INTRODUCTION
Combination therapies for cancer have become a valid approach to alleviate chemotherapy resistance. Recently, cancer treatment involved the use of a minimum of two medications to enhance effectiveness and decrease toxicity [1,2]. By combining anti-cancer medications, specifically targets crucial pathways in an additive way, leading to better outcomes compared to using a single therapy. In addition, this method has the potential to decrease the development of drug resistance while providing various therapeutic benefits against cancer. These benefits include slowing the expansion of tumors and the likelihood of metastasis, halting the division of actively dividing cells, decreasing the number of cancer stem cells, and triggering apoptosis [2].
Natural bioactive compounds have been highly valuable remedies for the past 50 years in treating infectious diseases and cancer. Many natural products and synthetically modified natural product derivatives have been successfully developed for clinical use to treat human diseases in almost all therapeutic areas [3,4].
Coumarin (COMs), (Fig. 1A) are substantially present in nature and show a wide range of beneficial and varied range of biological actions [5]. These compounds are found as secondary metabolites in various kinds of plants, particularly in concentrated amounts found in the tonka bean, which serves as the source of their name (coumarou, a French term meaning tonka bean) [6]. The precise function of COMs is not fully understood, even though they have been suggested to play roles in the regulation of plant growth, inhibition of fungal growth, and inhibition of bacterial growth, and potentially serve as byproducts or metabolic waste. Several natural compounds containing a coumarin structure were found to exhibit diverse physiological effects. It is reasonable to anticipate that, resembling isomeric flavonoids, coumarins may impact the generation and elimination of oxidative species and free radicals and impact functions related to free radical-induced damage. Coumarin has the ability to reduce tissue edema and inflammation [7]. Coumarins can help fight tumors in various ways, such as by blocking certain enzymes, targeting specific cell signaling pathways, promoting cell death, and hindering the resistance of cancer to drugs. They also play a role in regulating cell processes and limiting the growth of blood vessels that support tumor growth [8,9]. Due to their outstanding biocompatibility, good capacity for regulating drug release, improved drug stability, and passive targeting capabilities through the enhanced permeability and retention (EPR) effect in tumor regions, liposomes are among the most widely utilized drug delivery systems in the world [10,11]. However, certain disadvantages restrict the use of liposomes in clinical settings, including issues with storing, transporting liquid formulation, and sustaining the drug levels in the desired target region. Therefore, some enhancements are required to provide liposomes that are more suited for therapeutic applications [12]. The aim of this study is the formulation and evaluation of anticancer activity of simple coumarin, and phenyl butyric acid (PBA) co-loaded into liposomes.
Butyrate compounds have numerous effects that include reducing inflammation of the intestinal lining, regulating the movement of fluid across the intestinal wall, improving the body’s ability to handle oxidative stress, and preventing the development of colon cancer [13]. In addition, an increasing number of studies suggest that butyric acid also has beneficial effects in preventing or inhibiting other types of cancer, by halting the growth of cancer cells and promoting their programmed cell death [14].
Similarly, phenylbutyrate (Fig. 1B) has the potential to provide favorable effects in various health conditions, including cancer, genetic metabolic disorders, neuropathies, diabetes, hemoglobinopathies, and urea cycle disorders. The mechanisms through which phenylbutyrate exerts these effects are diverse. Some of them involve the regulation of gene expression, acting as an inhibitor of histone deacetylases [15]. Others involve its ability to help proteins fold correctly, acting as a chemical chaperone. In addition, phenylbutyrate can facilitate the removal of toxic ammonia from the body, acting as an ammonia scavenger. It is important to note that the effects of phenylbutyrate can vary depending on the specific type of cell, which has led to the term “butyrate paradox”[16].
 | Figure 1. Chemical structure of A) Coumarin (COM) B) Phenyl butyric acid (PBA). [Click here to view] |
Overall, these findings highlight the wide range of beneficial effects associated with phenylbutyrate, suggesting its potential in therapeutic applications. Kusaczuk et al. [17] focused on the cellular and systemic effects of phenylbutyrate treatment, with particular emphasis on its three main mechanisms of action: ammonia scavenging, chaperoning, and histone deacetylase inhibition, its specific role in various human diseases has also been described In addition, PBA has been described as a compound with dual inhibitory effects of HDACs and pyruvate dehydrogenase kinases, with anticancer effects. Low membrane permeability and inadequate cellular absorption, however, prevent it from reaching the target organelle, which results in limited potencies against the targeted organs [18,19].
This study focuses on formulating liposomes loaded with natural compounds, coumarin (COM), and 4-PBA, which exhibit advanced techniques to achieve desired liposomal characteristics and stability over time. Results indicate promising antitumor effects in vitro, with the formulated liposomes demonstrating high encapsulation efficiencies and cytotoxicity against various cancer cell lines.
MATERIAL AND METHOD
PBA was obtained from ICT (Japan), Comarin (COM) was obtained from Sigma-Aldrich (USA). 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) was obtained from Avanti Polar Lipids, Inc. (Alabaster, USA) and Cholesterol (CHO) was obtained from Carbosynth (UK). Phosphate buffer saline (PBS) was purchased from LONZA® (USA). High performance liquid chromatography (HPLC) grade Methanol was acquired from Sigma-Aldrich (USA). HPLC-grade ethanol, acetonitrile, and chloroform were sourced from the carbon group (England). The remaining chemicals and solvents were of high analytical purity. No additional treatment was performed on any of the reagents or chemicals.
Liposomes preparation and characterization
Liposomes
Liposomes were prepared using the conventional ethanol injection technique (Fig. 2). Briefly, 4 mg of DPPC, CHO 0.5 mg, and 1.0 mg COM were dissolved in 0.3 ml of ethanol, which was then warmed at a 40°C water bath. PBS was heated using a hot plate at -50°C for 14 minutes with continuous string at 500 rpm. Then, the warm drug lipid ethanol solution was injected rapidly into the PBS with continuous string and heating. The mixture was left for 2 hours on the hot plate with continuous stirring until the complete evaporation of ethanol, which was confirmed with a decrease in the volume. The vesicle suspension was passed through a polycarbonate membrane multiple times, if necessary, using a Mini-Extruder (Avanti Polar Lipids, Inc. USA) at 50°C to obtain the proposed size (100 nm, 44 Whatman®). This process was repeated 13 times to obtain liposomes with low polydispersity and the desired size. After that, the liposomes were washed twice with a PBS solution through centrifugation to eliminate any remaining free COM and PBA. The encapsulated liposomes were then stored at 4°C [20].
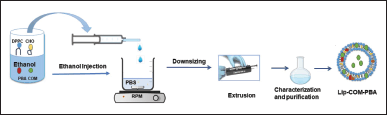 | Figure 2. Schematic representation of preparation of liposomes using ethanol injection method. [Click here to view] |
Liposomes characterization
Evaluation of the characteristics of the free liposome (Lipo-Free), PBA-loaded liposomes (Lipo-PBA), COM-loaded liposomes (Lipo-COM) as well as liposomes loaded with both PBA and COM (Lipo-PBA-COM) was performed through the use of Dynamic Light Scattering (DLS). Measurements include hydrodynamic diameter, Polydispersity index (PDI), and charge (ζ-Potential) for liposomes were measured by DLS. (Nano-ZS (Malvern Instruments Ltd., 45 Malvern, UK). The liposomal samples were diluted with deionized water at a ratio of 20:980 μl (v/v) to achieve a suitable counting rate. Before the measurement, all samples were placed in the specimen holder of a Zetasizer and allowed to reach room temperature equilibrium for 60 seconds.
In vitro stability of loaded liposomes
The Stability Assay of the loaded liposomes was performed at 4°C with storage times of 10 days. Liposome samples (10–20 μl) were collected at 4°C at different time intervals. The mean hydrodynamic diameter, Zeta potential, and PDI of the loaded liposomes were determined every day using a similar DLS method described before. All liposomes were diluted in Deionized Water before measurements.
In vitro release assay for COM and PBA Co-loaded liposomes
Encapsulated liposomes suspension of both COM and PBA (in 1 ml PBS) were added in a dialysis bag (molecular weight cut off [MWCO] 10 kDa, Thermo Fisher Scientific). The dialysis system was suspended in a release volume of 20 ml PBS, pH 7.4, at 37°C in 1 ml PBS, and was placed in a dialysis bag. At scheduled intervals, 200 μl of the release medium was collected for the HPLC assay. The same volume of fresh PBS buffer at the same temperature was added immediately to maintain a constant release volume. The length of the dialysis tubing was kept consistent for all methods to ensure that the surface area available for dialysis remained constant.
Chromatographic method and preparation of reference
The standard stock solution was prepared for COM and PBA (1.0 mg/ml) by dissolving 1.0 mg of each in 1.0 ml methanol. Solutions were ultrasonicated in a water bath for 10 minutes. From these stock solutions, a working standard of COM and PBA was prepared as a serial dilution. Finally, calibration curves were prepared composed of six standard Solutions with increasing concentrations for COM and PBA that were used in the linearity assay. All solutions were filtered through a 0.45 μm cellulose membrane and injected into the HPLC system (n = 3).
Chromatographic and HPLC conditions
Conditions analyses were performed on an HPLC system consisting of Shimadzu LC-2030 equipped with a UV Detector (Shimadzu Corporation, Kyoto, Japan). Separation was carried out on Thermo Scientific™ Hypersil™ BDS C18 HPLC Column, 100 Å, 5 µm, 4.6 × 150 mm stationary phase using mobile phase of Methanol: water 70:30; v/v) (pH = 2.8) [21]. Statistical acquisition, recording, 46, and chromatographic integration were achieved using LabSolutions version 5.92. The mobile phase was filtered through a nylon milli pore (0.2 μm) membrane filter, degassed before use, and pumped at a flow rate of 1.0 ml/ minute, and the injection volume was 10 μl. The analytical column was kept at 40°C. The chromatographic run time was set to 10 minutes. COM and PBA were detected at 210 nm. The calibration curve and HPLC chromatogram and conditions are shown in Figure 3.
Liposomes encapsulation efficiency (EE %) and loading efficiency (LO%)
Liposome suspensions (1 ml of COM and PBA) used for the evaluation were freshly prepared. To release the COM and PBA from the liposome’s Suspension, 200 µl of liposomes suspension was disrupted with 800 µl mobile phase or Acetonitrile then kept in bath sonication for 10 minutes and directly injected into HPLC.
The EE % and LO % of COM and PBA in liposomes were expressed as the percent of drug encapsulated inside liposomes and calculated using the following equations:
In vitro cytotoxicity (4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT assay)
New liposomes were prepared using the same Ethanol Injection Technique, by doubling the amounts of active ingredients COM and PBA. 3.4.1 Cell culture The parental MDA-231 (Breast cancer cell line), A549 (Lung cancer cell line), HT29 (Colorectal adenocarcinoma cell line), and Human dermal fibroblast cell lines were procured from the American type culture collection (, Manassas, VA, USA). The cells were cultivated as a monolayer and sustained in RPMI 1,640 medium (EuroClone, Italy) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (EuroClone, Italy), 1% PenicillinStreptomycin (EuroClone, Italy), and 2 mM L-glutamine. Incubation of the cells was carried out at 37°C in a 5% CO2 Tissue culture incubator (Memmert, Schwabach, Germany. Cell Viability Assay MTT). To determine the IC50 of Free COM, free PB, Free mix (COM-PB), Lipo-COM, and Lipo-PB on various cell lines, an MTT Assay was conducted using a 200 mm concentration of each treatment. MDA, A549, HT29, and Human dermal fibroblast cell lines were seeded at a density of approximately 9 × 103 cells/well in a 96-well plate (Corning, USA). Different concentrations of treatment ranging from 0.001 to 400 μm were administered to all cell lines. Subsequently, the cells were incubated at 37°C in a 5% CO2 incubator for 72 hours. Following incubation, the old media was removed, and 100 μl of fresh media containing MTT Assay salt (Bioworld, USA) was added to each well. The plates were then incubated at 37°C for an additional 3 hours. Afterward, 50 μl of Solubilization Solution Dimethyl sulfoxide was added to each well to assess cell viability. The absorbance of the solution was measured at 570 nm using a Glomax plate reader (Promega, USA).
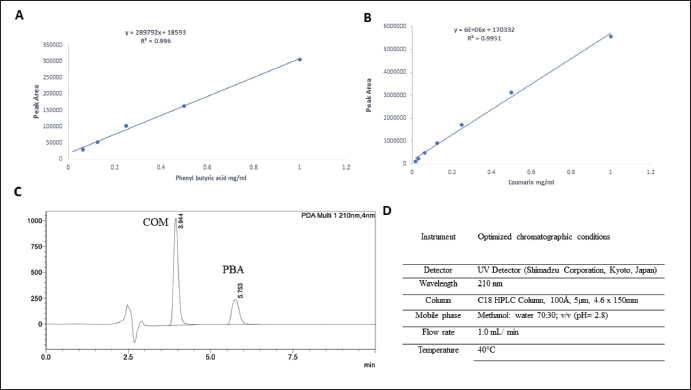 | Figure 3. Calibration curves of A) COM in ethanol, B) PBA in ethanol, C) Chromatogram of COM and PBA, D) HPLC conditions simultaneous method of development for COM and PBA. [Click here to view] |
Statistical analysis
Data were analyzed using Excel, GraphPad Prism version 8, CompuSyn version1. Values in the figures represent the mean of three independent experiments ± standard deviation.
RESULTS AND DISCUSSION
The current study revealed that the liposomes prepared using extrusion through a polycarbonate membrane achieved the desired size range of 100–200 nm with low polydispersity. The liposomes exhibited different particle sizes, PDI values, and ζ-potentials depending on the loaded drugs. The liposomes loaded with both PBA and COM demonstrated a size of particles of 155.6 ± 10.3 nm and a PDI of 0.086 ± 0.5, indicating a suitable size distribution for tumor tissue accumulation (Fig. 4A–C).
The validated newly developed HPLC analysis allowed for accurate and simultaneous quantification of the encapsulated drugs, and high encapsulation efficiencies were achieved for both PBA and COM. HPLC analysis was performed to determine the calibration curves for COM, PBA, and the mixed COM-PBA. The chromatograms demonstrated the elution of pure components, allowing for accurate quantification of the encapsulated drugs. The EE% and loading efficiency (LO%) of the liposomes were determined. The results showed high EE% values of 53% for Lipo-PBA and 25% for Lipo-COM, indicating efficient drug loading within the liposomes (Fig. 4D).
Moreover, the stability of the formulated liposomes was evaluated over a period of 10 days, (the time needed to perform cytotoxicity assay) and all liposomal formulations showed excellent stability in terms of hydrodynamic (Fig. 5A), diameter, charge (Fig. 5B), and PDI (Fig. 5C). The in vitro release of loaded COM from lip-COM and Lip-COM-PBA COM was assessed in physiological buffer (PBS, pH 7.4) at 37°C over a period of 72 hours at different intervals as shown in Figure 5D. The results of the load showed a biphasic release from liposomes with fast release at first 24 hours followed by sustained release [22]. The release behavior of PBA (at 24 hours> 50) was more than the release of COM (at 24 hours <50) either separated or co-loaded. These findings can be explained due to the differences in the structure of the two drugs, ionizability, and hydrophobicity [23].
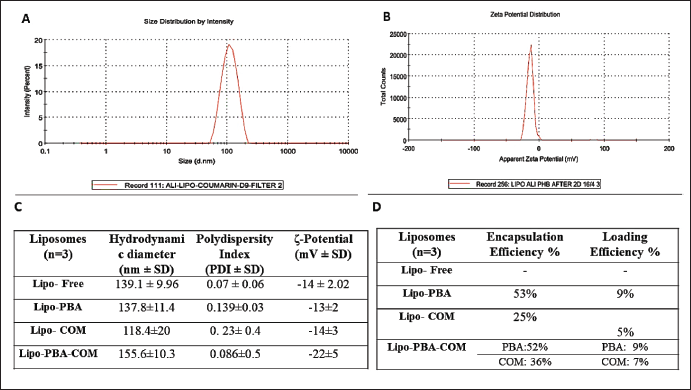 | Figure 4. A) DLS example size distribution, B) DLS example average zeta potential, C) Table showing average size, charge, EE%, and loading efficiency (LE %). [Click here to view] |
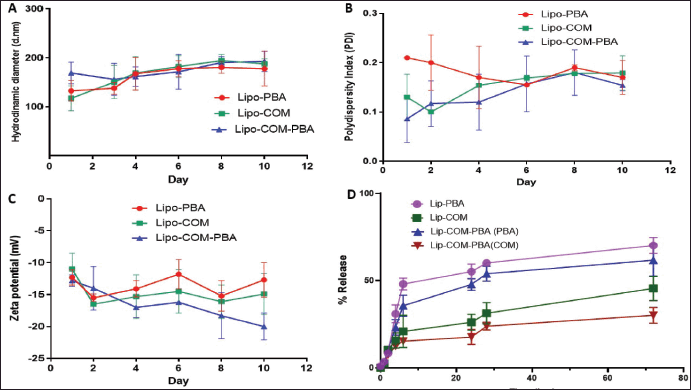 | Figure 5. Colloidal stability test for liposomes during 30 days at 4°C. A) Hydrodynamic diameter, B) Zeta potential, C) PDI, D) In vitro release curve of a COM and PBA loaded liposomes and free drug in phosphate buffer at 37°C (mean ± SD, n = 3). [Click here to view] |
In vitro cell viability studies were conducted to evaluate the cytotoxicity of the liposomal formulations on different cancer cell lines. The liposomes loaded with both COM and PBA exhibited the most effective inhibition on the MDA-MB-231 breast cancer cell line, in addition to the A59 lung cancer cell line. Lipo-COM demonstrated potent inhibition on HT29 colorectal cancer cells. The encapsulation of drugs inside liposomes increased their cytotoxicity, possibly due to enhanced penetration into tumor cells.
This was consistent with the findings of Zhao et al. [24] who tested the in-vitro and in-vivo cancer effects of pH-sensitive COM liposomes with Dox. The formulation demonstrated good tumor-controlling ability and high efficacy in reducing tumor growth and angiogenesis in living organisms [24].
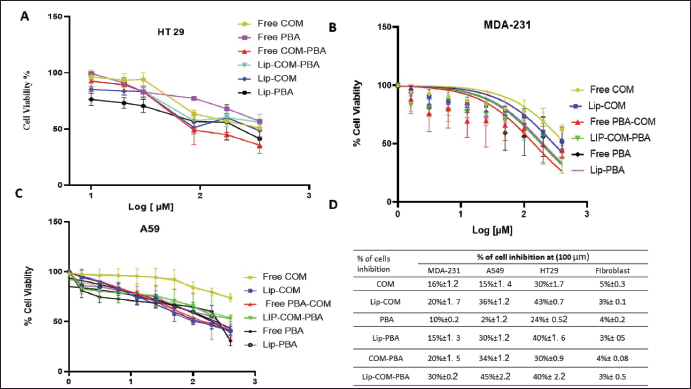 | Figure 6. The dose-response curve of (0.4–200 µM, n = 4) of free COM, PBA, Lip-COM Lip-PBA and their combinations against A) Colorectal cancer H T29 Cell lines, B) Breast cancer MDA-231 cancer cell line, C) Lung cancer cell lines, D) Table summarizes % of inhibition of the treatment against all cell lines (mean ± SD, n = 3). [Click here to view] |
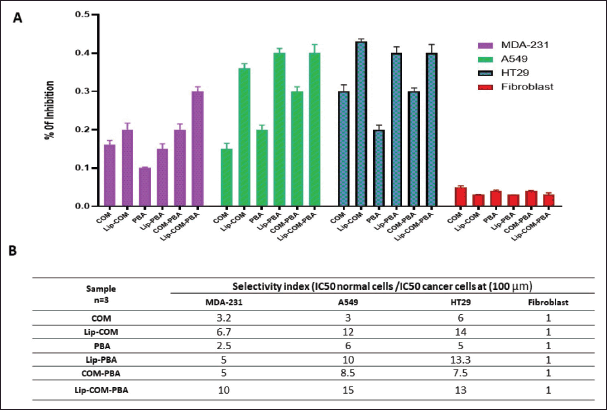 | Figure 7. A) Graph represents inhibition of the treated cells. B) Table of selectivity index for the three cell types (mean ± SD, n = 3). [Click here to view] |
Moreover, the stronger activity of Lipo-COM-PBA is comparable with prior research results where Tao et al. [25] designed a PBA-magnolol-COM conjugate. Ester bonds were broken upon entry into tumor cells, and the active drugs magnolol, COM, and PBA were released to start attacking cancerous tissue. The conjugate exhibited much stronger inhibitory potency toward A549, HepG2, A431, and MCF-7 cell lines compared to its individual original compound. according to in vitro anti-proliferation tests [25,26] (Fig. 6A–D).
The in vitro cell viability studies revealed the enhanced cytotoxicity of the liposomal formulations compared to free drugs, suggesting improved drug penetration into cancer cells especially since both drugs are lipophilic [26]. Finally, combination therapies involving liposomal formulations loaded with multiple drugs revealed a high selectivity index for cancer cells compared to normal fibroblast cell lines (Fig. 7A and B). This can be explained through the EPR effect in cancer therapy. ERP refers to the ability of certain drugs to preferentially accumulate in tumor tissues due to the leaky vasculature and impaired lymphatic drainage associated with the tumor. This selective accumulation enhances the drug‘s concentration within the tumor, contributing to improved therapeutic outcomes while minimizing systemic exposure and potential toxicity to normal tissues [27,28].
Regarding the viability study, it is complicated to understand cellular behavior and responses and researchers face challenges when translating findings from simplified 2D cell cultures to more intricate 3D environments or tissues within living organisms. Furthermore, findings from 2D cell cultures may not accurately reflect the dynamic interactions and complexities present in cancer tissues within animals but they are important for screening [29]. The gap between cell cultures and the in vivo environment emphasizes the necessity for comprehensive research that incorporates animal testing to bridge this divide. Therefore, it is recommended to confirm the in vitro study with in vivo experiment.
CONCLUSION
The liposomes loaded with both PBA and COM showed suitable particle size distribution for tumor tissue accumulation. The stability testing confirmed the long-term stability of the liposomal formulations, indicating their potential for storage and future clinical use. HPLC analysis allowed for accurate quantification of the encapsulated drugs, and high encapsulation efficiencies were achieved for both PBA and COM.
The in vitro cell viability studies revealed the enhanced cytotoxicity of the liposomal formulations compared to free drugs, suggesting improved drug penetration into cancer cells. Based on the findings of this study, several recommendations can be made for future research. First, in-vivo investigations are warranted to assess the therapeutic efficacy and pharmacokinetics of the liposomal formulations. Animal models can provide valuable insights into the biodistribution, tumor-targeting ability, and overall efficacy of liposomes in a more complex biological environment. In addition, further optimization of the liposomal formulations can be explored to enhance their drug-loading capacity and stability. Different lipid compositions, encapsulation techniques, or modifications of the liposomal surface can be investigated to improve the EE and prolong the stability of the liposomes. Moreover, more comprehensive toxicity investigations are warranted to assess the safety profile of the liposomal formulations. Assessing their potential side effects on normal cells and organs is crucial for the development of safe and effective therapeutic strategies. Future research should focus on in vivo studies, optimization of the liposomal formulations, comprehensive toxicity evaluations, and exploration of combination therapies with multiple drugs to further improve treatment outcomes.
LIST OF ABBREVIATIONS
COM, Coumarin; DLS, Dynamic light scattering; DMSO, Dimethyl sulfoxide; EE, Encapsulation efficiency%; HPLC, High performance liquid chromatography; IC50, Half-maximal inhibitory concentration; LE%, Loading efficiency%; MTT, (4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; PBA, 4-phenylbutyric acid; PDI, Polydispersity Index; WHO, World Health Organization.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Zargar A, Chang S, Kothari A, Snijders AM, Mao JH, Wang J, et al. Overcoming the challenges of cancer drug resistance through bacterial-mediated therapy. Chronic Dis Transl Med. 2019;5(4):258–66. CrossRef
2. Lafi Z, Alshaer W, Gharaibeh L, Alqudah DA, AlQuaissi B, Bashaireh B, et al. Synergistic combination of doxorubicin with hydralazine, and disulfiram against MCF-7 breast cancer cell line. PLoS One. 2023;18(9):e0291981. CrossRef
3. Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K, et al. A comprehensive review on nutraceuticals: therapy support and formulation challenges. Nutrients. 2022;14(21):4637. CrossRef
4. Nsairat H, Lafi Z, Al-Sulaibi M, Gharaibeh L, Alshaer W. Impact of nanotechnology on the oral delivery of phyto-bioactive compounds. Food Chemistry. 2023;424:136438. CrossRef
5. Küpeli Akkol E, Genç Y, Karpuz B, Sobarzo-Sánchez E, Capasso R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers (Basel). 2020;12(7):1959. CrossRef
6. Loncar M, Jakovljevic M, Šubaric D, Pavlic M, Buzjak Služek V, Cindric I, et al. Coumarins in food and methods of their determination. Foods. 2020;9(5):645. CrossRef
7. Stefanachi A, Leonetti F, Pisani L, Catto M, Carotti A. Coumarin: a natural, privileged and versatile scaffold for bioactive compounds. Molecules. 2018;23(2):250. CrossRef
8. Wu Y, Xu J, Liu Y, Zeng Y, Wu G. A review on anti-tumor mechanisms of coumarins. Front Oncology. 2020;10:592853. CrossRef
9. Rawat A, Vijaya Bhaskar Reddy A. Recent advances on anticancer activity of coumarin derivatives. Eur J Med Chem Rep. 2022;5:100038. CrossRef
10. Medina-Alarcón KP, Voltan AR, Fonseca-Santos B, Moro IJ, de Oliveira Souza F, Chorilli M, et al. Highlights in nanocarriers for the treatment against cervical cancer. Mater Sci Eng C Mater Biol Appl. 2017;80:748–59. CrossRef
11. Al-Azzawi H, Alshaer W, Esawi E, Lafi Z, Abuarqoub D, Zaza R, et al. Multifunctional nanoparticles recruiting hyaluronic acid ligand and polyplexes containing low molecular weight protamine and ATP-Sensitive DNA motif for doxorubicin delivery. J Drug Deliv Sci Technol. 2022;69:103169. CrossRef
12. Cheng R, Liu L, Xiang Y, Lu Y, Deng L, Zhang H, et al. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials. 2020;232:119706. CrossRef
13. Wu X, Wu Y, He L, Wu L, Wang X, Liu Z. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9(14):2510–7. CrossRef
14. Chen M, Jiang W, Xiao C, Yang W, Qin Q, Mao A, et al. Sodium butyrate combined with docetaxel for the treatment of lung adenocarcinoma A549 cells by targeting Gli1. OncoTargets Ther. 2020;13:8861–75. CrossRef
15. Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs in R D. 2011;11:227–49. CrossRef
16. Ryu SH, Kaiko GE, Stappenbeck TS. Cellular differentiation: potential insight into butyrate paradox? Mol Cell Oncol. 2018;5(3):e1212685. CrossRef
17. Kusaczuk M, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyric Acid: simple structure - multiple effects. Curr Pharm Des. 2015;21(16):2147–66. CrossRef
18. Kusaczuk M, Kretowski R, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 2016;37(1):931–42. CrossRef
19. Lumpkin CJ, Harris AW, Connell AJ, Kirk RW, Whiting JA, Saieva L, et al. Evaluation of the orally bioavailable 4-phenylbutyrate-tethered trichostatin A analogue AR42 in models of spinal muscular atrophy. Sci Rep. 2023;13(1):10374. CrossRef
20. Alshaer W, Zraikat M, Amer A, Nsairat H, Lafi Z, Alqudah DA, et al. Encapsulation of echinomycin in cyclodextrin inclusion complexes into liposomes: in vitro anti-proliferative and anti-invasive activity in glioblastoma. RSC Advances. 2019;9(53):30976–88. CrossRef
21. Marahatta A, Bhandary B, Lee M-R, Kim D-S, Lee YC, Kim S-R, et al. Determination of phenylbutyric acid and its metabolite phenylacetic acid in different tissues of mouse by liquid chromatography with tandem mass spectrometry and its application in drug tissue distribution. J Chromatogr B. 2012;903:118–25. CrossRef
22. Tefas LR, Sylvester B, Tomuta I, Sesarman A, Licarete E, Banciu M, et al. Development of antiproliferative long-circulating liposomes co-encapsulating doxorubicin and curcumin, through the use of a quality-by-design approach. Drug Des Devel Ther. 2017;11:1605–21. CrossRef
23. Miao ZL, Deng YJ, Du HY, Suo XB, Wang XY, Wang X, et al. Preparation of a liposomal delivery system and its in vitro release of rapamycin. Exp Ther Med. 2015;9(3):941–6. CrossRef
24. Zhao Y, Ren W, Zhong T, Zhang S, Huang D, Guo Y, et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J Control Release. 2016;222:56–66. CrossRef
25. Tao A, Song Z, Feng X, Hu B, Lei X. Magnolol–Coumarin– Phenylbutyric acid conjugates: an anticancer prodrug via multiple targets. IOP Conf Ser Earth Environ Sci. 2019;330(4):042054. CrossRef
26. Zhang L, Xu Z. Coumarin-containing hybrids and their anticancer activities. Eur J Med Chem. 2019;181:111587. CrossRef
27. Li J, Gu Y, Zhang W, Bao CY, Li CR, Zhang JY, et al. Molecular mechanism for selective cytotoxicity towards cancer cells of diselenide-containing paclitaxel nanoparticles. Int J Biol Sci. 2019;15(8):1755–70. CrossRef
28. Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771. CrossRef
29. Kapalczylska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci. 2018;14(4):910–9.