INTRODUCTION
Naloxone (NLX) which is marketed as Narcan injection is used for the treatment of opioid intoxication. It is approved worldwide and is on the WHO model list of Essential Medicines as a specific antidote. It has opioid adversary effect by blocking the property of narcotic drugs, in a prescribed overdose of drug causing death worldwide. Precisely, NLX has a more tendency toward μ opioid receptors acting as inverse agonist. This causes the quick elimination of opioid drugs on to these binding sites. It works by blocking the effects of the opioid in the brain.
NLX was Naloxone is available in the form of Naloxone hydrochloride injection and can administered by different route of administration which includes sub-cutaneous, nasal spray, intramuscular, and infusion through intravenous. However, the perfect way for administration, is to be intravenous (IV) route (Mycyk et al., 2003; Panchagnula et al., 2004).
NLX hydrochloride is chemically 4,5α-Epoxy-3,14-dihy-droxy-17-(prop-2-enyl) morphine-6-one hydrochloride and avail-able in dihydrate form. The molecular mass of dihydrate form is 363.84 g/mol. The drug substance is official in USP and Ph Eur monographs, while the drug product Naloxone hydrochloride injection is official only in USP monograph. NLX is marketed as NARCAN® which is a sterile liquid formulation indicated for reversing the effect of over dose of opioid pain relievers.
A total of seven impurities were reported for Naloxone hydrochloride injection which includes both process and degradants. Literatures are available for quantifying these listed impurities. Several methods have been reported to identify NLX and its related impurities using techniques like high-performance liquid chromatography (HPLC), liquid chromatography–mass spectrometry (LC–MS). (Huang et al., 2016). Any new impurities than mentioned above may have a significant impact on the safety and efficacy of the product. Different techniques are available for isolation and characterization of unknown impurities. Some techniques for the identification of unknown impurities include fractional collector, preparative two-dimensional LC, which uses heart cutting technology in isolating the impurity at particular retention time and determine the mass and nuclear magnetic resonance (NMR) spectra of the impurity etc.
During stability study of the NLX HCl product as per International Council for Harmonization guidelines (ICH) Q1A (R2) stability guidelines, two additional degradants were found to be increasing with time. Evaluation of the marketed formulations when stored at accelerated conditions showed the formation of the forth-mentioned impurities when analyzed at the end of 6 months.
The aim of this study is to identify the conditions to enrich these unknown impurities (Impurity-1 and Impurity-2), isolate, and structurally identify these impurities to predict the possible degradation pathway.
MATERIALS AND METHODS
Samples, chemicals, and reagents
The samples required for analysis, Naloxone hydrochloride injection USP 2 mg/2 ml, were formulated and manufactured at the Dr. Reddy’s IPDO, Innovation Plaza (Dr. Reddy’s Limited, Hyderabad, India). Impurities enrichment and isolation process were done at Dr. Reddy’s IPDO facility. The chemical and solvent system used for the analysis, i.e., Trifluoro acetic acid (MS grade), 1-Octane sulfonic acid sodium salt (AR grade), Ortho-phosphoric acid (ACS grade, 88% w/w), Tetrahydrofuran (gradient grade) Acetonitrile (Merck-gradient grade), Hydrochloric acid 36% (AR grade), Sodium chloride (AR grade), and Milli-Q Water.
HPLC (analytical-LC)
Chromatographic separation was achieved on Waters HPLC system configured with Waters 2695 separation module and 2996 photo diode array detector with Empower 3 software. The chromatographic system comprises stationary phase of Phenomenex Luna C18, (250 × 4.6) mm, 5 μm Particle size column. Solvent system A comprises integrating buffer with tetrahydrofuran in the fractions of (96:04) %v/v (preparation of buffer: dissolve 1.1 g of 1-Octane sulfonic acid sodium salt per 1 l of water, pH was regulated to 2.00 ± 0.05 with diluted OPA) and Solvent system B comprises homogenous mix of acetonitrile: Tetrahydrofuran 96:04 (%v/v) ratio.
The flow rate was maintained at 1.5 ml/minute with a column temperature of 50°C. Injection volume of 50 μl is selected for better peak sensitivity. The UV-Vis detection was set to 230 nm. Data acquirement time is 60 minutes and pump mode is set to gradient. The gradient program is as follows: time (minute)/%A(v/v): %B(v/v): T0.01/95:05, T1/95:05, T16/89:11, T22/89:11, T45/70:30, T50/30:70, T53/95:05, T60/95:05.
Stability studies
Following ICH (2003), Naloxone hydrochloride injection 2 mg/2 ml filled in 3 ml glass pre-filled syringe, were exposed to following environments to assess the stability profile in influence of regulated temperature and humidity circumstances i.e., real time stability conditions at 25 ± 2°C / 60 ± 5%RH accelerated stability conditions at 40 ± 2°C / 75 ± 5%RH, over a range of time intervals (from and initial to 24 months for real time storage conditions and from initial to 6 months at accelerated conditions, respectively), while marketed formulations were only stored at accelerated stability condition for 6 months
All samples were withdrawn periodically from their corresponding stability conditions and analyzed using reversed-phase -HPLC method mentioned in HPLC (analytical-LC) section.
Stress studies
Forced degradation / stress studies were executed to understand the chemical behavior of the product. NLX HCl was subjected to different stress forms like acidic, basic and thermal hydrolysis, peroxide, and photolytic oxidation.
Preparative liquid chromatography
Agilent 1200 series preparative liquid chromatograph configured with UV-VIS detector and fraction collector was used for purification. A Phenomenex Gemini NX C18 250 mm × 30 mm, particle size of 5 μm column was selected for the separation of degradation products. The solvent system A comprises 0.1% TFA with water and solvent system B comprises 0.1% TFA with acetonitrile. Flow rate of 20 ml/minute and UV-Vis detection at 230 nm. Pump mode set to gradient with following gradient program: time (minute)/A (v/v):B (v/v):T0.0/100:00, T25/30:70, T40/10:90, T60/10:90.
LC-MS/MS analysis
Performed LC-MS/MS analysis on triple quadruple mass spectrometer (AB SCEIX QTRAP 4500) coupled with a Shimadzu ultra HPLC with LC-30 AD pumps, SIL-30 AC auto-injector, column oven of CTO-20AC with a detector of SPD-M20A. Analyst software was used for the generation of mass spectral data. Ultra-high pure nitrogen gas was preferred for curtain and auxiliary gas. The mass spectral source conditions were optimized as below. The turbo ion spray voltage: 5,500 mV; source temperature: 550°C. The scan rate was fixed as 200 Da/S with unit mass resolution. Zero air was used as nebulization gas. The mass spectral data was acquired by selecting Q1 MS Q1 with the scan range from m/z 100 Da–1,000 Da in 0.1 amu steps with 2.0 seconds dwell time. The chromatographic separation of samples was achieved by using Phenomenex Luna C18(2), (250 × 4.6) mm, with particle size of 5 μm column. The solvent system A comprises 0.1% TFA with milli-Q water and solvent system B comprises of 0.1% TFA with Acetonitrile. UV detection of 230 nm and flow rate set to 1.5mL/min. The total run time was 48 min. The optimized gradient program: time (minute)/A (v/v): B (v/v): T0.0/95:05, T1/95:05, T16/89:11, T22/89:11, T33/75:25, T38/75:25, T38.1/15:85, T43/15:85, T43.1/95:05, T48/95:05.
Nuclear magnetic resonance (NMR)
One-dimensional (1H, 13C, DEPT-135) and various two-dimensional (2D) Nucleo-Magnetic resonance experiments such as 1H-1H gDQF-COSY, 1H-1H, ROESY, 1H-13C gHSQC, and 1H-13C gHMBC) were performed on Bruker Avance III HD cryo 600 MHz NMR spectrometer (600 and 150 MHz resonance frequency for 1H and 13C, respectively.
Mass spectrometry
Mass and MS/MS spectrums were collected on AB SCIEX QTRAP 4500 mass spectrometers with electron spray ionization (ESI) source. Source temperature as 500°C, ion spray voltage as 5,500 V (IS). Nitrogen gas was selected as curtain gas, nebulizer gas (GS1) and collision gas, zero air used as heater gas (GS2), delustering potential was 60 V (DP), collision energy was 18 V (CE) and CXP was 8 V. Detection of ions by electrospray ionization, +ve ion mode by direct infusion of sample solution in to source.
Elemental composition by Q-TOF
Elemental composition was performed on SYNAPT G2. Si Q TOF (Water solutions) equipped with interface ESI source and Masslynks (version 4.1) was operation and processing software. Capillary voltage was 1,000 V, source temperature 120°C, cone voltage 25 V, and desolvation temperature of 300°C. Samples solution was directly infused using Hamilton syringe, recorded mass spectra and elemental composition was deduced from mass data by applying the DBE: min = −100.0, max =100 and tolerance is 10.0 PPM.
RESULTS AND DISCUSSION
Stability study
Stability readings depicted fact that the two degradation impurities, viz., Impurity-I and Impurity-II, were gradually increasing at accelerated condition of 40 ±2°C/ 75 ± 5%RH with different time intervals. Figure 1 shows the chromatograms representing the enhancement of the degradation impurities under the stability conditions at the initial and third month accelerated conditions, i.e., 40 ± 2°C / 75 ± 5%RH timepoints.
The two degradation impurities (impurity-I and impurity-II) at different time points or intervals were tabulated for both in-house and marketed formulations in Tables 1 and 2.
Forced/stress degradation studies
To get better insight into the enhancement of the impurities I and II under accelerated conditions, stressed degradation studies were carried out under different environments. The compiled data are presented in Table 3. From the degradation data, it is evident that the unknown impurities I and II are increasing in acid stress sample under thermal stress condition.
Impurity enrichment
As it was evident from Forced/stress degradation studies section, the impurities in question were raising in acidic environment, Naloxone HCl API is stressed with 1 N HCl under thermal stress for 7 days. The solution was then neutralized with 1 N NaOH. The net concentration achieved was 20 mg/ml. A part of resultant solution was then diluted to 1 mg/ml and injected into chromatographic parameters mentioned in above section to check the % w/w impurity-I and impurity-II in the degradation solution. % Level of impurities obtained is tabulated in table-3 with its representative chromatogram as shown in Figure 2. As considerable levels of % impurities I and II were achieved, the degradation solution is subjected to impurity isolation
Impurity isolation
This degradation solution which was prepared in Impurity enrichment section was loaded in to a preparative column using the parameters prescribed in Stress studies section. Fractions collected with above ≥ 90% impurity stock solutions were collected and concerted on rotavapor to remove the solvents. The aqueous solution solutions having impurities I and II were then subjected to lyophilization separately using freeze dryer lyophilizer (Virtis advantage 2 XL). The impurities I and II were obtained as an off-white powder, with chromatographic purity of >90%.
Structural elucidation of degradation impurity-I:
The ESI mass spectrum of NLX and degradation impurity-Ishows protonated molecular ion peaks (M+H)+ at m/z 328.1 and 342.1, respectively, in positive ion mode. The latter is 14 amu more than protonated molecular ion peak of Naloxone. The ESI mass spectrum of NLX confirmed molecular ion at m/z 212.14 and produced three major daughter ions at m/z 208, 194, and 192.
The MS/MS of degradation impurity is showing loss of hydroxy group from the molecular ion peak of impurity-I. The major fragmentation ions of degradation impurity-I and naloxone from MS/MS are represented in Figure 3a and b and elemental composition was deduced from SYNAPT G2. Si Q TOF was C19H20NO5.
Moreover in 1H-NMR and 13C-NMR spectra of the degradation impurity-I, the majority of protons and carbons show the chemical shift (δ) values similar to those in Naloxone hydrochloride. However, some structural modifications observed at parochiral center of C12, C15, and C17 in Naloxone form degradation impurity-I and the same was identified by change in proton and carbon chemical shift (δ) values of parochiral centers at C12 and C15. The degradation impurity-I in DMSO-d6 solution initially exists Enol form, where it is slowly converted to Keto form during the NMR collection. However, initial structural signatures are corresponding to Enol form only. The Keto and Enol form of the impurity-I is deduced from one-dimensional (1D) NMR and different 2D-NMR experimental data. For NMR (1D and 2D), comparative data of impurity-I and Naloxone hydrochloride is given in Table 4. Based on collective structural characterization data, the proposed structures of degradation impurity-I are shown in Figure 3b with that of Naloxone in Figure 3a.
Structural elucidation of degradation impurity-II
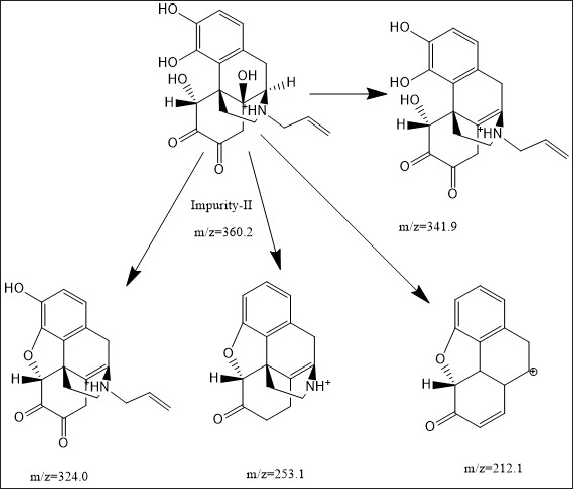
The ESI mass spectrum of Naloxone and degradation impurity-II shows protonated molecular ion peaks (M+H)+ at m/z 328.1 and 360.2, respectively, in positive ion mode, which is 32 amu more than NLX. The MS/MS of degradation impurity is showing loss of two hydroxyl groups from the molecular ion peak of impurity-II. The major fragmentation ions of degradation impurity-II and naloxone from MS/MS are shown in Figure 5 and elemental composition was deduced from SYNAPT G2. Si Q TOF was C19 H22 N O6. Moreover, in 1H- NMR and 13C-NMR spectra of the degradation impurity-II, the majority of protons and carbons show the chemical shift (δ) values similar to those in Naloxone hydrochloride. However, some structural modifications observed at prochiral center of C12, C15, and C17 in Naloxone form degradation impurity-II and the same was identified by the change in proton and carbon chemical shift (δ) values of parochiral centers at C12 and C15. The degradation impurity-II in DMSO-d6 solution initially exists Keto form, where it is slowly converted to Enol form during NMR collection. However, initial structural signatures are corresponding is Keto form only. The Keto and Enol form of the impurity-II is deduced from 1D NMR and different 2D NMR experimental data. For NMR (1D and 2D), comparative data of impurity-II and Naloxone hydrochloride is shown in Table 5. Based on the all-structural characterization data, the proposed structures of degradation impurity-II are shown in Figure 6 with that of Naloxone in Figure 3a.
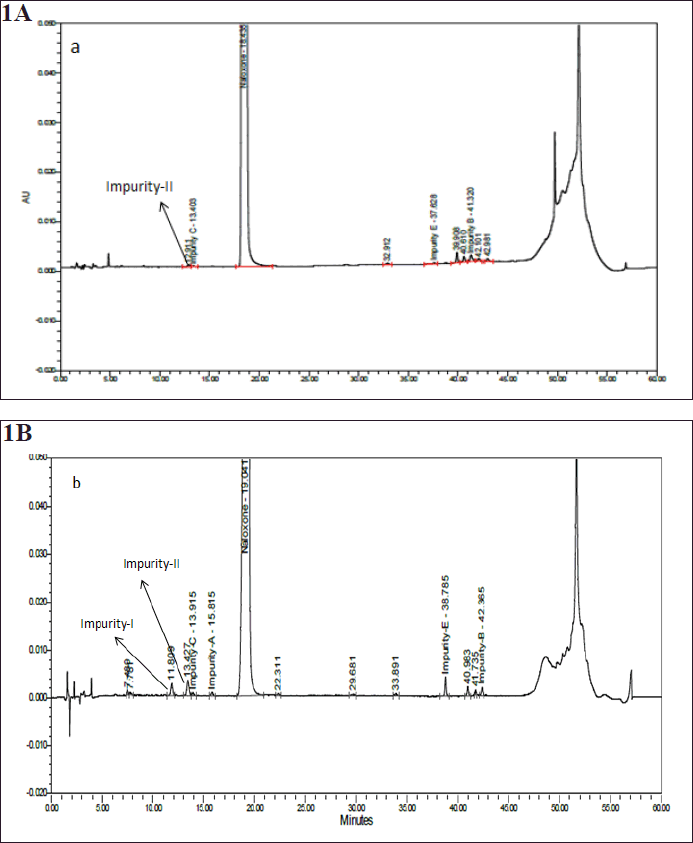 | Figure 1. Typical stability chromatograms of Naloxone hydrochloride injection at initial (a); and 3rd month accelerated stability conditions at 40 ± 2°C /75 ± 5% RH (b). [Click here to view] |
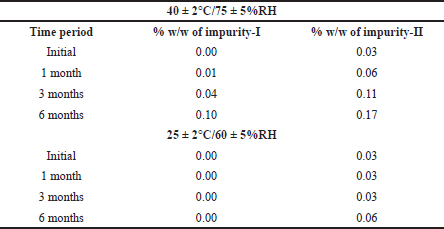 | Table 1. Formation of two degradation impurities at different storage conditions in in-house formulation. [Click here to view] |
 | Table 2. Formation of degradation impurities in NARCAN®. [Click here to view] |
 | Table 3. % Impurity levels in Naloxone HCl after 7 days stress with 1 N HCl. [Click here to view] |
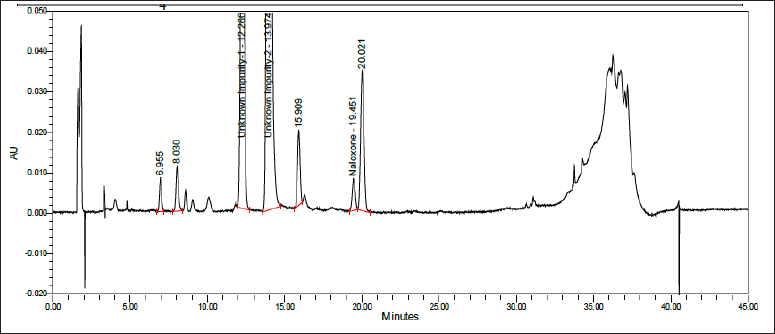 | Figure 2. Typical chromatogram exhibiting Naloxone HCl with acid stress for 7 days. [Click here to view] |
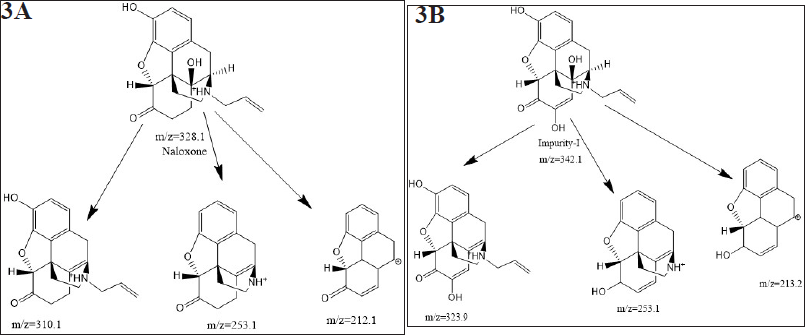 | Figure 3. (a) Mass fragmentation pattern (MS/MS) of Naloxone (b) Mass fragmentation pattern (MS/MS) of Naloxone impurity-I. [Click here to view] |
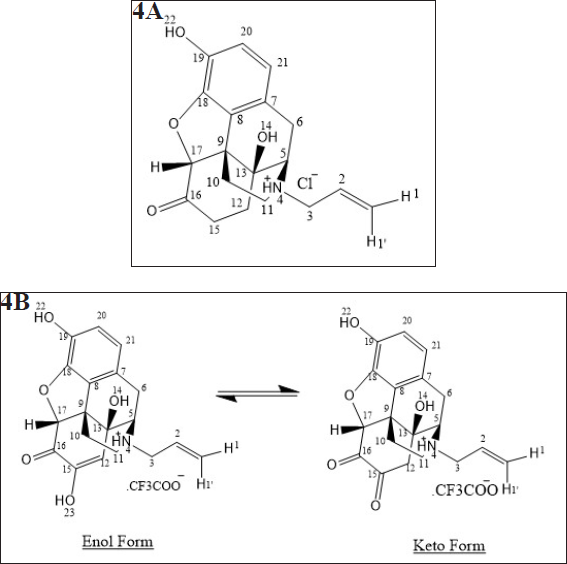 | Figure 4. (a) Chemical structures of Naloxone (b) Proposed Chemical structures of Naloxone impurity-I. [Click here to view] |
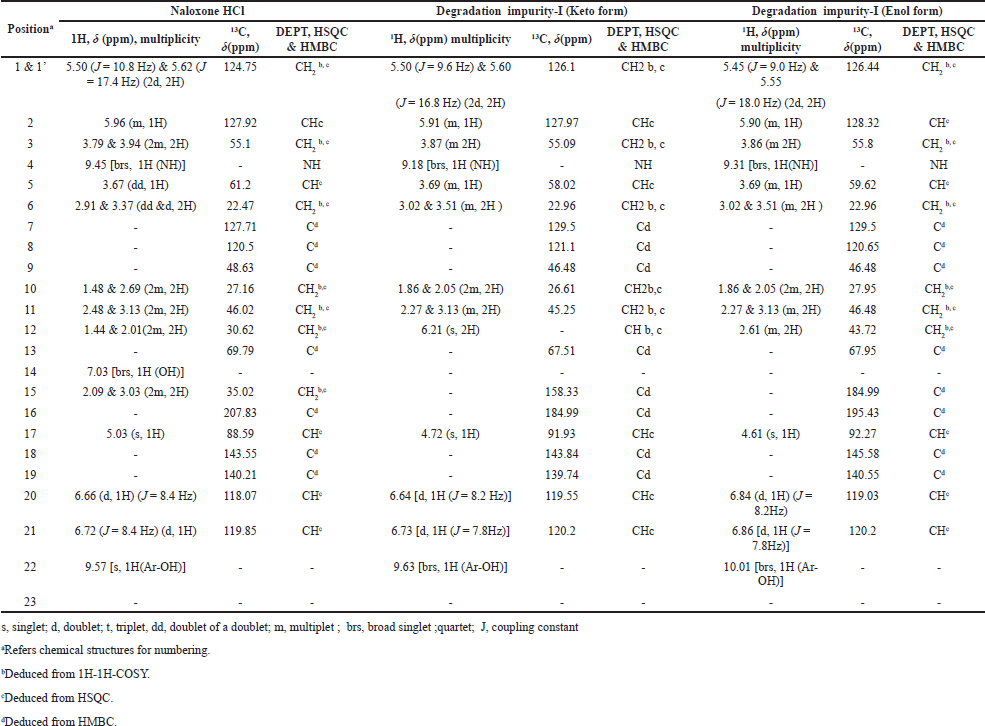 | Table 4. NMR assignments of Naloxone Hydrochloride and degradation impurity-I. [Click here to view] |
 | Figure 5. Mass fragmentation pattern of impurity-II. [Click here to view] |
 | Figure 6. Chemical structures of Impurity-II. [Click here to view] |
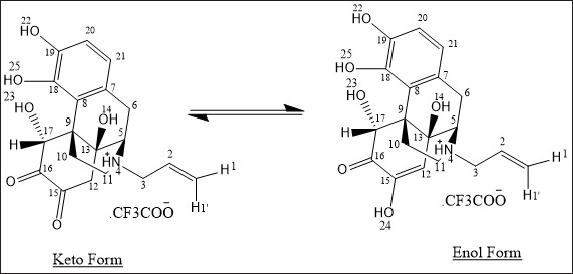
Mechanism for formation of impurity-I and II
Based on the above elucidation, the probable mechanism of formation of both impurities, impurity-1 and impurity-2, are shown in Figure 7a and b.
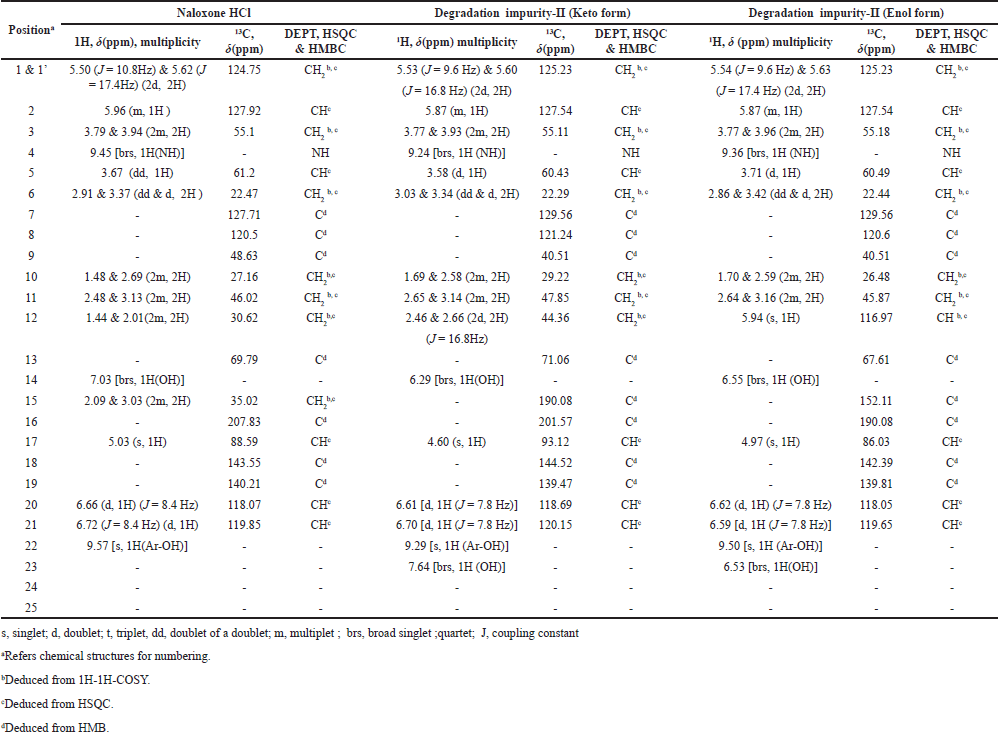 | Table 5. NMR assignments of Naloxone Hydrochloride and degradation impurity-II. [Click here to view] |
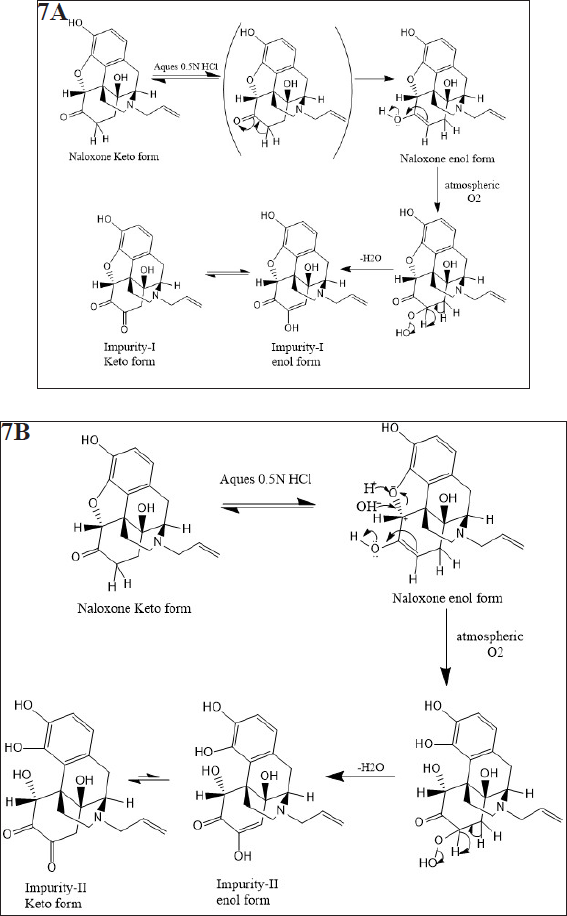 | Figure 7. (a) Proposed Mechanism for formation of degradation impurity-I (b) Proposed Mechanism for formation of degradation impurity-II. [Click here to view] |
CONCLUSIONS
From the above experiments, the unknown and unspecified impurities of Naloxone hydrochloride injection were identified in accelerated stability conditions. These impurities were enriched by forcedly degrading the product at extreme condition. The enriched impurities were isolated and characterized using NMR and LC-MS techniques to predict their structures. These experimental studies revealed that the two impurities were acid degradants in the presence of atmospheric oxygen (acid born oxidative impurities) and probable mechanistic pathways were designed in Figure 7a and b.
Furthermore, the author recommends for in silico assessments for qualifying the safety levels of these unknown impurities (impurity-1 and impurity-2)
ABBREVIATIONS
USP: United States Pharmacopeia
LC-MS/MS: Liquid Chromatography with 2 mass spectrophotometer
DEPT: Distortionless Enhancement by Polarization Transfer
HSQC: Heteronuclear Single Quantum Coherence
HMBC: Heteronuclear Multiple Bond Correlation
ACS: American Chemical Society
OPA: Orthophoshporic acid
TFA: Trifluro acetic acid
CXP: Exit cell potential
Q-TOF: Quadrupole time-of-flight
DMSO Dimethyl sulfoxide
ACKNOWLEDGEMENT
The authors specially acknowledge Dr. Reddy’s laboratories to perform all type of analytical work and Ranga Rao K for providing his valuable insights in drafting the article.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004–2008. J Pain Palliat Care Pharmacother, 2010; 24(3):293–7. CrossRef
Desai S, Patel A, Gabhe SY. Isolation and characterization of impurities present in 8-chlorotheophylline. Indian J Pharm Sci, 2011; 73(1):79–84; doi:10.4103/0250-474X.89762 CrossRef
Drugs C on. Naloxone dosage and route of administration for infants and children: addendum to emergency drug doses for infants and children. Pediatrics, 1990; 86(3):484–5.
Gamal M. Analytical review: analytical techniques for hyoscine N butyl bromide. Analyst, 2020; 145; doi:10.1039/D0AN00076K. CrossRef
Goodrich PM. Naloxone hydrochloride: a review. AANA J, 1990; 58(1):14–6. Erratum in: AANA J 1990 Jun;58(3):216. PMID: 2180244.
Gupta K, Prasad A, Nagappa M, Wong J, Abrahamyan L, Chung FF. Risk factors for opioid-induced respiratory depression and failure to rescue: a review. Curr Opin Anaesthesiol, 2018; 31(1):110–9. CrossRef
Huang C-q, Zuo H-y, Cao G-h, Chang L. Determination of related substances in naloxone hydrochloride injection by HPLC. J Shenyang Pharm Univ, 2016; (10):778–82.
ICH Guideline, “Stability Testing of New Drug substances and Products,” in Proceedings of International Conference on Harmonization, Topic Q1A (R2), Geneva, Switzerland, February 2003.
Jarzyna D, Jungquist CR, Pasero C, Willens JS, Nisbet A, Oakes L, Dempsey SJ, Santangelo D, Polomano RC. American Society for Pain Management Nursing guidelines on monitoring for opioid-induced sedation and respiratory depression. Pain Manag Nurs, 2011; 12(3):118–145.e10. CrossRef
Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth, 2001; 87(1):62–72. CrossRef
Koo CY, Eikermann M. Respiratory effects of opioids in perioperative medicine. Open Anesth J, 2011; 5(1):23–34. CrossRef
McPherson M. Strategies for the management of opioid-induced adverse effects. Adv Stud Pharm, 2008; 5(2):52–7.
Mycyk MB, Szyszko AL, Aks SE. Nebulized naloxone gently and effectively reverses methadone intoxication. J Emerg Med, 2003; 24(2):185–7. CrossRef
Panchagnula R, Sharma P, Khandavilli S, Varma MVS. RP-HPLC method and its validation for the determination of naloxone from a novel transdermal formulation. Farmaco, 2004; 59(10):839–42. CrossRef
Pattinson KTS. Opioids and the control of respiration. Br J Anaesth, 2008; 100(6):747–58. CrossRef
Ramachandra B. Development of impurity profiling methods using modern analytical techniques. Crit Rev Anal Chem, 2017; 47(1):24–36. doi: 10.1080/10408347.2016.1169913. Epub 2016 Apr 12. PMID: 27070830. CrossRef
Ryan SA, Dunne RB. Pharmacokinetic properties of intranasal and injectable formulations of naloxone for community use: a systematic review. Pain Manag, 2018; 8(3):231–45; doi: 10.2217/pmt-2017-0060. Epub 2018 Apr 23. PMID: 29683378. CrossRef
Tenenbein M. Continuous naloxone infusion for opiate poisoning in infancy. J Pediatr, 1984; 105(4):645–8. CrossRef
The British Pharmacopoeia 2007. The Stationary Office (TSO). Health Ministry, London, UK, 2006.
Venkatesan P, Valliappan K. Impurity profiling: theory and practice. J Pharm Sci Res, 2014; 6(7):254–9.