A validated stability indicating HPLC method for the determination of Valsartan in tablet dosage forms
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 97-99

Quantification of Colchicine in Seed and Tuber samples of Gloriosa superba by High Performance Liquid Chromatography method
Kavina .J, R.Gopi, R.Panneerselvam
Pages: 116-119

High Performance Liquid Chromatography (HPLC) Method Development and Validation Indicating Assay for Ciprofloxacin Hydrochloride
Sani A. Ali, Chijioke C. Mmuo, Rafat O. Abdulraheem, Sikirat S. Abdulkareem, Emmanuel T. Alemika, Musa A. Sani, Mohammed Ilyas
Pages: 239-243

Development of validated liquid chromatographic method for estimation of levocetirizine from pharmaceutical dosage forms
Chaitanya Prasad MK, Vidyasagar G, Sambasiva Rao KRS, Madhusudhanareddy Induri, Ramanjeneyulu S
Pages: 95-97

Evaluation of Transdermal Formulations: A Technical Note
Mudasir Mohamad, Roheena Jan
Pages: 37-40

A comparative study of the in-vitro dissolution profiles of paracetamol and caffeine combination in different formulations using HPLC
M.E.M. Hassouna, Y.M. Issa, A.G. Zayed
DOI: 10.7324/JAPS.2012.2531Pages: 52-59

Antioxidative effect of Melastoma Malabathticum L Extract and Determination of its Bioactive Flavonoids from Various Location in Malaysia by RP-HPLC with Diode Array Detection
Sundram Karupiah and Zhari Ismail
DOI: 10.7324/JAPS.2013.30204Pages: 019-024

Stability Indicating HPLC Method for the Determination of Hydrochlorothiazide in Pharmaceutical Dosage form
Sachin Bhagwate and N. J. Gaikwad
DOI: 10.7324/JAPS.2013.30215Pages: 088-092

Stability-indicating HPLC-DAD method for the determination of Granisetron hydrochloride in its pharmaceutical preparations
Mokhtar Mabrouk, Hamed El-Fatatry, Ismail Hewala and Ehab Emam
DOI: 10.7324/JAPS.2013.3633Pages: 189-202

Therapeutic Role of Coenzyme Q10 in Brain Injury during Experimental Diabetes
Jihan Hussein, Dina Abo El-matty, Zakaria El-Khayat and Yasmin Abdel-Latif
DOI: 10.7324/JAPS.2013.3636Pages: 213-217

Simultaneous Determination of Hyoscine, Ketoprofen and Ibuprofen in Pharmaceutical Formulations by HPLC - DAD
Rasha A. Shaalan, Rim S. Haggag, Saeid F. Belal and Mahmoud Agami
DOI: 10.7324/JAPS.2013.3708Pages: 038-047

Stability indicating chromatographic techniques for the determination of pipoxolan HCl
Hala E. Zaazaa, Afaf O. Mohamed, Mohamed Abdelkawy and Maha A.Hawwam
DOI: 10.7324/JAPS.2013.31011Pages: 066-073

Green Tea Attenuates Experimental Hepatitis in Context of Oxidative Stress
Jihan Hussein, Eman Refaat, Safaa Morsy, Dalia Medhat and Fatma Oraby
DOI: 10.7324/JAPS.2013.31222Pages: 124-128

High Performance Liquid Chromatographic Determination of the Ternary Mixture of Caffeine, Dipyrone and Drotaverine Hydrochloride in Tablet Dosage Form
Tarek S. Belal, Essam F. Khamis, Fawzy A. El Yazbi and Mohamed M.A. Hamdy
DOI: 10.7324/JAPS.2014.40605Pages: 033-039

Antinociceptive, anti-gastric ulcerogenic and anti-inflammatory activities of standardized egyptian pomegranate peel extract
Rola Milad Labib, Sherweit Hamed El-Ahmady
DOI: 10.7324/JAPS.2015.50109Pages: 048-051

Development and Validation of RP-HPLC Method for the Simultaneous Determination of Trimethoprim, Sulfadimidine Sodium and Tylosin Tartrate in injectable solution formulation
Mashhour Ghanem, Saleh Abu-Lafi
DOI: 10.7324/JAPS.2015.50117Pages: 094-098

RP-HPLC Method for the Simultaneous Estimation of Ambroxol Hydrochloride and Fexofenadine Hydrochloride In bulk and in a Tablet Mixture
Prathyusha Buchupalli, Srinivas Medidi
DOI: 10.7324/JAPS.2015.50211Pages: 074-080

Effect of Plumbago zeylanica administration on brain neurotransmitter level in Wistar albino rats
Muthukumarasamy Baskaran, Latha Shanmugam, Vijayakumar Raman
DOI: 10.7324/JAPS.2015.50409Pages: 053-057

A novel stability indicating HPLC-method for simultaneous determination of atenolol and nifedipine in presence of atenolol pharmacopeoial impurities
Hisham Hashem, Ibrahim Adel Ehab, Elhenawee Magda
DOI: 10.7324/JAPS.2015.50804Pages: 017-025

Pharmacokinetics of Chloroquine and Metronidazole in Rats
Kudirat Bola Mustapha, Moji T Bakare-Odunola, Garba Magaji, Obiageri O. Obodozie-Ofoegbu, David D Akumka
DOI: 10.7324/JAPS.2015.50814Pages: 090-094

Phenylalanine enhances Quercetin content in In vitro cultures of Abutilon indicum L.
Ramgopal Rao Sajjalaguddam, Anitha Paladugu
DOI: 10.7324/JAPS.2015.501014Pages: 080-084

A Validated High Performance Liquid Chromatography Method for the Simultaneous Analysis of Guaifenesin, Ambroxol and Loratidine in Bulk and Liquid Dosage form
Gugulothu Sailaja, Bollikolla Hari Babu
DOI: 10.7324/JAPS.2015.501210Pages: 061-066

Test purchase of new synthetic tryptamines via the Internet: Identity check by GC-MS and separation by HPLC
Magdalena Taschwer, Edith Ebner, Martin G Schmid
DOI: 10.7324/JAPS.2016.600105Pages: 028-034

Quinine-Loaded Polymeric Nanoparticles: Validation of a simple HPLC-PDA Method to Determine Drug Entrapment and Evaluation of its Photostability
Luana Roberta Michels, Lisiane Bajerski, Tamara Ramos Maciel, Letícia Marques Colomé, Sandra Elisa Haas
DOI: 10.7324/JAPS.2016.60202Pages: 009-015

Validated, Ultra High Efficiency RP-HPLC and Stability Indicating Method for Determination of Tranylcypromines Sulphate in Bulk and in Tablet Dosage Forms
Gamal H. Ragab, Hanaa M. Saleh, Magda M. EL-Henawee, Omnia F. Elsayed
DOI: 10.7324/JAPS.2016.60209Pages: 064-071

Development and Validation of a Stability Indicating HPLC Method for the Simultaneous Analysis of Esomeprazole and Itopride in Bulk and In Capsules
M. Nageswara Rao, K. B. M. Krishna, B. Hari Babu
DOI: 10.7324/JAPS.2016.60210Pages: 072-080

Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India
Tapan Seal
DOI: 10.7324/JAPS.2016.60225Pages: 157-166

Simultaneous estimation of Esomeprazole and Tadalafil in pharmaceutical formulations using High Performance Liquid Chromatography
Mohammed Hamad, Ahmed Al-Sharqawi, Wael Abu Dayyih, Eyad Mallah, Tawfiq Arafat
DOI: 10.7324/JAPS.2016.60407Pages: 052-059

Determination of Benzalkonium Chloride in Ophthalmic Solutions by Stability-Indicating HPLC Method: Application to a Stability Study
Hashem AlAani, Yasmin AlNukkary
DOI: 10.7324/JAPS.2016.60513Pages: 080-089

Development and validation of a stability indicating HPLC-diode array-fluorescence method for the determination of meclofenoxate hydrochloride and p-chlorophenoxyacetic acid
Marwa Said Moneeb, Feda Elgammal, Suzy Mohamed Sabry
DOI: 10.7324/JAPS.2016.60701Pages: 001-011

Phytochemical Study and Antioxidative Property of Ethanolic Extract from Termitomyces clypeatus
Payel Mitra, Narayan Chandra Mandal, Anirban Roy, Krishnendu Acharya
DOI: 10.7324/JAPS.2016.60718Pages: 120-124

Total salinity stress on physico-chemical characterization of lecithin isolated from soya bean oil seeds grown in the coastal region of south, India
Pragasam Antony, Preeti N. Tallur, Sikandar I. Mulla, Vinayak M. Naik
DOI: 10.7324/JAPS.2016.60805Pages: 030-035

Biological activities of four Parmotrema species of Malaysian origin and their chemical constituents
Vinoshene Pillai Rajan, Saranyapiriya Gunasekaran, Surash Ramanathan, Vikneswaran Murugaiyah, Mohd. Wahid Samsudin, Laily B. Din
DOI: 10.7324/JAPS.2016.60806Pages: 036-043

Anti-mycobacterial assessment and characterization of 5-O-caffeoylquinic acid methyl ester and rutin from Pavetta crassipes
Patricia O. Odumosu, William John Lough, Davis Yakubu, Keith Thomas, Gemma Williamson, Nicolas Haroune
DOI: 10.7324/JAPS.2016.601001Pages: 001-007

Investigation of possible pharmacokinetic interaction of metformin with sugar replacement sweeteners in rats
Riad Awad, Eyad Mallah, Israa Al-Ani, Wael Abu Dayyih, Zainab Zakarya, Tawfiq Arafat
DOI: 10.7324/JAPS.2016.601029Pages: 210-215

Pharmacognostic standardization of a well known edible mushroom Volvariella volvacea
Krishnendu Acharya, Sandipta Ghosh, Ipshita Kundu
DOI: 10.7324/JAPS.2016.601129Pages: 185-190

Preparative HPLC fractionation of Cinnamomum cassia Water Extract and their in-vitro Antimalarial Activities
Mutaz Akkawi, Saleh Abu-Lafi, Hanadi Attieh, Qassem Abu-Remeleh, Sadam Makhamra, Mutaz Qutob
DOI: 10.7324/JAPS.2017.70117Pages: 129-134

Pharmacokinetics of a new imidazoline receptor agonist in rat plasma after intragastric and intravenous administration
Kulikov Aleksandr, Avtina Tatyana, Pokrovsky Mikhail, Korokin Mikhail
DOI: 10.7324/JAPS.2017.70302Pages: 006-008

Comparative study on the phenolic content, antioxidant properties and HPLC fingerprinting of the leaf extracts of Clerodendrum volubile P. Beauv
Olorunfemi R. Molehin, Omotade I. Oloyede, Aline A. Boligon
DOI: 10.7324/JAPS.2017.70322Pages: 135-140

Phytochemical study and anti-inflammatory effect of Psychotria stenocalyx (Rubiaceae)
Gustavo Silva Queiroz, Ana Beatriz Gobbo Luz, Marcus Vinícius Pereira dos Santos Nascimento, Sergio Scherrer Thomasi, Antonio Gilberto Ferreira, Eduardo Monguilhott Dalmarco, Ines Maria Costa Brighente
DOI: 10.7324/JAPS.2017.70425Pages: 168-173

Solid-phase extraction for RP-HPLC/UV determination of ziprasidone at presence its main metabolite in urine
Sophia Davydovych, Iryna Halkevych, Olha Korobova, Svitlana Humenyuk
DOI: 10.7324/JAPS.2017.70501Pages: 001-006

Quality assessment and antioxidant study of Pleurotus djamor (Rumph. ex Fr.) Boedijn
Krishnendu Acharya, Somanjana Khatua, Saswata Ray
DOI: 10.7324/JAPS.2017.70614Pages: 105-110

Influence of extraction process on antioxidant activity and rutin content in Physalis peruviana calyces extract
Maria Isabel Cardona, Reina Marcela Toro, Geison M. Costa, Luis Fernando Ospina, Leonardo Castellanos, Freddy A. Ramos, Diana Marcela Aragón
DOI: 10.7324/JAPS.2017.70623Pages: 164-168

Validation of analytical method by HPLC for determination of dapsone in polymeric nanocapsules based on crude rice brain oil
Letícia Marques Colomé, Gaya Mengue Freitas, Janaina de Medeiros Bastiani, Thais Carla Brussulo Pereira, Lisiane Bajerski, Eduardo André Bender, Sandra Elisa Haas
DOI: 10.7324/JAPS.2017.70734Pages: 230-233

Development and validation of a stability-indicating RP-HPLC method for the detection and quantification of azithromycin in bulk, and self-emulsifying drug delivery system (SEDDs) formulation
Reem Abou Assi, Yusrida Darwis, Ibrahim M. Abdulbaq, Shaik Mohammed Asif
DOI: 10.7324/JAPS.2017.70903Pages: 020-029

Chemical composition and biological activities of methanol extract from Macrocybe lobayensis
Somanjana Khatua, Sandipta Ghosh, Krishnendu Acharya
DOI: 10.7324/JAPS.2017.71021Pages: 144-151

Evaluation Antioxidant and cytotoxic activities of novel chitooligosaccharides prepared from chitosan via enzymatic hydrolysis and ultrafiltration
Sanaa T. El-Sayed, Nagwa I. Omar, El-Sayed M. El-Sayed, Wafaa G. Shousha
DOI: 10.7324/JAPS.2017.71107Pages: 050-055

New approaches in protecting against atherosclerosis in experimental model of postmenopause
Dalia Medhat, Mona A. El-Bana, Magdi N. Ashour, Ehsan Badawy, Yasser Diab, Jihan Hussein
DOI: 10.7324/JAPS.2017.71114Pages: 090-096

Estimation of Bosentan Monohydrate in Male Rabbit Plasma by using RP-HPLC Method
Revathi Mannam, Indira Muzib Yallamalli
DOI: 10.7324/JAPS.2017.71116Pages: 106-109

Validated Eco-Friendly Chromatographic Methods for Simultaneous Determination of Sacubitril and Valsartan in Spiked Human Plasma and in Pharmaceutical Formulation
Amal Mahmoud Abou Al Alamein
DOI: 10.7324/JAPS.2018.8202Pages: 011-017

Chemical study and evaluation of antioxidant activity and α-glucosidase inhibition of Myrciaria strigipes O. Berg (Myrtaceae)
Rafael D. Faitanin, João V. D. Gomes, Patrícia M. Rodrigues, Luís Fernando T. de Menezes, Álvaro C. Neto, Rita C. R. Gonçalves, Rodrigo R. Kitagawa, Dâmaris Silveira, Claudia M. Jamal
DOI: 10.7324/JAPS.2018.8317Pages: 120-125

Application of Chemometrics for the simultaneous estimation of stigmasterol and β-sitosterol in Manasamitra Vatakam-an ayurvedic herbomineral formulation using HPLC-PDA method
Srikalyani Vemuri, Mohan Kumar Ramasamy, Pandiyan Rajakanu, Rajappan Chandra Satish Kumar, Ilango Kalliappan
DOI: 10.7324/JAPS.2018.8701Pages: 001-009

Screening of two glucocorticoids in non-prescription skin whitening creams purchased via internet in Iraq by HPLC method
Mohanad Naji Sahib
DOI: 10.7324/JAPS.2018.8713Pages: 078-084

Validation of a simple isocratic HPLC-UV method for rifampicin and isoniazid quantification in human plasma
Laura Carolina Luciani-Giacobbe, María Laura Guzman, Rubén Hilario Manzo, María Eugenia Olivera
DOI: 10.7324/JAPS.2018.8715Pages: 093-099

Development and Validation Method for Simultaneous Analysis of Retinoic Acid, Hydroquinone and Corticosteroid in Cream Formula by High-Performance Liquid Chromatography
Elvi Rahmayuni, Harmita Harmita, Herman Suryadi
DOI: 10.7324/JAPS.2018.8913Pages: 087-092

Liquid Chromatography and Fourier Transform Infrared Spectroscopy for quantitative analysis of individual and total curcuminoid in Curcuma longa extract
Ratna Wulandari, Sudjadi, Sudibyo Martono, Abdul Rohman
DOI: 10.7324/JAPS.2018.8916Pages: 107-113

Ethyl acetate fraction of garlic (Allium sativum) inhibits the viability of MCF7 and HepG2 through induction of apoptosis and G2/M phase cell cycle arrest
Amira M. Shaban, Ola Hammouda, Laila Abou Ghazala, Mai Raslan, Mohammed A. El-Magd
DOI: 10.7324/JAPS.2018.8920Pages: 142-150

A post-market quality assessment of first-line, fixed-dose combination antiretrovirals in South Africa
Kim Ward, Reem Suleiman, Yunus Kippie, Admire Dube
DOI: 10.7324/JAPS.2019.90213Pages: 097-104

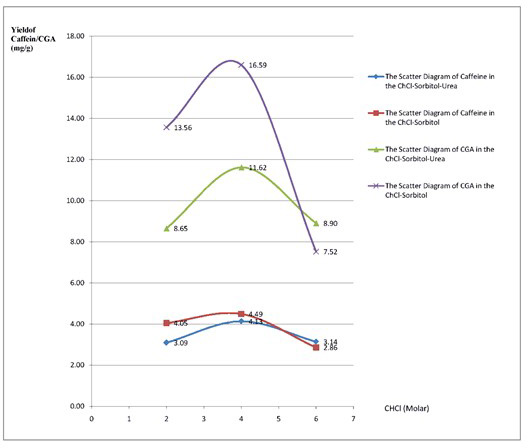
Application of the natural deep eutectic solvent choline chloride-sorbitol to extract chlorogenic acid and caffeine from green coffee beans (Coffea canephora)
Erline Yuniarti, Fadlina Chany Saputri, Abdul Mun’im
DOI: 10.7324/JAPS.2019.90312Pages: 082-090
Bioanalytical HPLC method of Piper betle L. for quantifying phenolic compound, water-soluble vitamin, and essential oil in five different solvent extracts
Rayudika Aprilia Patindra Purba, Pramote Paengkoum
DOI: 10.7324/JAPS.2019.90504Pages: 033-039

Validation of an HPLC-MS method for the determinatin of vardenafil in rat urine
Liudmila Osypchuk, Iryna Halkevych, Sophia Davydovych, Yuriy Bidnychenko
DOI: 10.7324/JAPS.2019.90811Pages: 079-085
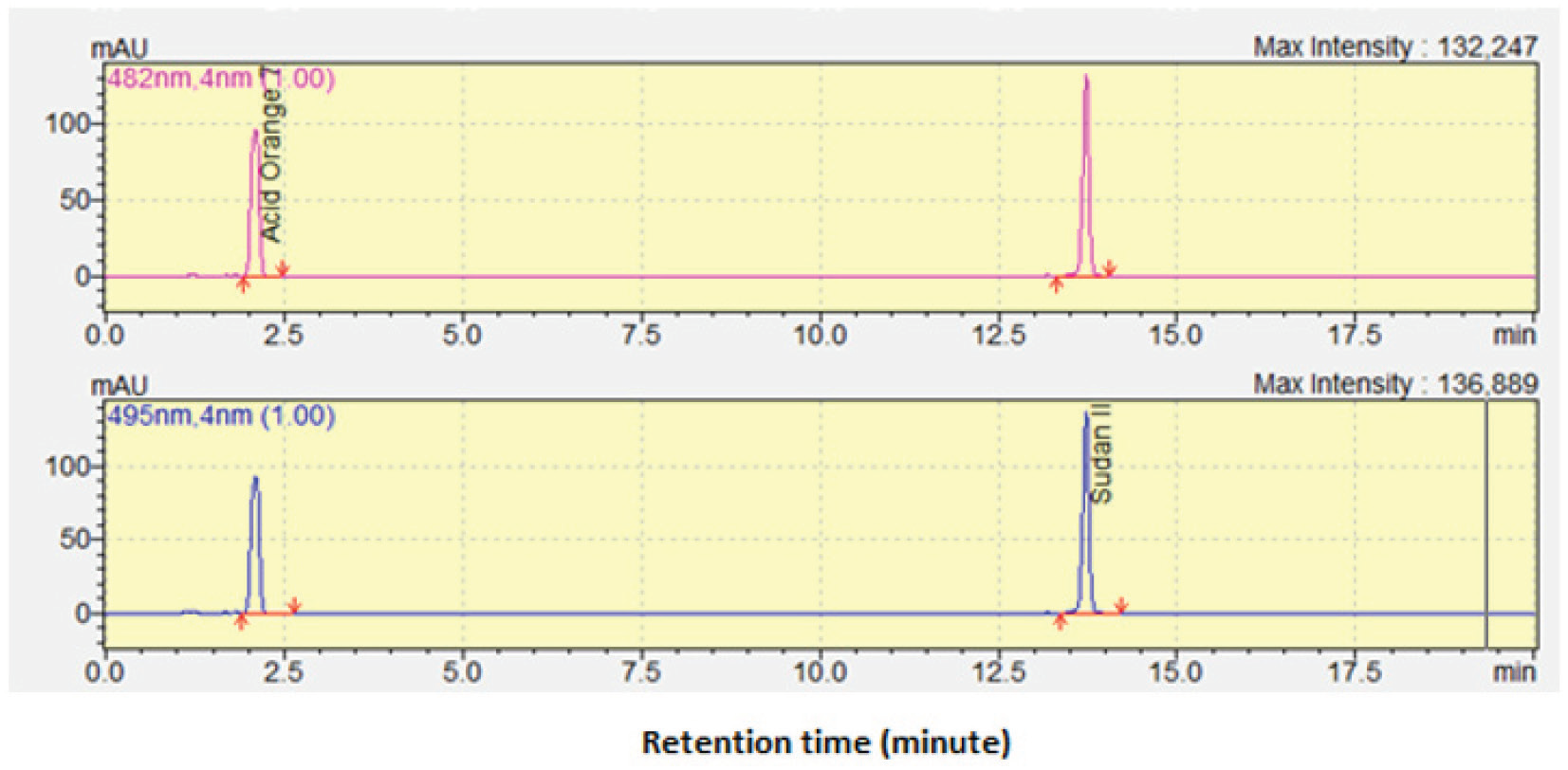
Application of FTIR spectroscopy and multivariate calibration for determination of Acid Orange 7 and Sudan II in blusher products
Novalina B. R. Purba, Abdul Rohman, Sudibyo Martono
DOI: 10.7324/JAPS.2019.91115Pages: 112-117
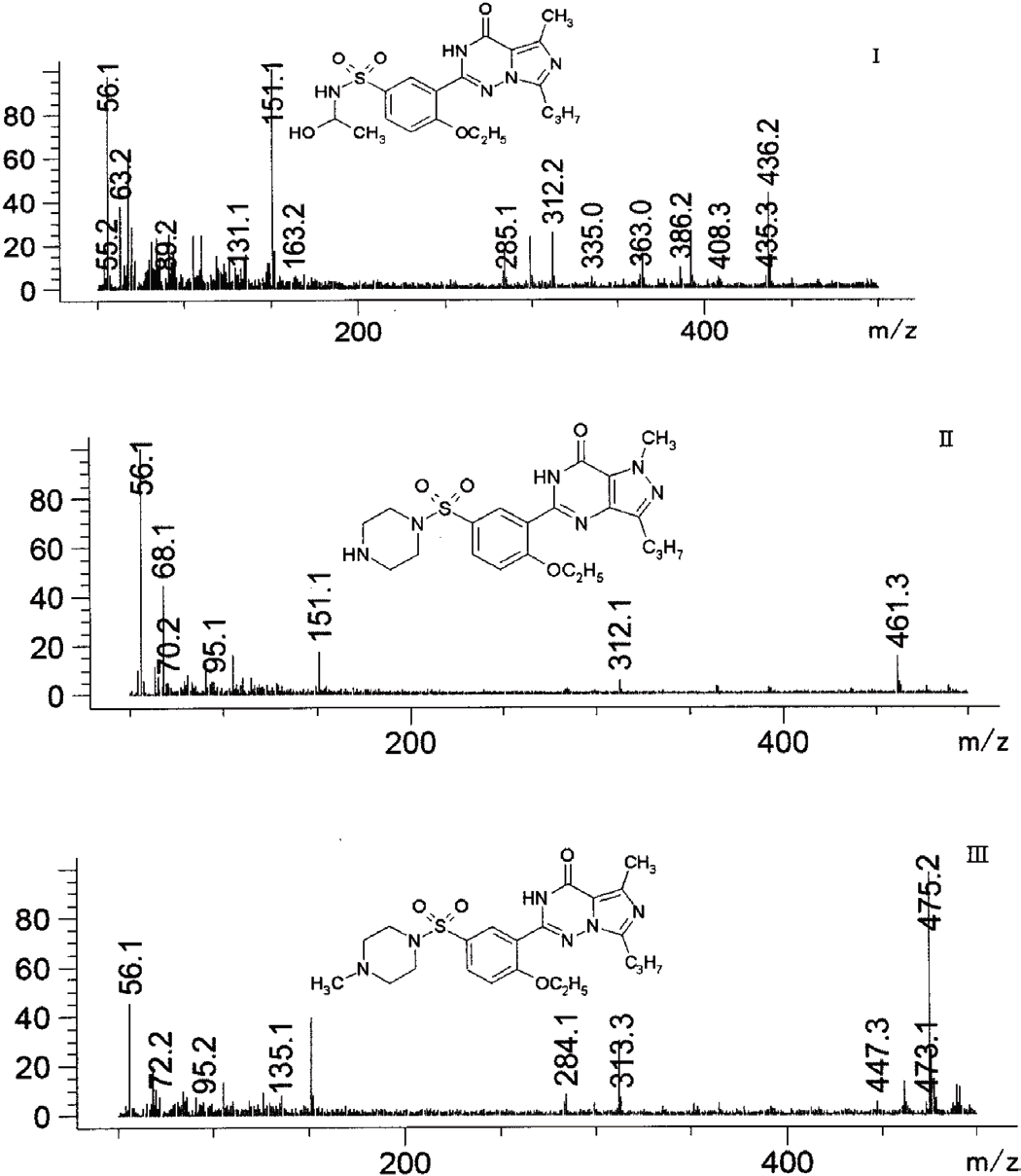
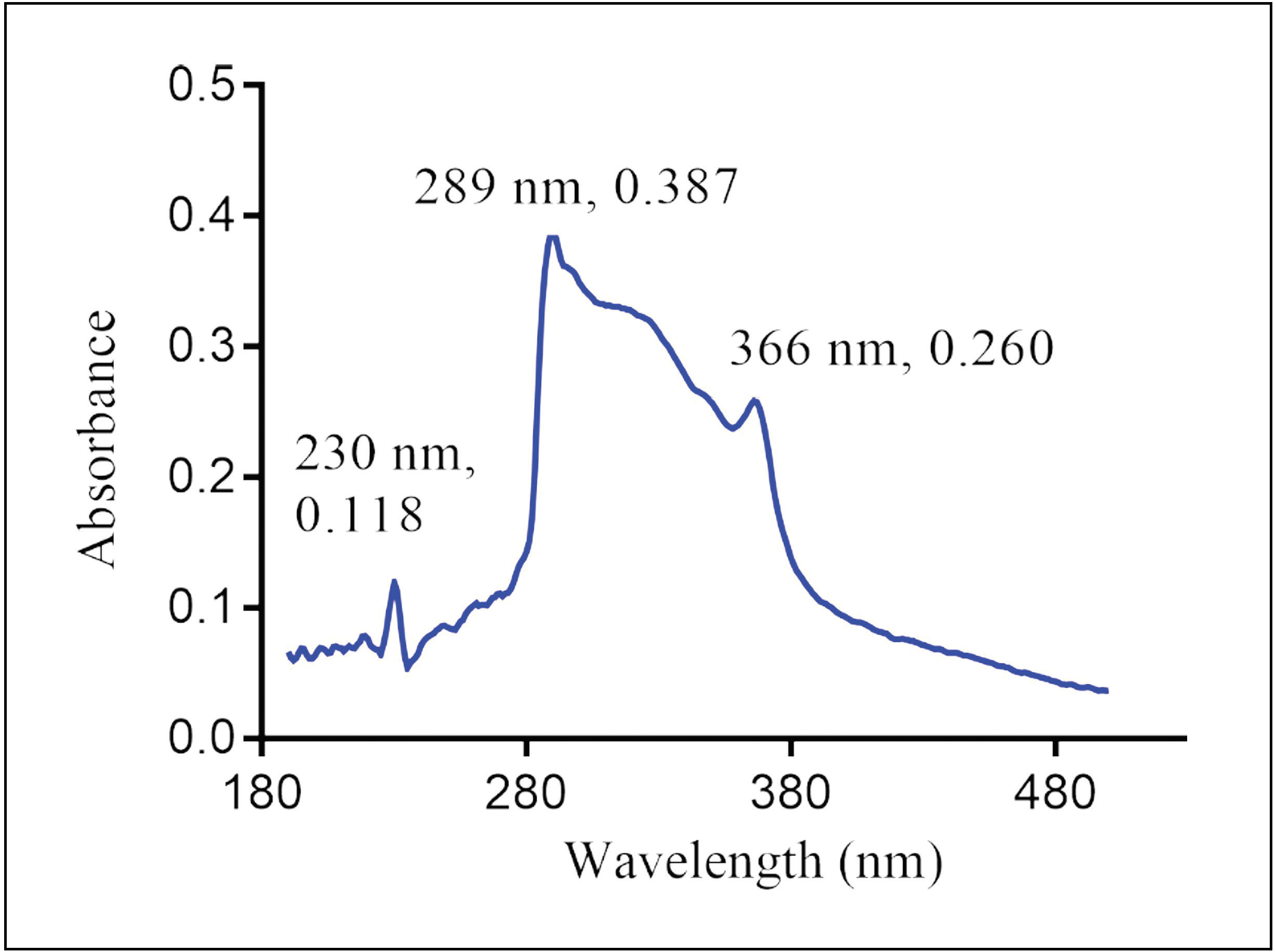
Forced degradation study of efonidipine HCl ethanolate, characterization of degradation products by LC-Q-TOF-MS and NMR
Charu P. Pandya, Sadhana J. Rajput
DOI: 10.7324/JAPS.2020.104012Pages: 075-099

Isolation, characterization, and validation of RP-HPLC method for the quantification of quercetin in Huberantha senjiana leaf extract
Rajakannu Pandiyan, Kaliappan Ilango
DOI: 10.7324/JAPS.2020.10515Pages: 110-118

Determination of shelf life of four herbal medicinal products using high-performance liquid chromatography analyses of markers and the Systat Sigmaplot software
Doris Kumadoh, Kwabena Ofori Kwakye, Noble Kuntworbe, Ofosua Adi-Dako, James Addy Appenahier
DOI: 10.7324/JAPS.2020.10610Pages: 072-080
Comparison of UV-spectrophotometric and RP-HPLC methods for estimation of deflazacort in solid dosage form
Manisha Puranik, Samta Shambharkar, Shantanu Nimbalkar, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2020.10711Pages: 082-088

Box–Behnken design-based HPLC optimization for quantitative analysis of chloramphenicol and hydrocortisone acetate in cream
Kusnul Khotimah, Sudibyo Martono, Abdul Rohman
DOI: 10.7324/JAPS.2020.10916Pages: 134-139

Isolation of catechins from Cycas armstrongii Miq. of an Egyptian origin
Ahmed Ismail, Mohamed M. Radwan, Hossam M. Hassan, Abeer S. Moawad, Mona H. Hetta
DOI: 10.7324/JAPS.2021.110119Pages: 158-162

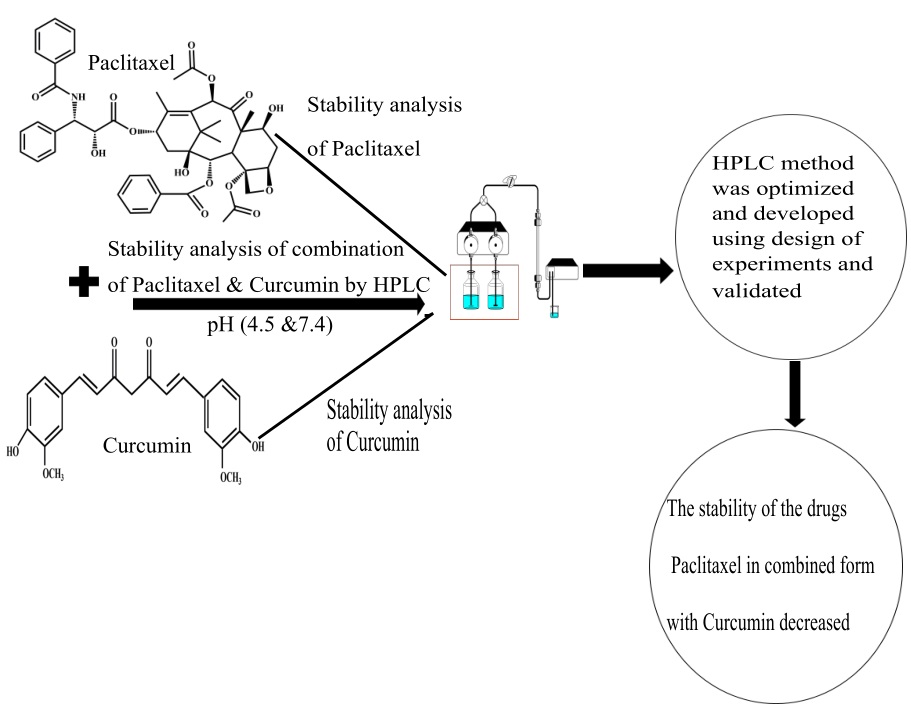
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
HPLC-FTIR spectroscopy combined with multivariate calibration for analysis of Andrographolide in Andrographis paniculata extract
Abdul Rohman, Hanifah Luthfianasari, Irnawati, Sugeng Riyanto, Mohamad Rafi, Bambang Prajogo, Muhammad Bachri Amran
DOI: 10.7324/JAPS.2021.110505Pages: 032-038

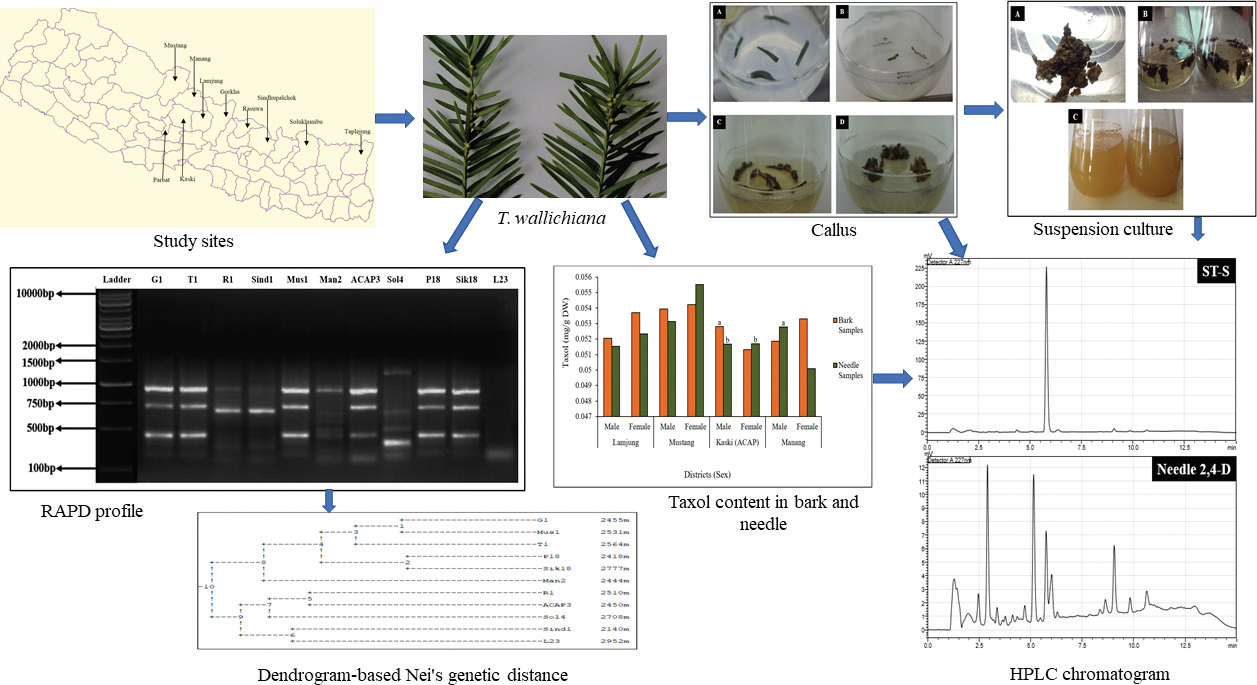
Establishment of regenerative callus, cell suspension system, and molecular characterization of Taxus wallichiana Zucc. for the in vitro production of Taxol
Dhurva Prasad Gauchan, Sudina Bhuju, Janardan Lamichhane, Rajani Shakya, María Rosario García-Gil
DOI: 10.7324/JAPS.2021.110603Pages: 022-034
Metabolic fingerprinting of Melastoma malabathricum L. extracts using high-performance liquid chromatography-diode array detector combined with chemometric data analysis
Dian Mayasari, Yosi Bayu Murti, Sylvia Utami Tunjung Pratiwi, Sudarsono Sudarsono
DOI: 10.7324/JAPS.2021.110906Pages: 048-056

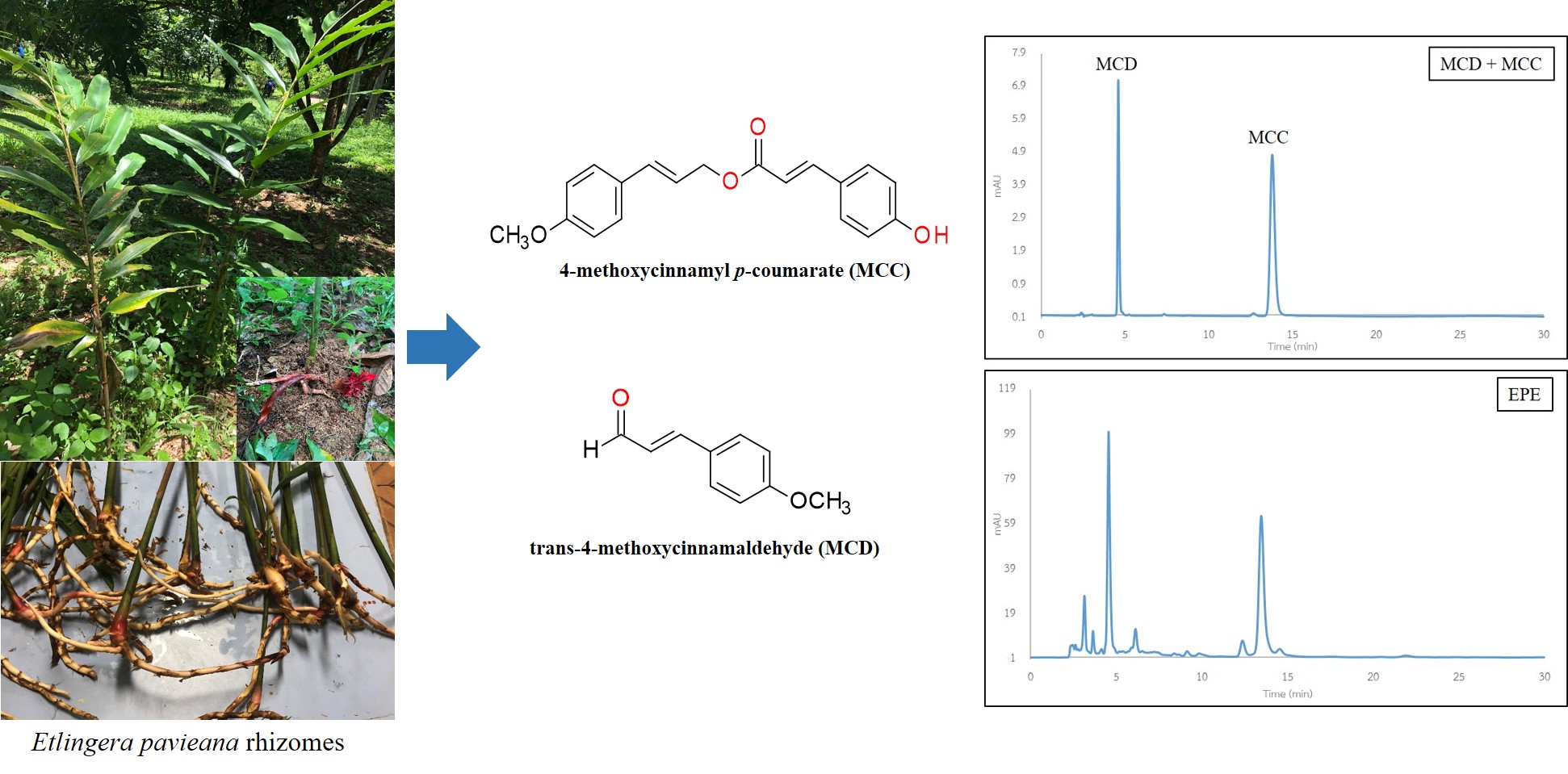
Validation of quantitative RP-HPLC-DAD method and extraction optimization of 4-methoxycinnamyl p-coumarate and trans-4- methoxycinnamaldehyde in Etlingera pavieana rhizomes
Klaokwan Srisook, Chartchai Malapong, Petchrat Sawai, Ekaruth Srisook
DOI: 10.7324/JAPS.2021.1101005Pages: 029-034
Development and validation of a stability indicating related substances of Opicapone by reverse phase high performance liquid chromatography and its degradation
Ramachandran Dittakavi, Neeharika Tirumalasetty
DOI: 10.7324/JAPS.2021.120219Pages: 179-186

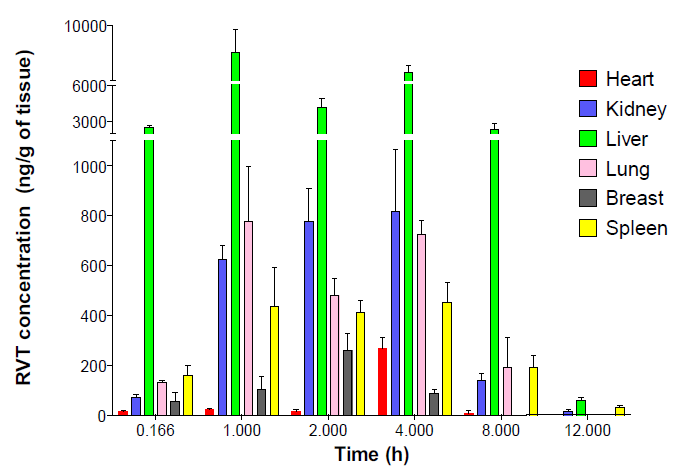
Bioanalytical RP-HPLC method validation for resveratrol and its application to pharmacokinetic and drug distribution studies
Shivaprasad Gadag, Reema Narayan, Yogendra Nayak, Usha Yogendra Nayak
DOI: 10.7324/JAPS.2021.120216Pages: 158-164
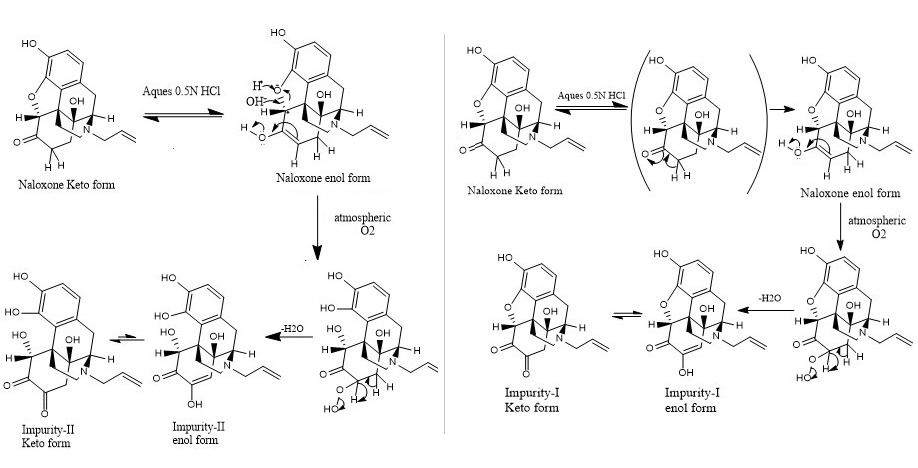
Acid borne oxidative impurities of Naloxone hydrochloride injection: Enrichment, isolation and characterization
Praveen Basappa, Venugopala Rao Dama, M. S. Uma Shankar
DOI: 10.7324/JAPS.2022.121201Pages: 001-011
Simultaneous determination of Brivaracetam and its isomers in the Brivaracetam drug by RP-HPLC
Palaniappan Ilayaraja, Murugan Maniavannan, Paramasivam Parthiban
DOI: 10.7324/JAPS.2022.120915Pages: 127-138

A stability-indicating reverse phase-HPLC method development and validation for newly approved drug, Belzutifan in bulk and pharmaceutical dosage forms
Dumpala Sravya, Banoth Ramya Kuber
DOI: 10.7324/JAPS.2023.114351Pages: 233-240
A novel chiral HPLC and LC-MS/MS method development for the triazole antifungal compound
R. Sangamithra, S. N. Meyyanathan, B. Babu
DOI: 10.7324/JAPS.2023.118532Pages: 001-008
_.jpg)
Development of volumetric absorptive microsampling for analysis phenytoin levels and its application to monitoring therapy in epilepsy patients
Rizka Mardhiani, Yahdiana Harahap, Winnugroho Wiratman, Fitri Octaviana, Irwandi Jaswir
DOI: 10.7324/JAPS.2023.125948Pages: 075-081

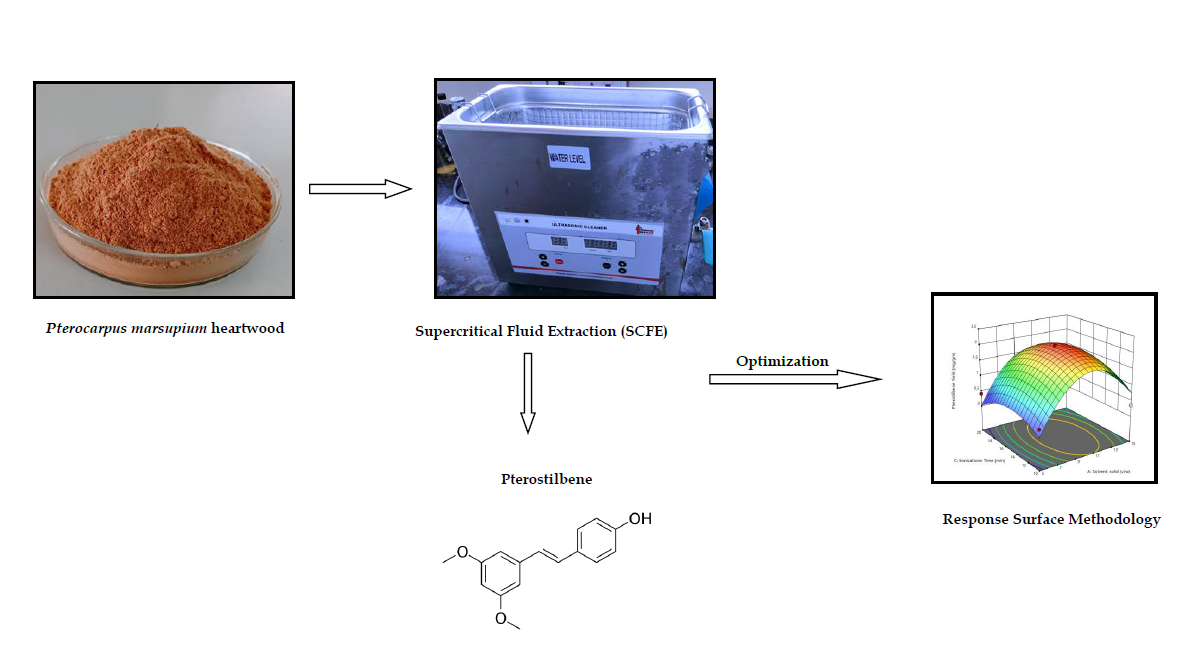
Optimization of ultrasound assisted extraction using response surface methodology for estimation of Pterostilbene in Pterocarpus marsupium
Kanchan Dilip Nikam, Sachin Shivling Bhusari, Pravin Shridhar Wakte
DOI: 10.7324/JAPS.2023.146949Pages: 201-206
Bioanalytical and validation high-performance liquid chromatography method for simultaneous quantification cefotaxime and ciprofloxacin in human plasma
Luh Putu Mirah Kusuma Dewi, Djoko Wahyono, Ika Puspitasari, Rizka Humardewayanti, Endang Lukitaningsih
DOI: 10.7324/JAPS.2024.153492Pages: 221-229
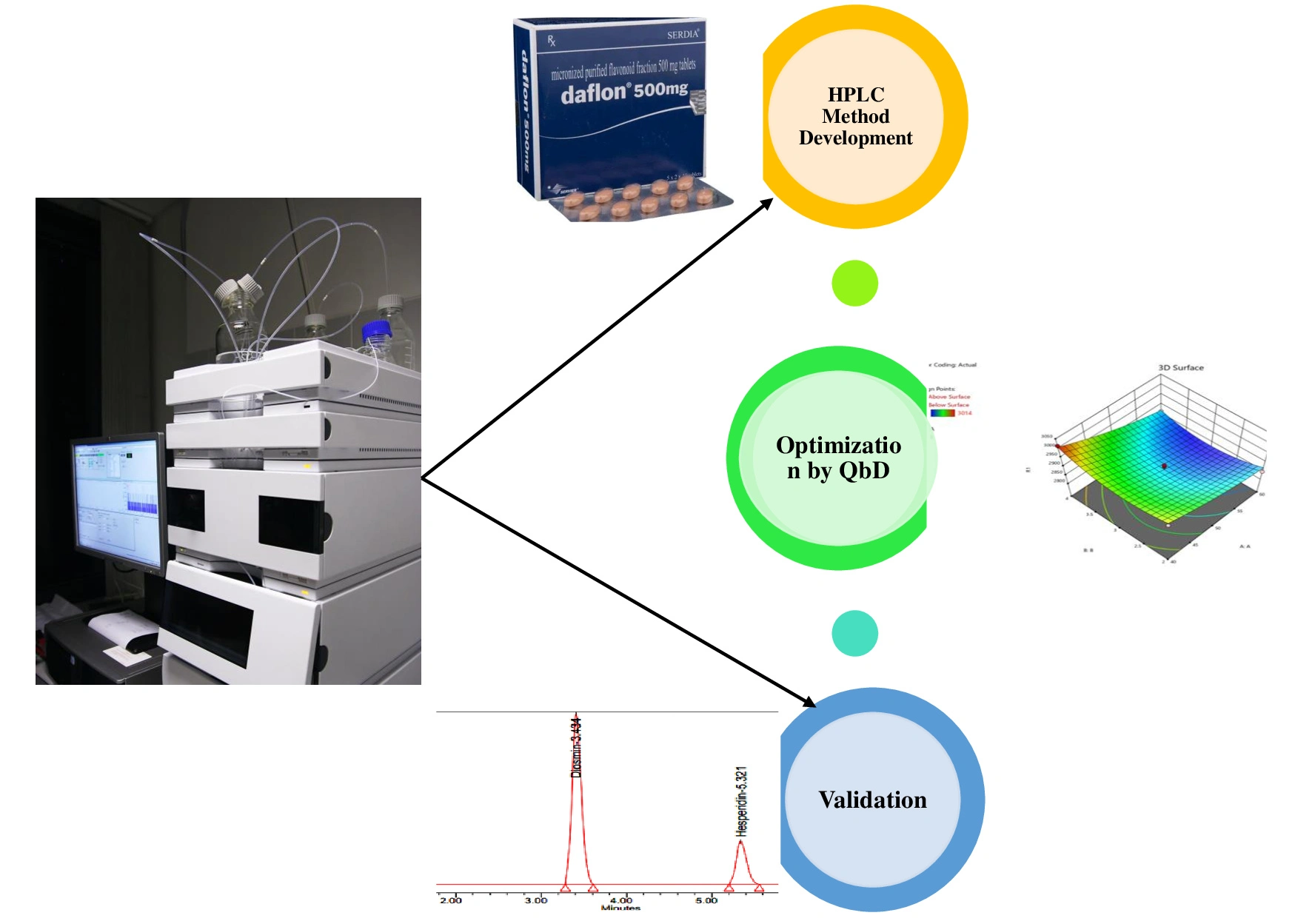
Development and optimization of a simple, robust RP-HPLC technique for analysis of diosmin and hesperidin using quality by design
Guttha Hemalatha, Adikay Sreedevi, Kaveripakam Sai Sruthi, Poreddy Swetha
DOI: 10.7324/JAPS.2024.162037Pages: 095-101
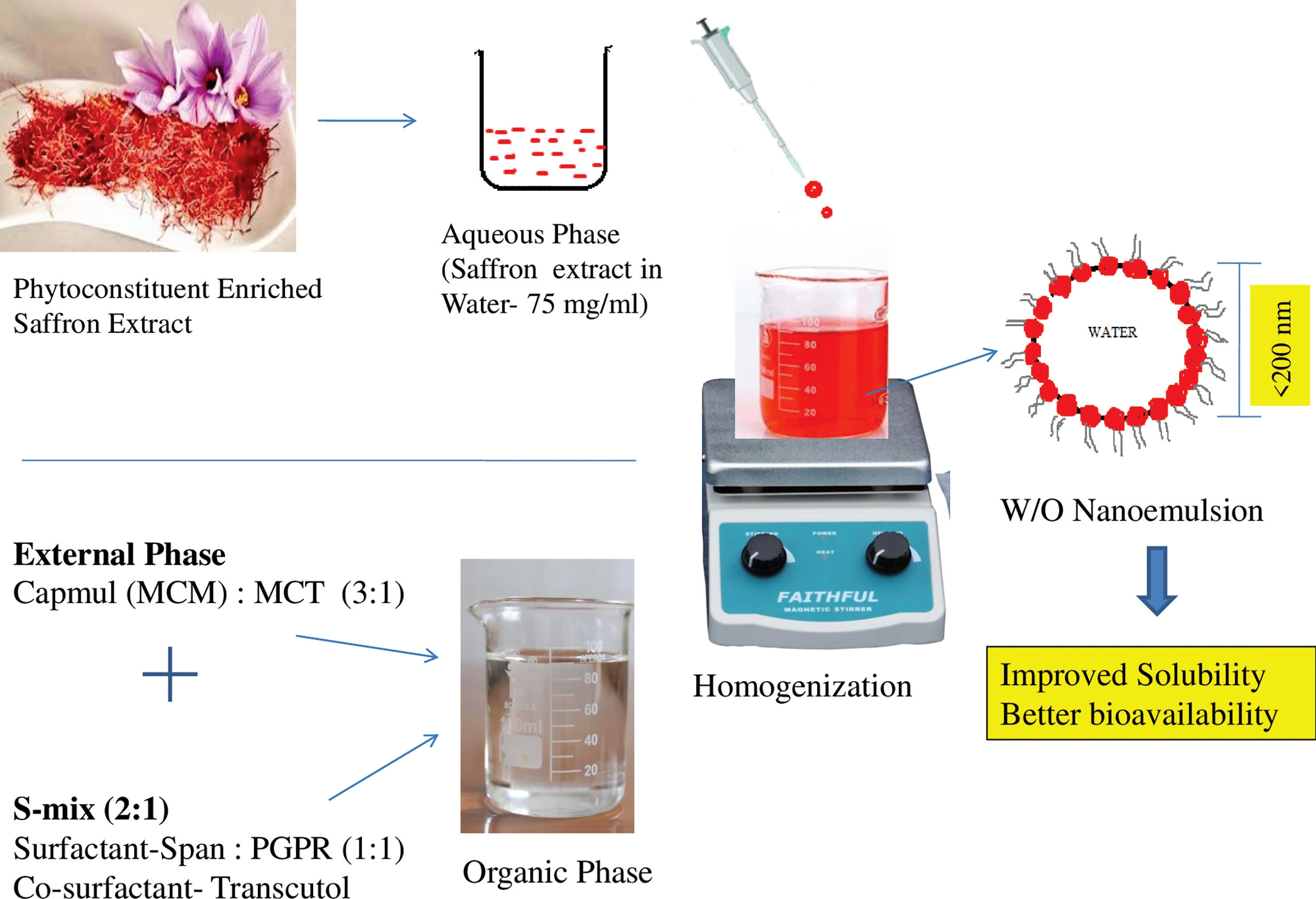
Fabrication of carotenoid enriched Crocus sativus w/o nanoemulsion: Optimization and pharmaceutical characterization
Namrata Aashish Parikh, Komal Patel, Abhay Dharamsi
DOI: 10.7324/JAPS.2024.158623Pages: 225-238
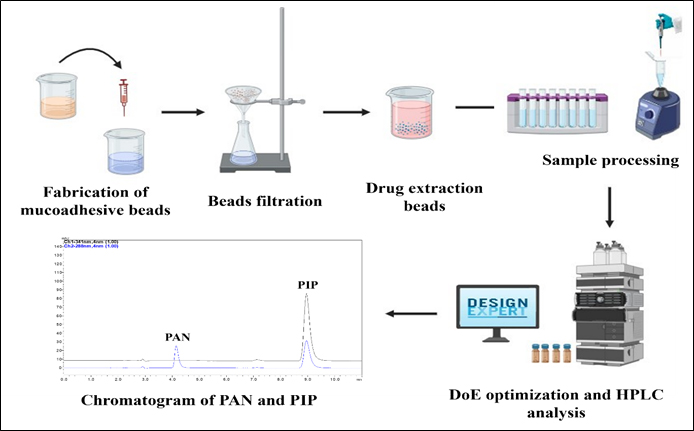
Box-Behnken guided development of an ecofriendly RP-HPLC analytical method for simultaneous quantification of pantoprazole sodium and piperine co-loaded mucoadhesive GRDDS formulation for H. pylori eradication
Ashutosh Gupta, Shiran Shetty, Srinivas Mutalik, Prerana D. Navti, Moumita Saha, Sudheer Moorkoth
DOI: 10.7324/JAPS.2024.179147Pages: 098-110
Anticancer activity of liposomal formulation co-encapsulated with coumarin and phenyl butyric acid
Ali Allateef, Naeem Shalan, Zainab Lafi
DOI: 10.7324/JAPS.2024.181335Pages: 208-215
_.jpg)
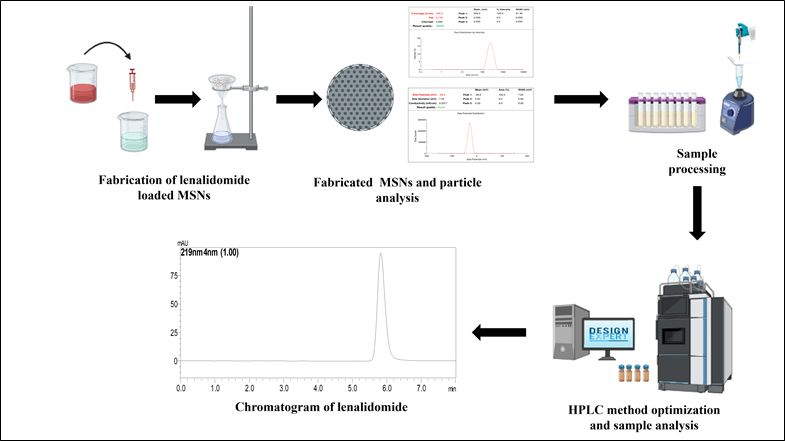
Central composite design aided optimization and validation of developed an eco-friendly HPLC method for the quantification of Lenalidomide loaded mesoporous silica nanoparticles
Ashutosh Gupta, Rachana S. P, Sudheer Moorkoth, Namdev Dhas
DOI: 10.7324/JAPS.2024.189998Pages: 089-101
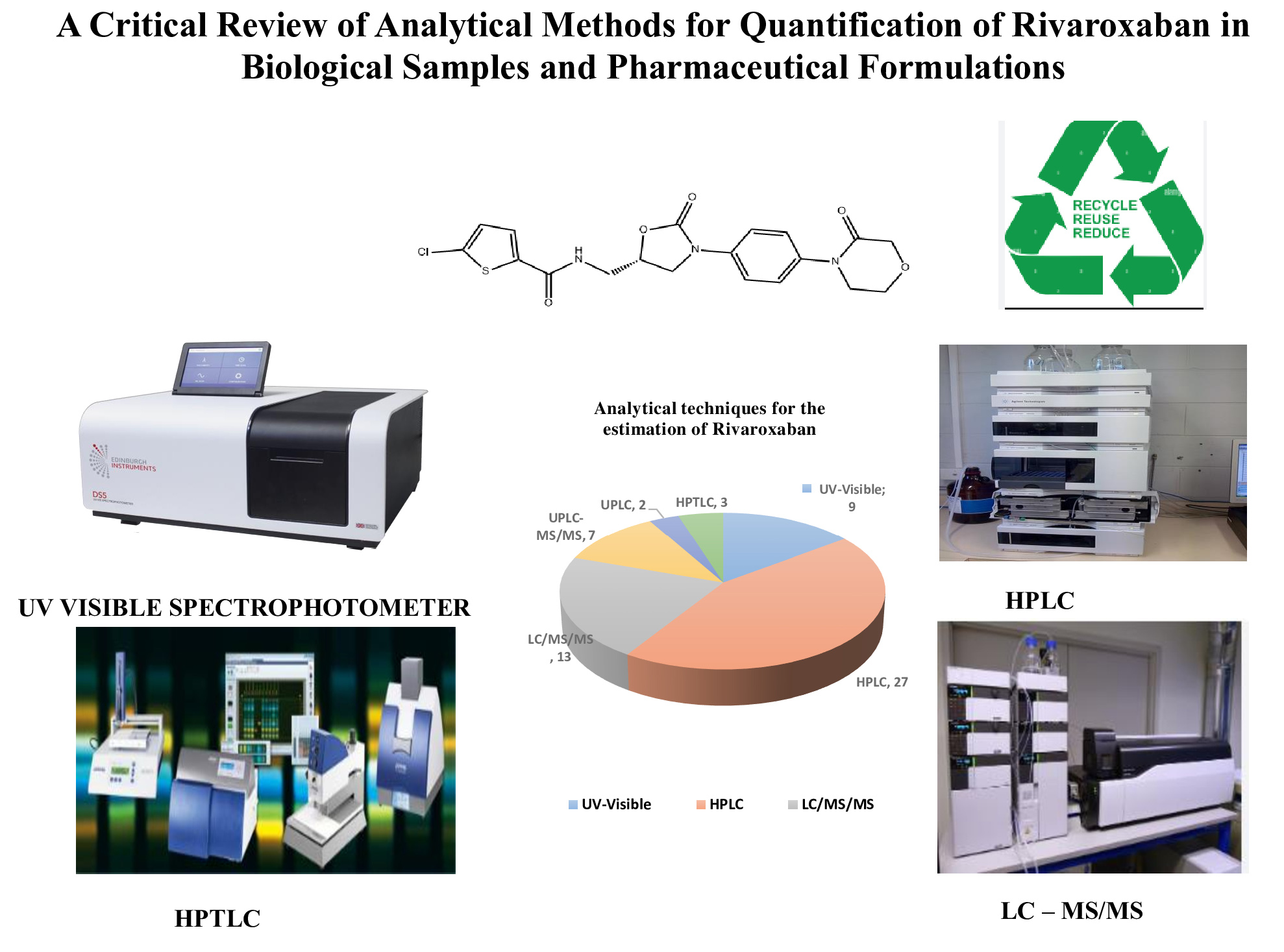
A critical review of analytical methods for quantification of rivaroxaban in biological samples and pharmaceutical formulations
Ajitha Azhakesan, Kishore Kumar Pasupuletti, Narendra Pentu
DOI: 10.7324/JAPS.2025.210368Pages: 067-085
Development and validation of a reverse phase-high performance liquid chromatography method for estimation of Thiamine diphosphate in whole blood and dried blood spot
Ysphaneendra Mallimoggala, Monalisa Biswas, Nihaal Maripini, Varashree Bolar Suryakanth, Revathi P. Shenoy, Leslie Edward S. Lewis
DOI: 10.7324/JAPS.2025.213923Pages: 240-252
_.webp)
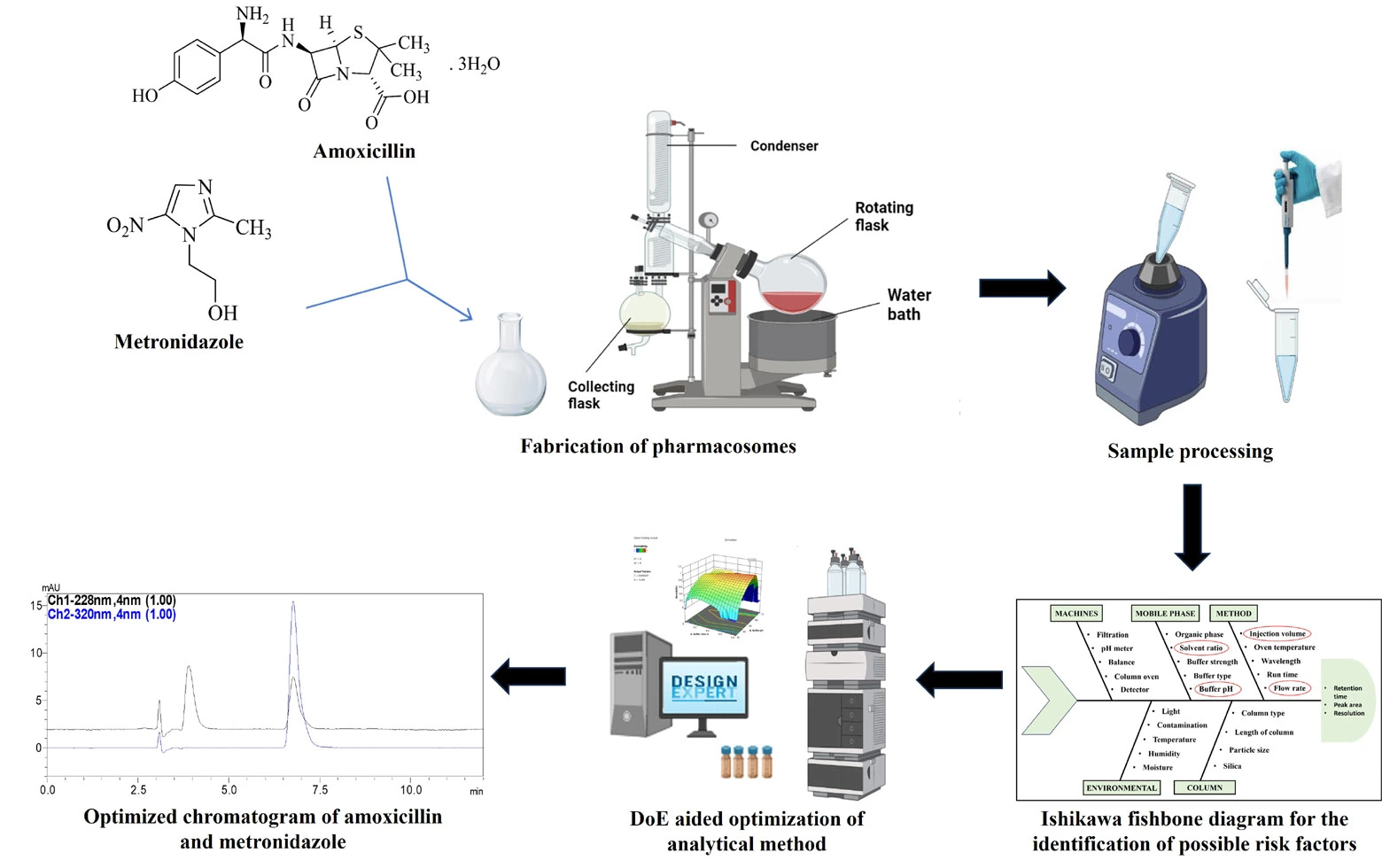
Analytical study of amoxicillin and metronidazole co-loaded pharmacosomes for H. pylori eradication using DoE-based modeling of an HPLC-PDA technique
Ashutosh Gupta, Moumita Saha, Shivani Kunkalienkar, Abhishek Jha, Pranav Kulkarni, Megha Samanth, Sathvik Chennamsetty, Princia Annie Dsouza, Srinivas Mutalik, Sudheer Moorkoth
DOI: 10.7324/JAPS.2025.226214Pages: 094-109
Advancing relugolix analysis: A comparative study and AQbD-driven method optimization with stability testing
Priyanka Nagar, Arvind Kumar Sharma, Robin Kumar, Chhaya Chauhan, Rini Singhal, Minakshi Garg
DOI: 10.7324/JAPS.2025.239585Pages: 055-070
_.jpg)