INTRODUCTION
Pterostilbene (PTB) is a naturally occurring compound in the heartwood of Pterocarpus marsupium and blueberries (Lin et al., 2009; Roupe et al., 2006). It is a vital component of blueberries and P. marsupium (Adrian et al., 2000; Breuil et al., 1999; Fuendjiep et al., 2002; Pezet and Pont, 1998). It was first isolated from Pterocarpus santalinus (Seshadri, 1972). It mostly grows in Sri Lanka and India. It is also known as Sarfaka in Sanskrit, Vijaysaar in Hindi, and Indian Kino in English. Pterocarpus marsupium heartwood is frequently used to treat diabetes mellitus (Gupta and Gupta, 2009; Mallavadhani and Sahu, 2003; Manickam et al., 1997; Mishra et al., 2013; Pari and Amarnath, 2006, 2008; Perera, 2016). Other pharmacological activities of different parts of P. marsupium, such as antioxidant (Amorati et al., 2004; Denise and David, 2013; Rimando et al., 2002; Stivala et al., 2001) hypolipidemic, and antifungal activities have also been reported (Daniel et al., 2013; Ferrer et al., 2005; Roberti et al., 2003; Tolomeo et al., 2005).
PTB, a naturally occurring isoflavone derivative, has been reported to have many therapeutic benefits, including anticancer activity. It was proven to be an effective anticancer drug in a number of cancers (Denise and David, 2012). It is a type of phenolic compound found in a traditional Ayurvedic beverage from India called Darachchasava, which is used to cure cardiovascular and other conditions (Paul et al., 1999).
Ultrasonic-assisted extraction (UAE) is becoming a more common way to extract active compounds from plant material (Wakte et al., 2011). By creating cavitation in the solvent, which occurs as a result of ultrasonic waves passing through it, ultrasound increases the effectiveness of single-molecule extraction (Giacometti et al., 2018; Jang et al., 2017; Pham et al., 2020; Yusoff et al., 2022; Zheng et al., 2020). As a result of improved solvent penetration into the sample, there is a higher release of matrix components into the solvent as a result of increased extraction. To create an effective ultrasound-assisted extraction method, it is necessary to maximize the experimental conditions because a number of process variables, such as ultrasound power, process temperature, and sonication time, affect the extraction efficiency (Ali et al., 2018; Aourabi et al., 2020; Fan et al., 2020; González-Silva et al., 2022; Pan et al., 2012).
One of the most important steps in the setup of a high throughput laboratory is the design of experiment. In order to maximize the benefits of a system, process, or product, it is necessary to optimize its performance. The phrase “optimization” has been frequently used in analytical chemistry to describe the process of identifying the conditions at which to execute a technique in order to obtain the optimal response. In the past, optimization in analytical chemistry has been done by keeping track of how one factor at a time affects an experimental result. Only one parameter is altered; all other parameters are maintained at their constant level. The term “one-variable-at-a-time” refers to this optimization method. The fact that the interacting effects between the variables under study are not taken into account is its major drawback. The whole impact of the parameter on the response is therefore not represented by this technique (Lundstedt et al., 1998). One-factor optimization also has a limitation in that it necessitates more experiments to complete the study, which adds to the time and cost of the project as well as the number of reagents and materials used.
In order to efficiently estimate the first- and second-order coefficients of the mathematical model, Box and Behnken (1960) described how to choose points from the three-level factorial arrangement. As a result, and mostly due to the enormous number of variables, these designs are more effective and cost-effective (Bhusari et al., 2020).
The advantages of response surface methodology (RSM) over traditional one-variable-at-a-time optimization, such as the generation of large amounts of information from a small number of experiments and the capability of evaluating the interaction effect between the variables on the response, have led to its widespread and consolidated use in the optimization of conventional extraction techniques today. The symmetrical second-order experimental design that is still most frequently used for the development of analytical techniques is the central composite design. Three-level factorial designs are not frequently used, and when they are typically only used to optimize two variables because they perform poorly with more variables.
The objective of this research was to enhance the extraction of PTB from P. marsupium by applying RSM to optimize the variables of the ultrasound-assisted extraction method, such as the soaking time, solvent to solid ratio, and sonication time by using Box Behnken Design (BBD).
MATERIAL AND METHODS
Plant material
Pterocarpus marsupium heartwood was collected in Aurangabad, Maharashtra. At the Botanical Survey of India in Pune, the material that had been obtained had been authenticated. The collected leaves were washed and dried in a micro tray dryer (S. B. Panchal & Company). To obtain consistent particle size, dried leaves were pulverized in a mixer grinder (Devika mixer grinder) and sieved through a 120 mesh sieve. The obtained powder was then utilized for extraction.
Chemicals
High performance liquid chromatography (HPLC) grade acetonitrile (ACN) procured from Rankem. Distilled ethanol was used for the study. The PTB was purchased from Sigma Aldrich (India) Pvt. Ltd.
Sample preparation
0.5 g of plant samples were put into an extraction vessel along with ethanol and sonicated using an ultrasonic bath (Labman Scientific Instruments Ltd.), under various experimental conditions. Schematic representation of UAE showed in Figure 1. Collected were the liquid fractions of the extraction. Prior to HPLC analysis, samples were filtered after extraction.
HPLC analysis
Chromatographic analysis was conducted using HPLC (Agilent Technologies). The system was equipped with a G1329B autosampler. The analysis made use of an Agilent Technologies G1315F variable wavelength detector. HPLC grade water was obtained using a “Extra pure” water purification equipment (Lab link). Using an ultrasonicator, the mobile phase was degassed (PCi Analyticals). Chemicals were weighed using a Vibra HT (Essae) analytical balance. HPLC grade water was prepared using Lab Link “Extra pure” water purification equipment. For HPLC analysis, a mobile phase with water: ACN ratio of 35:65 v/v was utilized. It was filtered through a 0.22 m filter and ultrasonically degassed for 10 minutes.
All analyses were performed at room temperature under isocratic conditions. The sample was filtered with a 0.2 m filter prior to analysis. For 10 minutes, the mobile phase was run at a flow rate of 1 ml/minute. Ultraviolet detection was used to monitor the column eluent at 306 nm, and the injection volume was 20 µl.
By comparing the PTB peak’s retention time to that of standards, the PTB peak was identified, and the concentration was then determined using calibration curves. The assay mean values for each experiment, which were carried out in triplicate, are used to express the results.
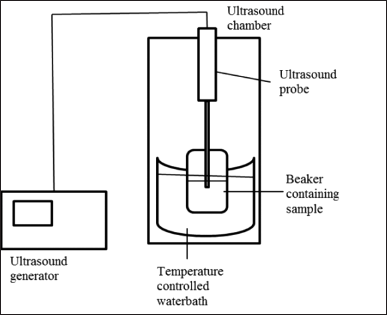 | Figure 1. Schematic representation of UAE. [Click here to view] |
Experimental design
The application of the BBD was conducted in the form of preliminary experiment observations. This resulted in 15 treatments with three center points for evaluation. The solvent-to-solid ratio (A), the soaking time (B), and the sonication time (C) were chosen as independent factors (Table 1). A model was created using Design Expert software, which also enabled us to move toward the optimization of operating conditions. This design was used to determine the ideal conditions under which P. marsupium PTB extraction could be successsfully accomplished.
RESULTS AND DISCUSSION
HPLC analysis
The PTB was detected at 306 nm and representative HPLC chromatograms are shown in Figure 2. For PTB concentrations from 1 to 5 ng/ml, the regression equation was = 6.69740× + 0.0.426, which presented good linearity (R = 0.999).
Statistical analysis and model fitting
In the present BBD, a total of 15 runs were required for each of the three independent parameters. The experimental factorial design parameters and PTB extraction yield values are shown in Table 2. According to the ANOVA results (Table 3), it is possible to determine the experiment’s response surface.
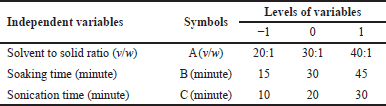 | Table 1. The independent variables affecting the extraction procedure. [Click here to view] |
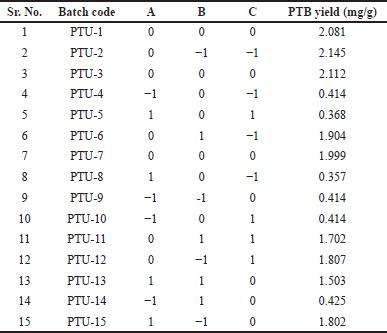 | Table 2. BBD of three independent variables. [Click here to view] |
The goodness-of-fit of the linear regression model can be measured using the adjusted determination coefficient. R adj is also called adjusted R2 adj, as it is calculated through a standard way of adjusting for multiple sources of noise. The R adj value, calculated with 947.8860 is very nearly 1, and therefore there is an almost perfect fit between observed and predicted values. The calculated p-value for the lack of fit was 0.3462.
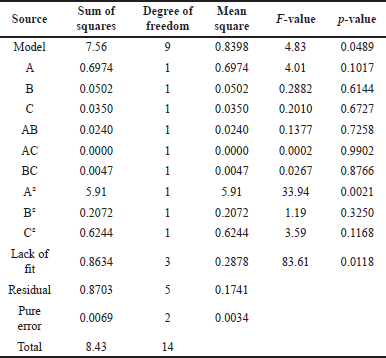 | Table 3. The ANOVA analysis for the extraction of PTB. [Click here to view] |
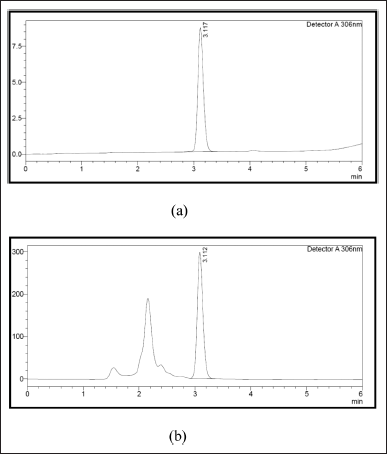 | Figure 2. Chromatograms of PTB (a) standard and (b) extract. [Click here to view] |
In order to assess the fitness of the model, we used the lack of fit test (p > 0.05) to assess the model’s accuracy in predicting variance. The F-value (df) is shown in this column with a p-value below 0.05 considered significant. The significance of associated variables would increase as df and p-value decreased. According to F-test, F = 29.75 and p = 0.001), evidenced high importance for this model.
RSM for optimization of extraction conditions
Based on estimations, the RSM provides empirical relationships between PTB extraction yield and test variables. The maximum PTB extraction yield has been estimated using a linear regression model with calibration data including response variables (extraction yield) and test variables. The multiple regression equation used here to relate the experimental variables of PTB extraction yield is as follows:
Y = + 2.06 + 0.2952 A −0.0792B −0.0661C −0.0774AB + 0.0027AC + 0.0341BC −1.26A² +0.2369B² −0.4112C²
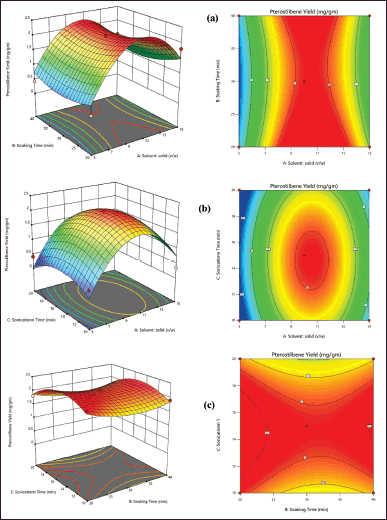 | Figure 3. Contour plots and response surface plots show the influence of variables on the response Y (PTB yield). [Click here to view] |
Where Y is the yield of PTB (mg/g), and A, B, and C are the coded variables for the solid-solvent ratio, soaking time, and sonication time, respectively. The solvent: solid ratio (A), soaking time (B), and sonication time (C) of PTB were found to influence the extraction yield of PTB as seen in Figure 2. The outcome of the experiment on PTB extraction yield showed that the increase in each factor of extraction was proportional to each other, as seen in Figure 3(a). The 3D response surface plot and 2D contour plot are graphical representations of how each factor affected PTB yield, which visually shows the relationship between them.
The extraction yield of PTB is shown in Figure 3(b) as a function of solvent: solid ratio, sonication time, and at a fixed soaking time using a 3D response surface plot and contour plot. Within the selected experimental range, higher solvent-to-solid ratios and longer sonication times led to higher yields of PTB. When the solvent: solid ratio was constant, the extraction yields of PTB affected by various sonication times and soaking times are shown in Figure 3(c). It was highly significant (p 0.001) how the solvent: solid ratio interacted with different sonication times and soaking times.
CONCLUSION
The extraction of PTB from P. marsupium with the use of ultrasound was studied in the current study applying the RSM. The experimental results indicate that the solvent: solid ratio, soaking time, and sonication time were all important process factors that had an impact on the extraction of PTB. The best conditions for extracting PTB were determined to be the following: solvent-to-solvent ratio of 10:1, 20 minutes soaking time, and 10 minutes sonication time. PTB achieved an experimental yield of 2.14 mg/g in these circumstances, which is quite similar to the expected yield value of 2.07 mg/g. The experimental results agreed with the RSM model predictions, proving the model’s effectiveness and how effectively RSM was employed. The combination of factors in the optimized batch gave the maximum percentage yield of PTB. Therefore, it will serve to be the base for the commercial extraction of PTB from heartwood of P. marsupium.
ACKNOWLEDGMENT
1. It is highly acknowledged that DST-DPRP, Govt. of India (Ref: VI-D&P/626/2018-19/TDT) sanctioned to Dr. Sachin S. Bhusari, Department of Chemical Technology for the proposed research work is highly appreciated.
2. The authors are also thankful to Science and Engineering Research Board, Department of Science and Technology, Government of India, New Delhi for providing extramural research grant wide sanction no. EMR/2017/000859 sanctioned to P.I. Dr. Pravin S. Wakte, Department of Chemical Technology for the support in developing this project.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest involved.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adrian M, Jeandet P, Douillet-Breuil AC, Tesson L, Bessis R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J Agric Food Chem, 2000; 48(12):6103–5.
Ali A, Lim XY, Chong, CH, Mah SH, Chua BL. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT, 2018; 89:681–8.
Amorati R, Lucarini M, Mugnaini V, Pedulli GF. Antioxidant activity of hydroxystilbene derivatives in homogeneous solution. J Org Chem, 2004; 69(21):7101–7.
Aourabi S, Sfaira M, Mahjoubi F. Optimization of ultrasound-assisted extraction of polyphenol content from Zea mays Hairs (waste). Sci World J, 2020; 2020:1–10.
Bhusari S, Ansari I, Chaudhary A. Development of Darunavir proliposome powder for oral delivery by using Box–Bhenken design. Drug Dev Ind Pharm, 2020; 46(5):732–43.
Box GEP, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics, 1960; 2:455–75.
Breuil ACD, Jeandet P, Adrian M, Bessis R. Changes in the phytoalexin content of various Vitis spp. in response to ultraviolet C elicitation. J Agric Food Chem, 1999; 47(10):4456–61.
Daniel M, Corey L, Krista D, Justin J, Marion R, David D. Analysis of safety from a human clinical trial with Pterostilbene. J Toxicol, 2013; 1:1–5.
Denise M, David M. Pterostilbene and cancer: current review. J Surg Res, 2012; 173(2):53–61.
Denise M, David M. A review of Pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev, 2013; 2013:1–15.
Fan S, Yang G, Zhang J, Li J, Bai B. Optimization of ultrasound-assisted extraction using response surface methodology for simultaneous quantitation of six flavonoids in Flos Sophorae Immaturus and antioxidant activity. Molecules, 2020; 25(8):1767.
Ferrer P, Asensi M, Segarra R, Ortega A, Beniloch M, Obrador E, Varea MT, Asensio G, Jordá L, Estrela JM. Association between Pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia, 2005; 7(1):37–47.
Fuendjiep V, Wandji J, Tillequin F, Mulholland DA, Budzikiewicz H, Fomum ZT, Nyemba AM, Koch M. Chalconoid and stilbenoid glycosides from Guibourtia tessmanii. Phytochemistry, 2002; 60(8):803–6.
Giacometti J, Žauhar G, Žuvi? M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods, 2018; 7(9):149.
González-Silva N, Nolasco-González Y, Aguilar-Hernández G, Sáyago-Ayerdi SG, Villagrán Z, Acosta JL, Montalvo-González E, Anaya-Esparza LM. Ultrasound-assisted extraction of phenolic compounds from Psidium cattleianum leaves: optimization using the response surface methodology. Molecules, 2022; 27(11):3557.
Gupta RR, Gupta RS. Effect of Pterocarpus marsupium in streptozotocin-induced hyperglycemic state in rats: comparison with glibenclamide. Diabetol Croat, 2009; 38(2):39–45.
Jang S, Lee AY, Lee AR, Choi G, Kim HK. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Integr Med Res, 2017; 6(4):388–94.
Lin HS, Yue BD, Ho PC. Determination of Pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr, 2009; 23(12):1308–15.
Lundstedt T, Seifert E, Abramo L, Thelin B, Nystrom A, Pertensen J, Bergman R. Experimental design and optimization. Chemometr Intell Lab Syst, 1998; 42:3–40.
Mallavadhani UV, Sahu G. Pterostilbene: a highly reliable quality- control marker for the ayurvedic antidiabetic plant ‘Bijasar’. Chromatographia, 2003; 58:307–12.
Manickam M, Ramanathan M Jahromi, MAF, Chansouria JPN, Ray AB. Antihyperglycemic activity of phenolic from Pterocarpus marsupium. J Nat Prod, 1997; 60(6):609–10.
Mishra A, Srivastava R, Swayam P, Gautam S, Kumar A, Maury R, Arvind K. Antidiabetic activity of heart wood of Pterocarpus marsupium Roxb. and analysis of phytoconstituents. Indian J Exp Biol, 2013; 51:363–74.
Pan G, Yu G, Zhu C, Qiao J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason Sonochem, 2012; 19(3):486–90.
Pari M, Amarnath S. Effect of Pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci, 2006; 79:641–45.
Pari M, Amarnath S. Effect of Pterostilbene on lipids and lipid profiles in streptozotocin–nicotinamide induced type 2 diabetes mellitus. J Appl Biomed, 2008; 6:31–7.
Paul B, Masih I, Deopujari J, Charpentier C. Occurrence of resveratrol and Pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol, 1999; 68:71–6.
Perera HKI. Antidiabetic effects of Pterocarpus marsupium (Gammalu). Eur J Med Plants, 2016; 13(4):1–14.
Pezet R, Pont V. Identification of Pterostilbene in grape berries of Vitis vinifera. Plant Physiol Biochem, 1988; 26(5):603–7.
Pham DC, Nguyen HC, Nguyen TH, Ho HL, Trinh TK, Riyaphan J, Weng, CF. Optimization of ultrasound assisted extraction of flavonoids from Celastrus hindsii leaves using response surface methodology and evaluation of their antioxidant and antitumor activities. BioMed Res Int, 2020; 2020:1–9.
Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Cancer chemopreventive and antioxidant activities of Pterostilbene, a naturally occurring analogue of resveratrol. J Agric Food Chem, 2002; 50(12):3453–7.
Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem, 2003; 46(16):3546–54.
Roupe, Remsberg, CM, Y´a˜nez, JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol, 2006; 1(1):81–101.
Seshadri TR. Polyphenols of pterocarpus and Dalbergia woods. Phytochemistry, 1972; 11(3):881–98.
Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, Forti L, Pagnoni UM, Albini A, Prosperi E, Vannini V. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem, 2001; 276(25):22586–94.
Tolomeo M, Grimaudo S, Cristina AD, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L, Barucchello R, Grisolia G, Invidiata F, Simoni D. Pterostilbene and 3′ hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol, 2005; 37(8):1709–26.
Wakte PS, Sachin BS, Patil AA, Mohato DM, Band TH, Shinde DB. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep Purif Technol, 2011; 79(1):50–5.
Yusoff IM, Taher ZM, Rahmat Z, Chua LS. A review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, saponins and proteins. Food Res Int, 2022; 22:111268.
Zheng S, Zhang W, Liu S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS One, 2020; 15(12):e0244749.