validated a sensitive and accurate RP-HPLC method for the estimation of RXN. The chromatographic conditions of all three methods were similar, whereas optimization was done by varying the ratio of the mobile phase (Acetonitrile: Water). A sensitive and less time-consuming method with good recovery was achieved by Amelia et al. [15] Sunny et al. [18] developed an Ion Pair-RP-HPLC method using triethylamine as the ion pair reagent which improved the separation efficiency and peak shape of RXN. The method was cost-effective (Triethyl Amine Buffer (pH: 11.06): Acetonitrile (85:15 v/v)) and less time-consuming (Rt 1.56 minutes) than the reported RP- HPLC methods. A sensitive method using a novel peak additive (Glacial acetic acid) in the mobile phase to improve the peak shape and efficiency of the method was developed by Meenakshi and Nageswara [19] Shivsankar et al. [20] developed an accurate, sensitive, precise, linear method but with a longer retention time (Rt) of 7.4 minutes. However, optimization of the mobile phase ratio would have resulted in a rapid method. Chandra shekar et al. [21] using the C18 column coupled with the guard column developed an RP-HPLC method that eluted RXN at 3.32 minutes. Awatade et al. [22] reported a method with a shorter run time and performed the qualitative analysis by IR Spectroscopy. A cost-effective and sensitive method using Buffer : Acetonitrile (70 : 30) as the mobile phase and C18 column was developed by Khan et al. [23] Chromatographic results obtained from Simultaneous estimation by Rupali et al. [24] (RXN with CPL) and Sarkis et al. [25] (RXN with aspirin) resulted in a rapid, simple, reliable, precise and robust methods. The majority of the methods used C18 as the column with isocratic mode by use of different buffers (Acetate and phosphate buffer), peak modifiers (Triethyl amine, glacial acetic acid), varying the aqueous/organic composition (Water, Buffer/Acetonitrile, methanol), pH (Acidic/Basic) of the mobile phase and detected RXN by UV/PDA detector (220–280 nm). Researchers to develop a method employing eco-friendly solvents that would meet the SDGs. Analytical quality by design (AQbD), an extension of QbD offers a robust approach in the development of analytical methods leading to higher quality products and improved patient safety. An exclusive AQbD method by Santos et al. [26] for the enantiometric estimation of S - RXN and its chiral impurity R- RXN by RP-HPLC using a chiral stationary phase. The method was selective, linear, precise, accurate, and sensitive with a LOQ of 0.68 µg/ml for S-RXN and 1.0 µg/ml for the chiral impurity [15–26]. The chromatographic condition of the RP-HPLC methods and their results are presented in Table 2.
STABILITY INDICATING METHOD
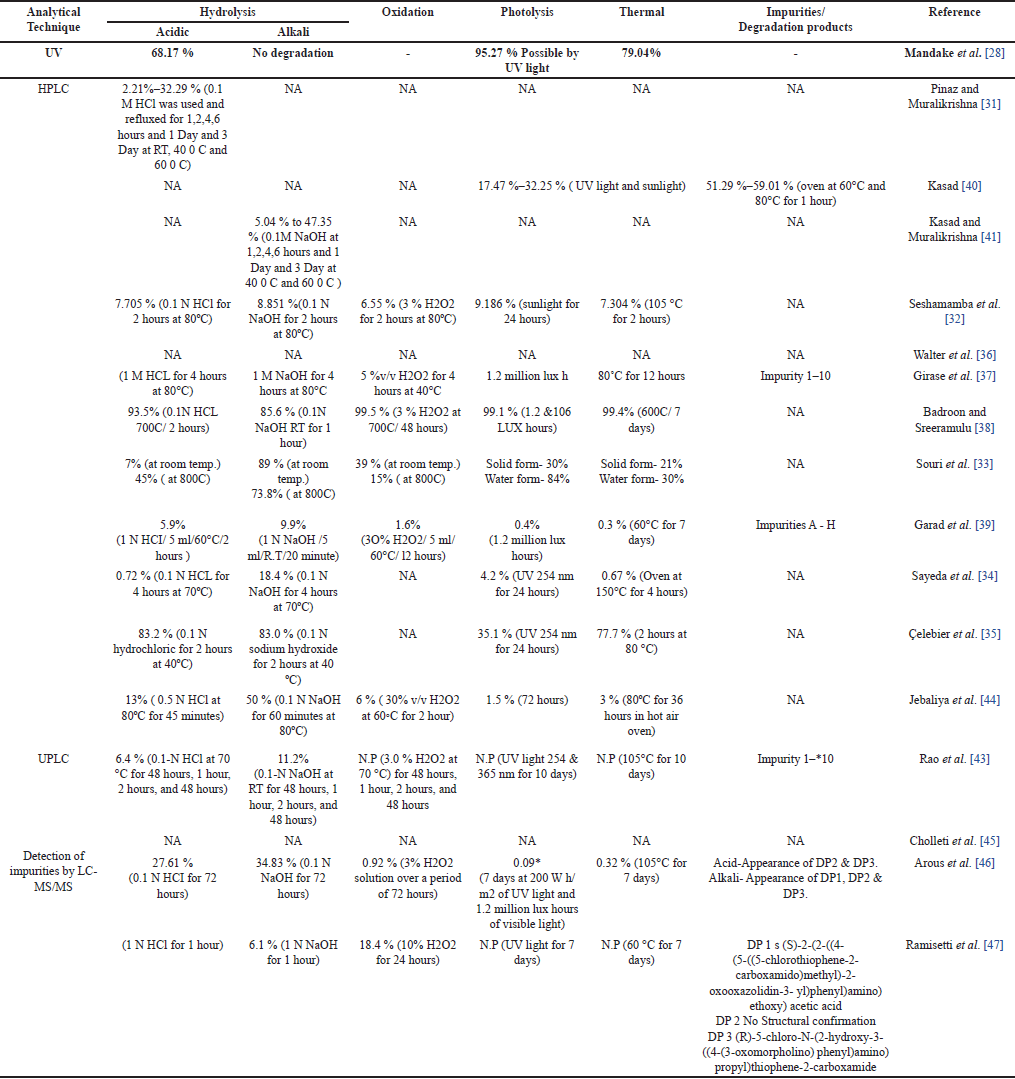
An ideal stability-indicating method discriminates the analyte and its degradation products (DPs) efficiently. Forced degradation studies aids in synthesis, formulation development, and packaging where knowledge of chemical behavior may be applied to enhance a therapeutic product [27].
UV Spectrophotometry
UV spectrophotometry is a cost-effective method that can be used for stress degradation studies of RXN and its degraded products. Girishchandra et al. [28] developed an accurate and reliable method in which the assay limit of RXN was as per the WHO limit (NLT 97 % and NMT 101.05 %). RXN degrade much especially on exposure to UV light and acidic medium but do not degrade in basic medium.
Chromatography methods
HPLC
HPLC methods can separate, detect, and quantify the various drug-related degradants of RXN formed on storage/manufacturing [29,30]. Hyphenated techniques (HPLC/UPLC–MS/MS) have become a golden standard in the characterization and quantitative analysis of degradants with improved selectivity, sensitivity, and efficiency. The chromatographic conditions of the stability-indicating methods are represented in Table 2. Kasad Pinaz et al. [31] developed and validated a simple, cost effective, less time-consuming, precise and accurate method in which the acidic (3 DP), basic (4 DP), photolytic, and thermal degradation peaks was clearly separated from RXN peak. However, characterization of the degradants would help researchers to understand the degradation pattern of RXN. Seshamamba et al. [32] Souri et al. [33] Zareen et al. [34] Çelebier et al. [35] performed a sensitive method with UV detection and isocratic elution. The methods were efficient in detecting the degradants. Seshamamba et al. [32] used a cyano column which has led to an efficient and sensitive than the other methods. Souri et al. [33] proposed a stability-indicating method that revealed the unstability of RXN in stress degradation conditions, also reported the dissolution profile which indicated >90% release of RXN within 45 minutes (Dissolution medium: 0.6% sodium lauryl sulfate in acetate buffer, Apparatus: paddle at 75 rpm). Walter et al. [36] developed a specific and stability-indicating method with an accuracy of 99.77%, hence the method can be applied for the analysis in human plasma and tablet dosage forms. Girase et al. [37] Badroon and Sreeramulu et al. [38] Sachin et al. [39] reported a stability-indicating analytical method using gradient elution and diode array detector (DAD) detection. The peak obtained during the forced degradation condition was homogenous and unaffected by degradation impurities [38]. Monitoring of the commercial route of synthesis and impurity profiling which is very important for the quality of RXN by RP-HPLC was performed by Girase et al. [37] and Sachin et al. [39] RXN was found to degrade significantly in Acid condition, but impurities (1–10) were well separated with an less RRT and the mass balance of all the peaks was found to be satisfactory. The method developed by Sachin et al. [39] eluted all the potential impurities of RXN (Impurity A-H) within 14 minutes [31–41].
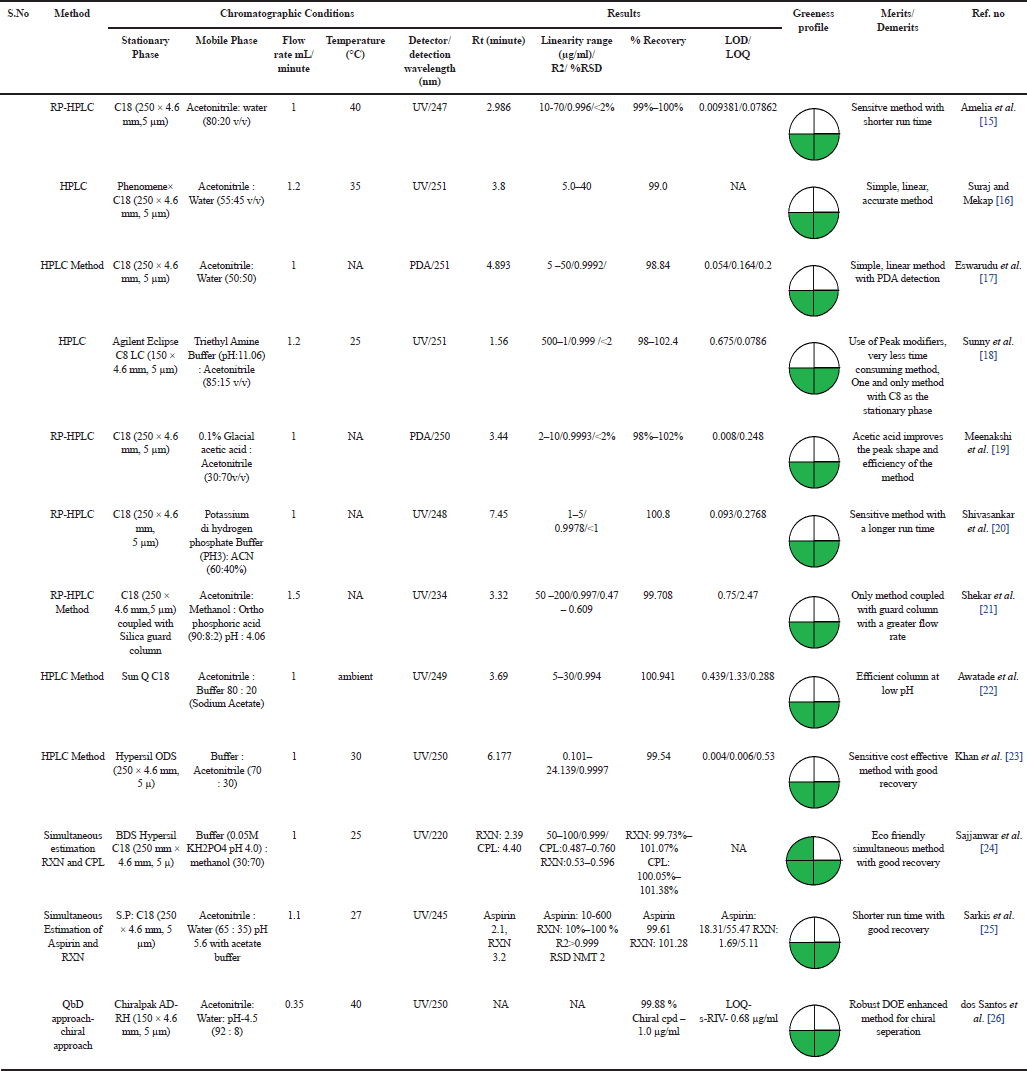 | Table 2. Estimation of Rivaroxaban by RP-HPLC. [Click here to view] |
UPLC
Ultra-performance liquid chromatography (UPLC) using shorter columns improves analyte resolution and sensitivity, lowers solvent consumption, and shortens run times. Rajan and Anver Basha et al. [42] and Rao et al. [43] reported a UPLC-DAD method for the estimation of RXN using gradient elution. Both the authors characterized the impurities. Rao et al. [43] developed a novel, rapid, stable, sensitive ion pair - UPLC - DAD method using ion pair reagents (octane sulphonic acid sodium) as the mobile phase for estimating RXN in tablet dosage forms. The anionic pair reagent improves the peak shape and retention of RXN and the impurities were characterized. Jabaliya et al. [44] transferred the HPLC method to UPLC owing to increased resolution, sensitivity, and reduction in run time [42–44]. The results of UPLC indicated an accurate, precise, linear method than the developed HPLC method. The method would be a great advantage if the impurities were characterized by UPLC-DAD.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
LC-MS/MS is an ideal technique for detecting nanomolar/picomolar quantities of various drugs, degradants, and food metabolites. Cholleti et al. [45] developed and validated an HPLC method and identified the degradants by LC-MS/MS method which separated enantiomers and all the process-related impurities of RXN. Arous et al. [46] investigated and found RXN susceptible to acid and base hydrolytic stress conditions. The authors identified, isolated, and characterized the degradation pathways using LC-MS, thin layer chromatography (TLC)-densitometry, NMR spectroscopy, and FT-IR. Three major DPs (DP-1, DP-2, DP-3) were detected, separated, and determined and two (DP-2 and DP-3) of them were further characterized by NMR spectroscopy and FT-IR. Ramisetti and Kuntamukkala et al. [47] presented a simple, rapid, and isocratic stability indicating HPLC method for the determination of RXN which detected 3 DPs formed under stress conditions. The structures of Process related impurities and the DPs (DP-1 and DP-3) were quantified by high-resolution ESI-MS/MS analysis and characterized by NMR spectroscopy (Process-related impurities and DP-3 [45–47].
The stability-indicating methods reported were highly sensitive with shorter Rt indicating a cost-effective green approach (Table 3). Most of the methods were stability indicating and detected the degradants employing a gradient program; however, characterization of the degradants formed in the HPLC method could be a better way ahead in these stress degradation studies. Characterization of impurities of RXN and degradation pathways by LC-MS/MS methods establishes biological safety which reveals the need and scope of impurity profiling of RXN in pharmaceutical research.
COMBINED ANALYTICAL TECHNIQUES
RXN is estimated by combined analytical techniques to indicate the accuracy of a method with other methods. These articles provide us the collective information in a single paper. Lories et al. [48] reported a HPLC, TLC, and UV method for the estimation of RXN in single dosage form. Method A : Stability indicating analytical method—HPLC method with phenomenex-C18, (150 × 4.6 mm, 5 µm) column and 1.2% w/v Potassium Dihydrogen Phosphate pH 3.5 ± 0.2 and Acetonitrile (70:30, v/v) as the mobile phase at a flow rate of 1.5 ml/minute with UV Detection at 280 nm to detect the impurities. RXN, Timolol Maleate degradant and Bimatoprost degradant eluted at 8.5, 5.3, and 4.2 minute, respectively. The Correlation Coefficient (R2), % recovery, LOD, and LOQ of Timolol Maleate degradant and Bimatoprost degradant were 0.999, 100.84, 0.62 µg/ml, 0.65 µg/ml, and 0.999, 99.67, 1.3 µg/ml, 3.15 µg/ml, respectively. Method B: TLC method used precoated Silica Gel GF254, 0.25 mm as the stationary phase and Chloroform: Isobutyl Alcohol (50:50 v/v) as the mobile phase with Densitometric detection at 280 nm. The Rf of RXN was 0.60 ± 0.02 with a % RSD of 0.50–4.80 µg/spot. Method C: First derivative method at a wavelength of 237.4 nm was linear over the range of 1.6–22.4 µg/ml. Method D: First derivative ratio method using acetonitrile as the solvent at a wavelength of 236 nm showed a R2, %RSD, %Recovery, LOD, LOQ of 0.9999, 1.072 %, 99.99 ± 0.242, 0.52 µg/ml and 1.85 µg/ml [48]. HPLC method emerged to be the sensitive and effective method in detecting the degradants at a short run time. Combinatorial methods help researchers explore the pros and cons of various methods from multiple angles and get more confined results.
BIO ANALYTICAL METHODS
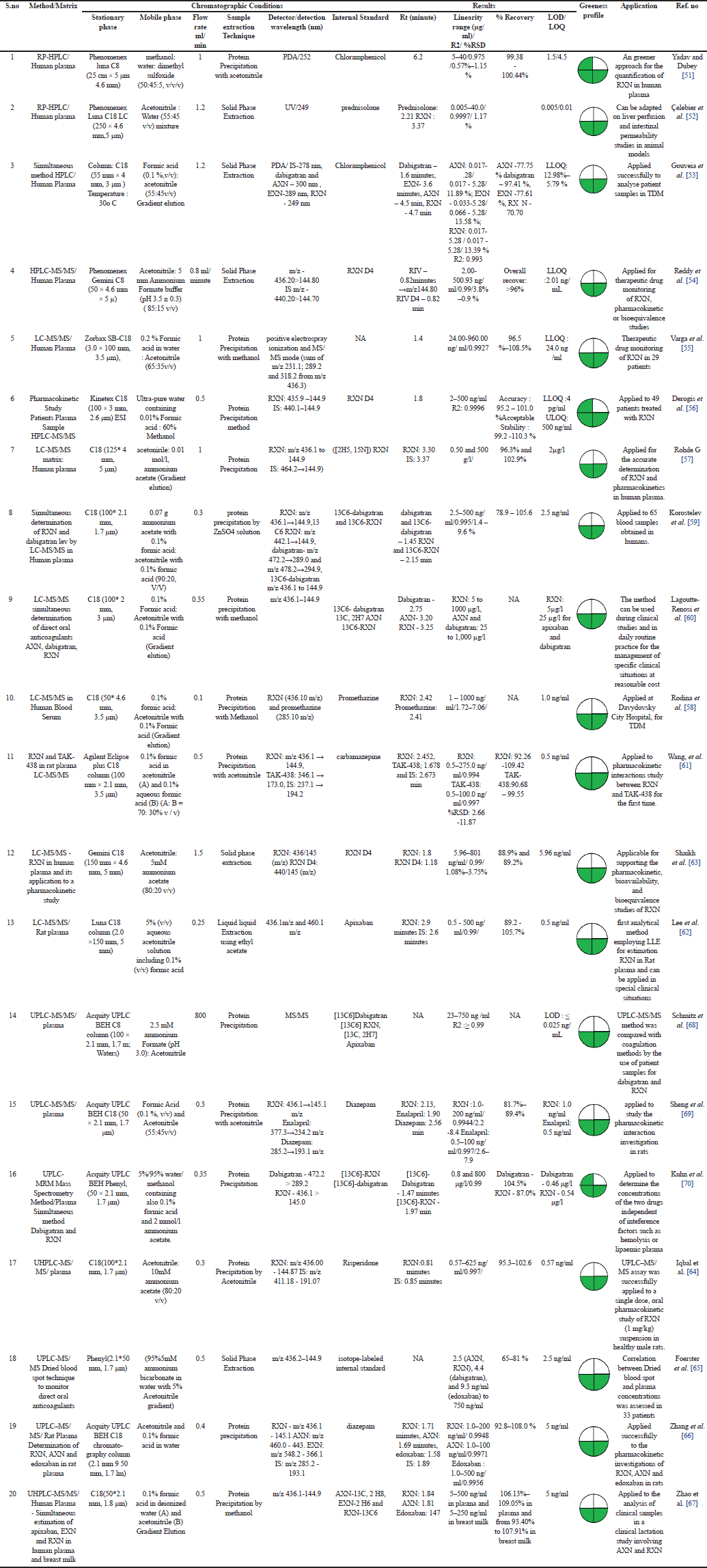
Bio-analytical method development is useful in the identity, purity, potency, and bioavailability of drugs. The data that are obtained from bioequivalence and pharmacokinetic studies are required for the successful evaluation of drugs in animal models and human trials. The development of effective bio-analytical methods is therefore crucial for the successful implementation of drug development. Hyphenated techniques have turned out to be quintessential in the assessment of pharmaceuticals in various biological samples. Bio-analytical methods employing HPLC/UPLC/hyphenated techniques are ephemeral and assist in controlling the quality of pharmaceuticals as well as in obtaining pharmacokinetic and toxicokinetic data of RXN in single and combined dosage forms. RXN was extracted from biological samples (Rat Plasma, human plasma/serum, patient samples) employing SPE, LLE, and protein precipitation. Numerous variations depending on the nature and amount of solvent/matrix in the extraction method will be optimized to achieve a better separation of RXN from the biological matrix. Structurally related internal standards used in the research were chloramphenicol, Prednisolone, carbamazepine, Apixaban (AXN), Diazepam, and Risperidone. Few researchers have used stable isotopically labeled (SIL) internal standards and deuterated internal standards which yield robust and reproducible results compared to structurally related internal standards.
UV Spectrophotometry
UV Spectrophotometry has emerged as an effective method for estimating the analyte from biological samples. Bhavyasri et al. [49] developed a novel UV Spectrophotometric bioanalytical method using human plasma as the matrix and detected RXN at 252 nm with Acetonitrile: Water (60: 40) as the diluent. De proteination of plasma with protein precipitation extraction using Acetonitrile depicted a good recovery (99.04 %–100.74 %), linear (1–20 ppm), LOD/LOQ (0.0121 µg/ml/ 0.0368 µg/ml), and precision (0.02%–0.03 %) which proved to be a simple, cost-effective and sensitive method in the estimation of RXN in spiked human plasma [49].
Chromatography Methods
HPTLC
HPTLC is the most flexible, reliable, and cost-efficient separation technique so become one of the most popular methods for bio analysis. Shukla et al. [50] reported a bioanalytical method for the estimation of RXN in human plasma. Protein precipitation with acetonitrile as the precipitating reagent gave a compact band of Rf at 0.44 minutes with a recovery of 66.95%–69.03 %. This method was applied to a pharmacokinetic study of RXN and estimated the Cmax (63.83 ng/ml) and tmax (47.3 ng/ml), area under the curve0-t (290 and 219.0 ng/ml/hour), and t1/2 (6.87 and 6.64 hours) for test and marketed formulation of RXN. Also, the study could be extended for the estimation of RXN in urine [50].
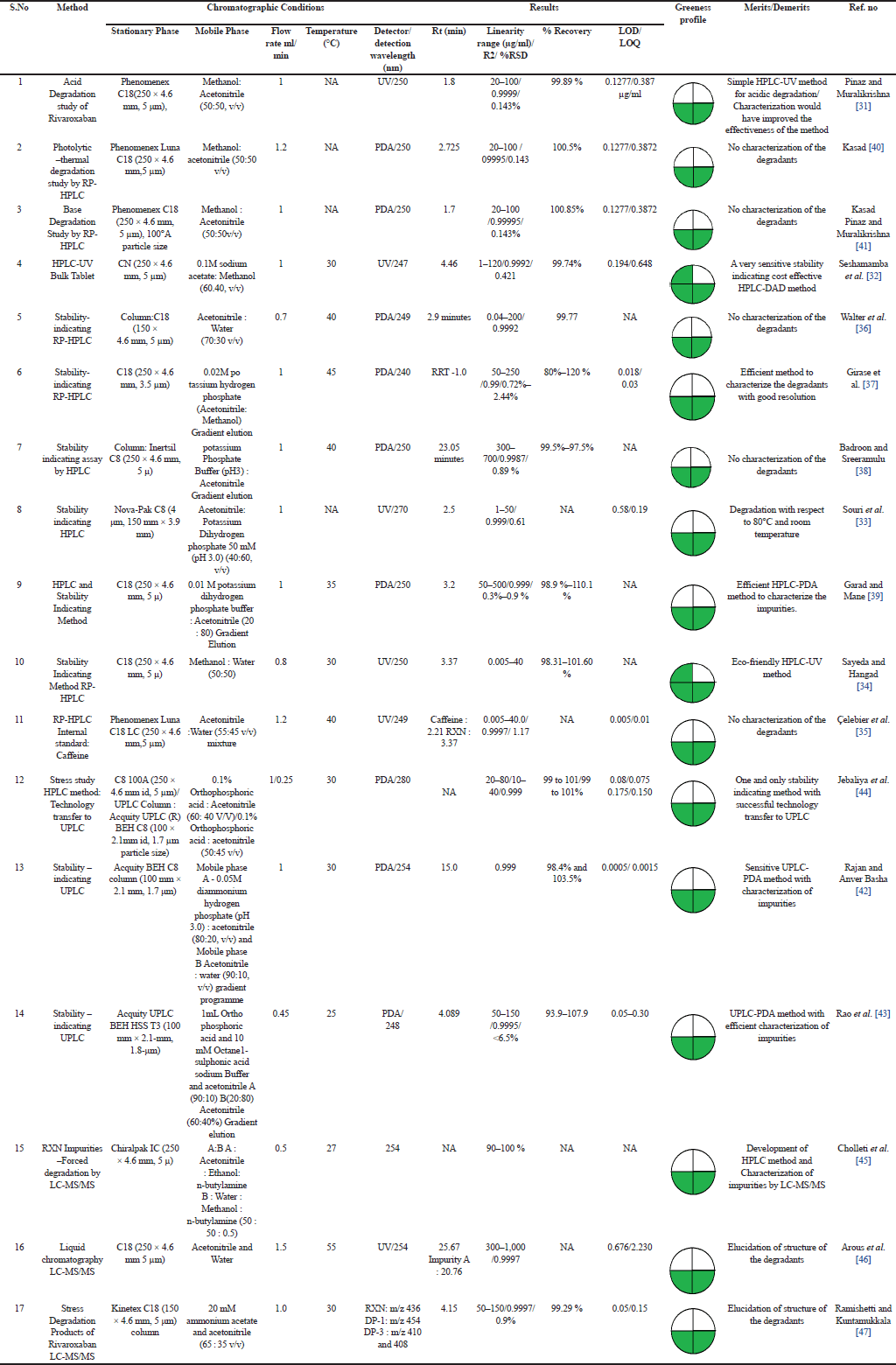 | Table 3. Stability indicating UV/HPLC/UPLC/MSMS methods for determination of rivaroxaban. [Click here to view] |
HPLC
Bioanalytical method development and validation using HPLC assist in performing quality control of pharmaceuticals as well as in obtaining pharmacokinetic and toxicokinetic data of drugs in less time. Protein precipitation which provides a quick sample clean up and better recovery was utilised by Yadav et al. [51] to develop a cost-effective bioanalytical method for the determination of RXN. Human plasma was spiked with RXN and the precipitating agent (acetonitrile) and centrifuged for 10 minutes at 1,000 rpm. The supernatant was injected into the HPLC and the results were monitored. Another author used SPE which effectively extracted RXN from human plasma with high recovery efficiency. Selective extraction of RXN based on their affinity to Phenomenex Strata-X 33-µm polymeric reversed phase cartridge facilitating the removal of interfering substances and enrichment of RXN by washing (methanol: water (50: 50, v/v)). RXN was eluted by methanol: glacial acetic acid (99: 1, v/v), followed by evaporation with a stream of nitrogen and finally reconstituted with the mobile phase before analysis [52]. This method was sensitive and increased the RXN concentration in samples by 7.5 times. Gouveia et al. [53] developed a fast and simple HPLC-DAD and applied to quantify the four currently marketed DOAC (AXN, EXN, dabigatran, and RXN). SPE using Oasis PRIME HLB cartridges (1cc/30 mg) for conditioning, the solid residue reconstituted with 0.1 % formic acid, and methanol (50:50, v/v) showed efficient recovery of all the anticoagulants. Also, the developed cost-effective HPLC-DAD bio analytical method can be more widely used in clinical laboratories than expensive LC–MS/MS [51–54].
LC-MS/MS
LC–MS/MS, a selective and sensitive technique used for pharmacokinetic studies, metabolites identification in biosamples. Reddy et al. [54] reported a precise, reproducible LC/MS/MS method that can be used for the TDM of RXN. RXN was found to be for about 5 freeze-thaw cycles and reconstituted samples were stable up to 72 hours post to extraction which indicates the effectiveness of the method. Varga et al. [55] Derogis et al. [56] Rohde [57] Rodina et al. [58] developed and validated a rapid, sensitive, high-throughput LC-MS/MS method for the estimation of RXN in human plasma and applied the method successfully in clinical studies. The authors adopted the protein precipitation method using methanol for the extraction of RXN from the biological matrix. The protein precipitation method was effective in extracting the analyte from clinical samples. Also incorporating gradient elution techniques leads to enhanced peak resolution, faster analysis times, and better detectability [56–58]. Simultaneous determination of RXN and dabigatran by LC-MS/MS in human plasma employing protein precipitation with zinc sulphate as the precipitating agent results in effective precipitation through hydrophobic interactions of protein molecules. Also, this method extracted the analyte by precipitating the proteins efficiently even when applied to 65 blood samples obtained from humans [59]. Simultaneous estimation of DOAC (AXN, dabigatran, RXN) in human plasma by LC-MS/MS employing protein precipitation technique was reported by Lagoutte-Renosi et al. [60] To compare the pharmacokinetics of RXN and TAK-438 in rats when being administered alone or being co-administered, Wang et al. [61] developed a simple, rapid and reliable method for simultaneous determination of the drugs in rat plasma by LC–MS/MS. Lee et al. [62] reported the first analytical method employing LLE for determining RXN in rat plasma which can be applied in special clinical situations. LLE is the classical method used for isolation, especially from water and biological fluid samples. Ethyl acetate, dichloromethane, and their mixtures are among the preferred extraction solvents. LLE was performed by mixing aliquot of a rat plasma and RXN with the IS and vortexed for a minute with the addition of Ethyl acetate. After centrifugation, the supernatant was collected, dried, and reconstituted with acetonitrile. The resulting solution was centrifuged and the supernatant solution was injected into the LC-MS/MS system and the results were monitored [55–63].
UPLC-MSMS
In recent years, a UPLC coupled with mass spectroscopy (MS) has been developed to estimate the biological samples, with better accuracy, sensitivity, precision, and high throughput. Iqbal et al. [64] developed UPLC/MS/MS method for the estimation of RXN in pharmaceutical dosage forms in the biological matrix (human Plasma) employing protein precipitation. All the other methods were simultaneous estimation of RXN in combined dosage forms by UPLC/MS/MS. Foerster et al. [65], Zhang et al. [66], Zhao et al. [67], Schmitz et al. [68], Sheng et al.[69], and Kuhn et al. [70] developed UPLC/MS/MS method for the estimation of RXN in combined pharmaceutical dosage forms in the biological matrix (human plasma/rat plasma) employing protein precipitation. Foerster et al. [65] developed a dried blood spot (DBS) assay that facilitates the sufficient extraction of all DOAC (AXN, dabigatran, EXN, and RXN). Consistent extraction recoveries and matrix effects of all samples were achieved in a dual extraction process of DBS (ultrasonic bath and shaking), which were treated with extraction solvent (methanol/water, 95/5 % v/v). The method was highly advantageous such that the patients can sample DBS at home within the dosing interval. Kuhn et al. [70] reported a rapid method with a run time of 2.5 minutes which was found to be one of the fastest methods in the estimation of DOAC. The method reported by Schmitz et al. [68] presented the highest precision for the DOAC compared to all the methods [64–70]. Table 4 overviews the LC parameters, validation, and application of the bioanalytical methods. All the UPLC/MS/MS methods were applied for the pharmacokinetics studies in patients, and the results obtained revealed the sensitiveness of the method. Most of the methods used protein precipitation as the major extraction technique followed by the SPE technique, the % recovery obtained with both methods was within the limits. Whereas, micromethods with a limited amount of extraction phase than the sample have emerged as an advantageous method than the usual methods. Syringe-based SPE (Microextraction by packed sorbent), disposable-pipette extraction, liquid-phase microextraction, thin-film microextraction, single-drop microextraction, sorbent-based microextraction techniques, dispersive liquid-liquid microextraction, electromembrane extraction and stirbar sorptive extraction are the numerous micromethods used in clinical investigations. In a nutshell, the incorporation of microsampling techniques and microextraction would be a tremendous spike in the area of bioanalytical research.
 | Table 4. Degradation Results of Rivaroxaban by UV/HPLC/LC-MS/ [Click here to view] |
GREENER ASSESSMENT OF THE REPORTED LIQUID CHROMATOGRAPHIC METHODS
Sustainable Development Goals 2030 were implemented to fulfill human development goals without exhausting resources for future generations [71,72]. Among the 17 SDGs, SDG Goal 12 (ensure sustainable consumption and production patterns) espouses Green analytical chemistry which deals with the development of efficient and sustainable chemical processes, thus reducing the environmental impacts [73]. NEMI, eco-scale assessments, green analytical procedure index, analytical method volume intensity, and analytical greenness metric are the several tools to evaluate the greenness of analytical methods. NEMI owing to its easiest and rapid greenness assessment, represented by a pictogram provides an adequate inference about the environmental effects of the investigated method. The circular pictogram divided into four quadrants (Fig. 6), where A = persistent, bio-accumulative, and toxic (PBT), B = hazardous, C = corrosive, and D = waste (run time × flow rate) employs the summary of the greenness of the method. The greenness profile of all the reported analytical methods for RXN was assessed and summarised in a tabular form, the quadrants were filled green for the greener methods while left blank for the non-greener ones. All the methods used acetonitrile (PBT and hazardous) and methanol (Not PBT but hazardous) as the mobile phase. Hence, the method with methanol as the mobile phase would show three of the quadrants green (PBT, corrosive, and waste), whereas acetonitrile as the mobile phase would show two quadrants green [74–77]. All the reported methods were found to be non-corrosive (pH 2–12) and generated a low amount of waste (less than 50 g). The greenness assessment using NEMI suggested that among the HPLC methods (12), one method was 75 % and the remaining 11 methods were 50 %. The stability indicating methods (18) was 75 % for 2 methods and 50 % for the remaining. The greenness profile of the bioanalytical methods was 75 % for 3 methods and 50 % for 17 methods. HPTLC methods reported a 100 % greenness profile among all the other analytical techniques. Subsequently, the replacement of toxic solvents like acetonitrile with ethanol or water could be more ecologically to make the existing analytical methods more sustainable meeting the requirement of SDG12. The reported methods pave a roadmap for future researchers that there is a need to use eco-friendly solvents for the chromatographic separation of RXN without compromising the sensitivity of the analytical methods.
 | Figure 6. Greeness assessment quadrants by NEMI. [Click here to view] |
 | Table 5. Bioanalytical methods for determination of rivaroxaban. [Click here to view] |
CONCLUSION
The review elaborates on the different analytical methods of RXN which will be helpful to the researchers. Validation of all the methods was performed as per ICH guidelines and found to be linear, accurate, precise, specific, and robust. Stability-indicating methods revealed the degradation profile of the drug. Protein precipitation was found to be the most preferred extraction technique adopted for sample pretreatment. Miniaturized novel sample extraction methods can be adopted in the future for the extraction of complex matrices. A substantial emphasis on examining the greenness profile by NEMI, which indicated 50% green for 90% of the methods. Also, concepts of “blueness” and “whiteness” assessment using the recently introduced blue applicability grade index and Red-Green-Blue 12 (RGB 12) algorithms can be incorporated. To put it briefly there is a pressing need for developing eco-friendly methods and applying them in the analytical techniques for the separation of RXN from biological matrices and finished drug products. Future trends incorporating miniaturized techniques would pave a roadmap for the estimation of RXN.
LIST OF ABBREVIATIONS
AQbD: Analytical Quality by Design; AXN: Apixaban; CPL: Clopidogrel; DAD: Diode Array Detector; DMSO: Dimethyl Sulphoxide; DOAC: Direct oral anticoagulants; DP:Degradation Product; EXN: Edoxaban; HPLC: High Performance Liquid Chromatography; HPTLC: High Performance Thin Layer Chromatography; LOD: Limit of Detection; LOQ: Limit of Quantification;
LC-MS/MS: Liquid Chromatography Tandem Mass Spectrometry; PBT: Persistent, Bioaccumulative, Toxic; R2: Correlation Coefficient; Rt: Retention Time; RXN: Rivaroxaban; TLC: Thin Layer Chromatography; UPLC: Ultra Performance Liquid Chromatography; UPLC-MS/MS: Ultra Performance Liquid Chromatography Tandem Mass Spectrometry; UV: Ultra- Violet.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. National Centre for Bio Technology Information. Pub chem compound summary for CID 9875401, Rivaroxaban, Bethesda, MD: National Centre for Bio Technology Information; 2020. Avaliable from: https://pubchem.ncbi.nlm.nih.gov/compound/RXN
2. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacokinetic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16.
3. Waheed SM, Kudaravalli P, Hotwagner DT. Deep vein thrombosis (DVT). [Internet]. Treasure Island, FL: Stat Pearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507708
4. Samama MM. The mechanism of action of rivaroxaban -an oral , direct factor Xa inhibitor -compared with other anticoagulants. Thromb Res. 2011;127(6):497–504. CrossRef
5. Recber T, Haznedaroglu IC, Celebier M. Review on characteristics and analytical methods of rivaroxaban. Crit Rev Anal Chem. 2022;52(4):865–77. CrossRef
6. Sekaran CB, Bind VH, Damayanthi MR, Sireesha A. Development and validation of UV spectroscopic method for determination of rivaroxaban. Sch Res Library Der Pharma Chem. 2013;5(4):1–56.
7. Çelebier M, Kaynak M, Alt?nöz S, ?ahin S. UV Spectrophotometric method for determination of the dissolution profile of rivaroxaban. Dissolution Technol. 2014;21:56–9.
8. Murali Krishna K. Spectrophotometric method for determination of rivaroxaban in bulk and tablet formulation and its validation. Inventi Rapid Pharm Anal Qual Assur. 2013;3(3):109–13; doi: https://doi.org/Inventi:ppaqa/699/13
9. Kasad Pinaz A, Muralikrishna KS. Area under curve spectrophotometric method for determination of rivaroxaban in bulk and tablet formulation and its validation. Asian J Res Pharm Sci. 2013;3(3):109–13.
10. Seshamamba BSV, Sekaran CB. Spectrophotometric analysis for the quantification of rivaroxaban in bulk and tablet dosage form. Int J Med Pharm Sci. 2017;7:21–34.
11. El-Bagary RI, Elkady EF, Farid NA, Youssef NF. A validated spectrophotometric method and thermodynamic studies for the determination of cilostazol and rivaroxaban in pharmaceutical preparations using Fe-phenanthroline system. Anal Chem Lett. 2017;7:676–88. CrossRef
12. Sharaf El-Din M, Ibrahim F, Shalan SH, Abd Ei -Aziz H. Spectrophotometric methods for simultaneous determination of rivaroxaban and clopidogrel in their binary mixture. Pharm Anal Acta. 2018;9:575.
13. Alam P, Ezzeldin E, Iqbal M, Anwer MK, Mostafa GA, Alqarni MH, et al. Ecofriendly densitometric RP-HPTLC method for determination of rivaroxaban in nanoparticle formulations using green solvents. Royal Soc Chem. 2020;10:2133–40.
14. Vaghela D, Patel P. High performance thin layer chromatographic method with densitometry analysis for determination of rivaroxaban from its tablet dosage form. Int J Pharm Pharm Sci. 2014;6(6):383–6.
15. Amelia M, Avachat Milind B, Yamgar. RP-HPLC development & validation for the estimation of rivaroxaban in bulk& tablet dosage form. World J Pharm Pharm Sci. 2017;6(8):1775–84.
16. Suraj S, Mekap SK. Assay comparison of Rivaroxaban by new HPLC method with an existing method in tablet dosage form. Pharm Biol Eval. 2017;4(3):180–2.
17. Eswarudu MM, Lalitha devi A, Pallavi K, Srinivasa Babu P, Nandini Priya S, Sabeeha Sulthana SK. Novel validated RP-HPLC method for determination of rivaroxaban in bulk and its pharmaceutical dosage form. Int J Pharm Sci Rev Res. 2020;64(1):183–7.
18. Sunny A, Dr. Sreedhar C, Srinivas Rao T, Akkama HG, Mahapatra A. Development of new analytical method and validation for quantitative estimation of Rivaroxaban in formulation & bulk drug. Int J Sci Res Edu. 2017;5(5):6469–78.
19. Meenakshi R, Nageswara Rao R. RP-HPLC method development and validation for determination of rivaroxaban in the pure and pharmaceutical dosage forms. J Chem Pharm Res. 2016;8(12):38–44.
20. Shivashankar V, GandhimathiM, Ravi TK. Development of validation RP HPLC method for estimation of rivaroxaban in pharmaceutical formulation. Int J Pharm Anal Res. 2015;4(4):106–410.
21. Sekhar KC, Vani PS, Lakkshami AD, Devi Ch LL, Barik A, Narendra D. A new method and validation for analysis of rivaroxaban in formulation by RP-HPLC.” Narendra Devanaboyina Res Desk. 2012;1(1):24–33.
22. Awatade PR, Veer VS, Kasabe AJ, Dhepe SD. Analytical method development and validation of rivaroxaban in bulk and pharmaceutical dosage form by using RP-HPLC. Int J Health Sci. 2022;6(S2):14688–702. CrossRef
23. Khan JA, Khaliq SA, Naeem S, Kashif M. Method development and validation of solid dosage form of rivaroxaban by RP-HPLC. J Int Res Med Pharm Sci. 2020;15(1):4–10. Ávailable from https://ikprress.org/index.php/JIRMEPS/article/view/5021
24. Sajjanwar R, Bhaskaran S, Kakati K, Jha SK. A validated reverse phase HPLC Method for simultaneous estimation of rivaroxaban and clopidogrel bisulphate in pharmaceutical application. J Appl Pharm Res. 2015;3(3):9–16.
25. Sarkis N, Bitar Y, Sarraj MM. Development and validation of RP-HPLC method for simultaneous estimation of aspirin and rivaroxaban in synthetic mixture. Res J Pharm Tech. 2020;13(11):5459–65. CrossRef
26. dos Santos NO, Wingert NR, Steppe M. Quality by design approach for enantiomeric evaluation by RP-HPLC method of rivaroxaban and its chiral impurity. Microchem J. 2023;192:108911. CrossRef
27. Patel Riddhiben M, Shri BM. Stability indicating HPLC method development-a review. Int Res J Pharm. 2011;2(5):79–87.
28. Mandake GR, Patil IS, Patil OA, Nitalikar MM, Mohite SK. UV spectroscopy analysis and degradation study of rivaroxaban. Asian J Res Pharm Sci. 2018;8(2):57–60. CrossRef
29. Chatwal GR. Instrumental methods of chemical analysis. Mumbai, India: Himalaya Publishing House; 2013. 2.624–39 pp.
30. Shah BP, Jain S, Prajapati KK, Mansuri NY. Stability indicating HPLC method development: a review. Int J Pharm Res Sci. 2012;3(9):2978–88.
31. Pinaz AK, Muralikrishna K. Method development and acid degradation study of rivaroxaban by RP-HPLC in Bulk. Asian J Pharm. 2013;3:62–5.
32. Seshamamba BSV, Satyanarayana PVV, Sekaran CB. Application of stability indicating HPLC method with UV detector to the analysis of rivaroxaban in bulk and tablet dosage form. Chem Sci Trans. 2014;3:1546–54. CrossRef
33. Souri E, Mottaghi S, Zargarpoor M, Ahmadkhaniha R, Jalalizadeh H. Development of a stability-indicating HPLC method and a dissolution test for rivaroxaban dosage forms. Acta Chromatogr. 2016 Sep;28(3):347–61.
34. Sayeda ZN, Hangad T. Development and validation of stability indicating assay method of rivaroxaban drug by HPLC. World J Pharm Res. 2022;11(7):1013–27.
35. Çelebier M, Reçber T, Koçak E, Altinöz S. RP-HPLC method development and validation for estimation of rivaroxaban in pharmaceutical dosage forms. Brazil J Pharm Sci. 2013;49:359–66.
36. Walter ME, Perobell R, Da Silva FS, Cardoso Junior CD, da Silva IS, Dalmora SL. Development and validation of a stability-indicating RP-HPLC method for the determination of rivaroxaban in pharmaceutical formulations. Lat Am J Pharm. 2015;34:1503–10.
37. Girase Y, Srinivasrao V, Soni D. Development and validation of stability-indicating RP-HPLC method for eivaroxaban and its impurities. SOJB. 2018;4:1–6. CrossRef
38. Badroon T, Sreeramulu J. Development and validation of stability indicating assay by HPLC method for estimation of eivaroxaban. Int J Pharma Bio Sci. 2019;8:2582–6. CrossRef
39. Garad SS, Mane DV. RP-HPLC stability indicating method development and validation for estimation of rivaroxaban in active pharmaceuticals ingredients. J Survey Fish Sci. 2023;10(4S):2261–70.
40. Kasad PA. Photolytic–thermal degradation study& method development of Rivaroxaban RP-HPLC. Int J Pharm Teach Res. 2013;5(3):1254–63.
41. Kasad PA, Muralikrishna S. Base degradation study and method development of rivaroxaban by RP-HPLC in bulk. Asian J Pharm Technol. 2013;3(3):109–13.
42. Rajan N, Anver Basha K. A stability-indicating ultra-performance liquid chromatographic method for estimation of related substances and degradants in rivaroxaban active pharmaceutical ingredient. J Pharm Res. 2014;8(11):1719–25.
43. Rao PSP, Cholleti VK, Reddy VR. Stability-indicating UPLC method for determining related substancesand degradants in rivaroxaban. Int J Res Pharm Sci. 2015;5(2):17–24.
44. Jebaliya H, Dabhi B, Patel M, Jadeja Y, Shah A. Stress study and estimation of a potent anticoagulant drug rivaroxaban by a validated HPLC method: technology transfer to UPLC. J Chem Pharm Res. 2015;7(10):65–74.
45. Cholleti V, Ravindra Kumar Y, Pasula A, Surya PRP. Stability-indicating HPLC method development and validation of rivaroxaban impurities and identification of forced degradation products using LC-MS/MS. Biomed Chromatogr. 2022 Sep;36(9):e5424. doi: 10.1002/bmc.5424
46. Arous B, Al-Mardini MA, Ghazal H, Al-Lahham F. Stability-indicating, method, for the determination, of rivaroxaban, and its degradation, products, using, LC-MS, and, TLC. Res J Pharm Technol. 2018;11(1):1–9.
47. Ramisetti NR, Kuntamukkala R. Development and validation of a stability indicating LC-PDA-MS/MS method for separation, identification and characterization of process related and stress degradation products of rivaroxaban. RSC Adv. 2014l;4:23155–67. CrossRef
48. Lories IB, Mostafa AA, Girges MA. High performance liquid chromatography, TLC densitometry, first derivative and first-derivative ratio spectrophotometry for determination of rivaroxaban and its alkaline degradates in bulk powder and its tablets. J Chromatogr Sep Tech. 2013;4(8):1–6.
49. Bhavyasri K, Dhanalakshmi C, Sumakanth M. Development and validation of ultra violet-visible spectrophotometric method for estimation of rivaroxaban in spiked human plasma. J Pharm Sci Res. 2020;12(9):1215–9.
50. Shukla AH, Shah PJ, Dedhiya PP, Vyas BA, Shah SA. Development and validation of a HPTLC method for rivaroxaban in human plasma for a pharmacokinetic study. Indian J Pharm Sci. 2020;82(2):315–20.
51. Yadav S, Dubey N. Development and validation of bio analytical method for estimation of rivaroxaban using HPLC-PDA in human blood plasma. J Drug Deliv Therap. 2017;7(7):123–5.
52. Çelebier M, Reçber T, Koçak E, Alt?nöz S, K?r S. Determination of RXN in human plasma by solid-phase extraction–high performance liquid chromatography. J Chromatogr Sci. 2016;54(2):216–20.
53. Gouveia F, Bicker J, Santos J, Rocha M, Alves G, Falcão A, et al. Development, validation and application of a new HPLC-DAD method for simultaneous quantification of apixaban, dabigatran, edoxaban and rivaroxaban in human plasma. J Pharm Biomed Anal. 2020 Mar 20;181:113109. CrossRef
54. Reddy GS, Reddy SL, Reddy LS. Development and validation of HPLC-MS/MS method for rivaroxaban quantitation in human plasma using solid phase extraction procedure. Orient J Chem. 2016;32(2):1145–54.
55. Varga A, ?erban RC, Muntean DL, T?tar CM, Farczadi L, Tilea I. Rapid liquid chromatography tandem mass spectrometry determination of rivaroxaban levels in human plasma for therapeutic drug monitoring. Revista Roman Med Lab. 2017;25(2):145–55. CrossRef
56. Derogis PB, Sanches LR, de Aranda VF, Colombini MP, Mangueira CL, Katz M, et al. Determination of rivaroxaban in patient’s plasma samples by anti-Xa chromogenic test associated to high performance liquid chromatography tandem MassSpectrometry(HPLC-MS/MS). PLoS One. 2017;12(2):1–14. CrossRef
57. Rohde G. Determination of rivaroxaban– a novel, oral, direct factor Xa inhibitor–in human plasma by high performance liquid chromatography–tandem mass spectrometry. J Chromatogr. 2008;872:43–50. CrossRef
58. Rodina TA, Melnikov ES, Aksenov AA, Belkov SA, Sokolov AV, Prokof’ev AB, et al. Development of an HPLC-MS/ MS method for quantitative determination of rivaroxaban in human blood serum. Pharm Chem J. 2018;52:372–7. CrossRef
59. Korostelev M, Bihan K, Ferreol L, Tissot N, Hulot JS, Funck-Brentano C, et al. Simultaneous determination of rivaroxaban and dabigatran levels in human plasma by high-performance liquid chromatography–tandem mass spectrometry. J Pharm Biomed Anal. 2014 Nov 1;100:230–5. CrossRef
60. Lagoutte-Renosi J, Le Poupon J, Girard A, Montange D, Davani S. A simple and fast HPLC-MS/MS method for simultaneous determination of direct oral anticoagulants apixaban, dabigatran, rivaroxaban in human plasma. J Chromatogr. 2018;1100–1101:43–9. CrossRef
61. Wang L, Gai S, Zhang X, Xu X, Gou N, Wang X. Simultaneous determination of rivaroxaban and TAK-438 in rat plasma by LC–MS/MS: application to pharmacokinetic interaction study. Bioanalysis. 2020;12:11–22. CrossRef
62. Lee HC, Kim DY, Choi MJ, Jin SK, Choi YS. A simple and efficient method to determine rivaroxaban in rat plasma using liquid-liquid extraction and LC-MRM. Mass Spectrom Lett. 2019;10:66–70.
63. Shaikh K, Mungantiwar A, Halde S, Pandita N. Liquid chromatography–tandem mass spectrometry method for determination of rivaroxaban in human plasma and its application to a pharmacokinetic study. J Mass Spectrom. 2019;20:91–105. CrossRef
64. Iqbal M, Khalil NY, Imam F, Khalid Anwer M. A validated high-throughput UHPLC-MS/MS assay for accurate determination of rivaroxaban in plasma sample. J Thromb Thrombolysis. 2015;39:79–88. CrossRef
65. Foerster KI, Huppertz A, Müller OJ, Rizos T, Tilemann L, Haefeli WE, et al. Simultaneous quantification of direct oral anticoagulants currently used in anticoagulation therapy. J Pharm Biomed Anal. 2018;148:238–44. CrossRef
66. Zhang Wl, Lou D, Zhang DT. Determination of rivaroxaban, apixaban and edoxaban in rat plasma by UPLC–MS/MS method. J Thromb Thrombolysis. 2016;42:205–11. CrossRef
67. Zhao Y, Couchman L, Kipper K, Arya R, Patel JP. A UHPLC-MS/MS method to simultaneously quantify apixaban, edoxaban and rivaroxaban in human plasma and breast milk: for emerging lactation studies. J Chromatogr B. 2020;1144:122095. CrossRef
68. Schmitz EM, Boonen K, van den Heuvel DJ, van Dongen JL, Schellings MW, Emmen JM, et al. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography - tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J Thromb Haemost. 2014 Oct;12(10):1636–46. CrossRef. PMID: 25142183
69. Sheng S, Luo SB, Mei YB, Guo J, Tong LJ, Zhang Q, et al. Simultaneous determination of RXN and enalapril in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic interaction study. Eur J Drug Metab Pharmacokinet. 2019 Apr;44(2):229–36. CrossRef
70. Kuhn J, Gripp T, Flieder T, Dittrich M, Hendig D, Busse J. UPLC-MRM mass spectrometry method for measurement of the coagulation inhibitors dabigatran and rivaroxaban in human plasma and its comparison with functional assays. PLoS One. 2015;10:e0145478. CrossRef
71. Ingle RG, Zeng S, Jiang H, Fang WJ. Current developments of bioanalytical sample preparation techniques in pharmaceuticals. J Pharm Anal. 2022;12(4):517–29. CrossRef
72. Sustainable development. Winnipeg, Canada: International Institute of Sustainable Development; 2024 Available from: https://www.iisd.org/mission-and-goals/sustainabledevelopment
73. U Nations. THE 17 Goals. [cited 2023 May 20]. Available from: https://sdgs.un.org/goals
74. Armenta S, Garrigues M. de la Guardia. Trends Anal Chem. 2008;27:497–511.
75. Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal Chem. 2020;92:10076–82.
76. Keith LH, Gron LU, Young JL. Green analytical methodologies. Chem Rev. 2007;107:2695–708.
77. Haneef J, Khan MD. Liquid chromatographic methods for the analysis of canagliflozin: concise overview and greener assessment. Anal Methods. 2023 Sep 21;15(36):4627–39. CrossRef