An unlimited scope for novel formulations as orally disintegrating systems: Present and future prospects
Reeta Rani Thakur, Mridul Kashi
Pages: 13-19

Design and development of Orally Disintegrating Tablets of Famotidine Prepared by Direct Compression Method Using Different Superdisintegrants
Mahaveer Pr. Khinchi, M.K.Gupta, Anil Bhandari, Natasha Sharma, Dilip Agarwal
Pages: 50-58

Development of pH-independent matrix type sustained release drug delivery system of propranolol hydrochloride
Haresh T Mulani, Bhumin Patel, Nehal J Shah
Pages: 83-92

Orally disintegrating tablets : formulation, preparation techniques and evaluation
Priyanka Nagar, Kusum Singh, Iti Chauhan, Madhu Verma, Mohd Yasir, Azad Khan, Rajat Sharma, Nandini Gupta
Pages: 35-45

Formulation and evaluation of fast dissolving tablets of Granisetron hydrochloride by vacuum drying technique
Basawaraj S.Patil, N.G.Raghavendra Rao
Pages: 83-88

Isolation of Mucilage from Cydonia vulgaris Pers. Seeds and its Evaluation as Superdisintegrant
Nisarg C Patel, Vaishali N Shah, Ashok N Mahajan, Dushyant A Shah
Pages: 110-114

A comprehensive review on fast dissolving tablet technology
V.Dinesh kumar, Ira Sharma, Vipin Sharma
Pages: 50-58

Levocetirizine orodispersible tablet by direct compression method
Gopal Satishkumar Gandhi, Dharmendra R. Mundhada , Shyamala Bhaskaran
Pages: 145-150

Bioequivalence studies on some selected brands of ciprofloxacin hydrochloride tablets in the Nigerian market with ciproflox® as innovator brand
Osonwa Uduma E., Agboke Ayodeji A., Amadi Rosemary C., Okorie, Ogbonna, Opurum Christian C
Pages: 80-84

Development and validation of RP-HPLC method for determination of content uniformity of rabeprazole sodium in its tablets dosage form
S. Elumalai, Kiran Aher, Girija Bhavar, Sachin Gupta
Pages: 165-170

Formulation and evaluation of orally disintegrating tablet containing tramadol hydrochloride by mass extrusion technique
Mansing G. Patil, Suhas M. Kakade, Sandhya G. Pathade
Pages: 178-181

High performance thin layer chromatographic method for estimation of deflazacort in tablet
Patel Satish A, Patel Natvarlal J.
Pages: 94-98

Development and validation of spectrophotometric method for simultaneous estimation of metoprolol succinate and olmesartan medoxomil in tablet
Vachhani Kevin H, Patel Satish A
Pages: 112-115

Development and validation of spectrofluorimetric method for estimation of deflazacort in tablets
Patel Satish A., Patel Natvarlal J.
Pages: 127-131

Bilayer tablet technology: An overview
Divya .A, K. Kavitha, M. Rupesh Kumar, Dakshayani S, Jagadeesh Singh SD
Pages: 43-47

Formulation and Evaluation of Oral disintegrated tablets of Alfuzosin Hydrochloride using superdisintegrants
Leela Manasa K, Ramana G, Digpati Roy
Pages: 161-165

Development of validated liquid chromatographic method for estimation of levocetirizine from pharmaceutical dosage forms
Chaitanya Prasad MK, Vidyasagar G, Sambasiva Rao KRS, Madhusudhanareddy Induri, Ramanjeneyulu S
Pages: 95-97

Formulation and Evaluation of Gastroretentive Floating Tablets of Domperidone Maleate
D. Saritha, D. Sathish, Y. Madhusudan Rao
DOI: 10.7324/JAPS.2012.2311Pages: 68-73

Binding Effect of Cassava Starches on the Compression and Mechanical Properties of Ibuprofen Tablets
Judith Chitedze, Maurice Monjerezi, JD Kalenga Saka, Jan Steenkamp
DOI: 10.7324/JAPS.2012.2402Pages: 31-37

Studies on directly compressed ondansetron hydrochloride mucoadhesive buccal tablets using gelatin, chitosan and xanthan gum along with HPMC K4M
Syed Amezuddin Azhar, Putta Rajesh Kumar, Vivek Sood, Somashekar Shyale
DOI: 10.7324/JAPS.2012.2517Pages: 100-105

Formulation and Evaluation of Bilayer Tablets of Diclofrenac Sodium with Ranitidine HCL for Sustained and Immediate Release
Prabhakar Shirse
DOI: 10.7324/JAPS.2012.2523Pages: 136-141

In Vitro Evaluation of Oral Extended Release Drug Delivery System for Metoprolol Succinate Using Kollidon SR
Mariyam Akter, Sujan Banik, Mohammad Salim Hossain
DOI: 10.7324/JAPS.2012.2539Pages: 188-192

Formulation and Evaluation of Sustained ReleaseMatrix Tablets of Glimepiride Based on Combinationof Hydrophilic and Hydrophobic Polymers
Mohd Abdul Hadi, V. Lokeswara Babu, Narottam Pal
DOI: 10.7324/JAPS.2012.2613Pages: 101-107

Formulation and Evaluation of Bi-layered SustainedRelease Matrix Tablets of Tramadol Hydrochloride
Sharmin Rahman, Md. Abdullah Al Masum, Florida Sharmin, S. M. Ashraful Islam, Md. Selim Reza
DOI: 10.7324/JAPS.2012.2638Pages: 129-134

Spectrophotometric method of estimation of atorvastatin calcium using sulfo-phospho-vanillinreaction
Shyni Bernard, Molly Mathew
DOI: 10.7324/JAPS.2012.2606Pages: 150-154

RP-HPLC Method Development for Estimation of Sildenafil Citrate in Tablets and in Seminal Fluid
Nitin Sharma, Praveen Rajput, Arti Thakkar, G. S. Sarma
DOI: 10.7324/JAPS.2012.2615Pages: 172-178

Simultaneous Estimation of Finasteride andTamsulosin Hydrochloride in Combined DosageForms by RP-HPLC-PDA Method
M. Sindhura, K. Raghavi, R. Prashanthi, Buchi N. Nalluri
DOI: 10.7324/JAPS.2012.2626Pages: 203-209

Formulation and Characterization of Fast Disintegrating tablets of Amlodipine using Super-disintegrants
Behin Sundara Raj, Punitha I.S.R., Suraj Dube
DOI: 10.7324/JAPS.2012.2819Pages: 118-123

Effect of Excipients on Oxcarbazepine Release from Modified Release Matrix Tablets
Buchi N. Nalluri, S. Vidyasagar, K. M. Maheswari
DOI: 10.7324/JAPS.2012.2826Pages: 150-158

In-vitro bioequivalence study of 8 brands of metformin tablets in Iran market
Parvin Zakeri-Milani, Peyman Nayyeri-Maleki, Saeed Ghanbarzadeh, Mahboob Nemati, Hadi Valizadeh
DOI: 10.7324/JAPS.2012.2834Pages: 194-197

Physico-Technical Properties of Tablets Formulated with Ethanolic Extract of Fresh Leaves of Combretum Micranthum G. Don
Osonwa, Uduma Eke, Umeyor, Chukwuebuka Emmanuel, Okon, Joel Etim, Agboke, Ayodele, Udosen, Victor Michael
DOI: 10.7324/JAPS.2012.21223Pages: 125-129

Development and In-Vitro Evaluation of Vinpocetine Loaded Bi-Layer Tablet Using Different Polymers
Mithilesh Kumar Jha, Md. Zakaria Faruki, Md. Habibur Rahman, Md. Mofizur Rahman, Md. Mesbah Uddin Talukder
DOI: 10.7324/JAPS.2012.21227Pages: 149-157

Formulation and Evaluation of Colon Targeted Drug Delivery System of Levetiracetam using Pectin as Polymeric Carrier
Amish Ashvinkumar Dangi, Ganure Ashok L, and Jain Divya
DOI: 10.7324/JAPS.2013.30115Pages: 078-087

Formulation and In-Vitro Characterization of Acyclovir Floating Matrix Tablets: A Factorial Design Study
Sadhana Shahi, Ashok Sonawane, Suhas Vanamore, Nityanand Zadbuke
DOI: 10.7324/JAPS.2013.3513Pages: 065-074

Development and evaluation of Methocel K15M based Theophylline floating tablets
Sayeeda Tasneem Chowdhury, A H M Mohidul Kabir, Nashid Karim, Md. Shahid Sarwar, Md. Delowar Hossain, Md. Shohel Hossain, Mohammad Safiqul Islam
DOI: 10.7324/JAPS.2013.38.S7Pages: S37-S41

Design and Development of Atenolol Matrix Tablet Employing Natural and Synthetic Polymers
Adity Bhowmik, Rajia Sultana Nijhu , Tajnin Ahmed, Sharmin Sultana
DOI: 10.7324/JAPS.2013.3919Pages: 103-108

Formulation and Optimization of Chronotherapeutic Drug Delivery from Carvedilol Sulphate Compression Coated Tablets by using Design of Experiment Approach
Vaishali Aggarwal, Ratendra Kumar, Rajiv Sharma, Yogendra Singh, Uday Veer Singh Teotia
DOI: 10.7324/JAPS.2013.31025Pages: 141-146

Orally Disintegrating Tablets: An Overview
Evren ALÄžIN YAPAR
DOI: 10.7324/JAPS.2014.40219Pages: 118-125

Effect of formulation parameters on Sumatriptan succinate Buccal Mucoadhesive Tablet: Quality by Design approach
Wable Arti J, Thokal Seema B, Mittal Sandeep S, Shirsat Ajinath E, Bhingare Chandrashekhar L, Ingle Pramod L
DOI: 10.7324/JAPS.2014.40418Pages: 103-111

Formulation and Evaluation of Repaglinide Buccal Tablet: Ex Vivo Bioadhesion Study and Ex Vivo Permeability Study
Biswajit Biswal, Nabin Karna, Bhavesh Bhavsar
DOI: 10.7324/JAPS.2014.40518Pages: 096-103

High Performance Liquid Chromatographic Determination of the Ternary Mixture of Caffeine, Dipyrone and Drotaverine Hydrochloride in Tablet Dosage Form
Tarek S. Belal, Essam F. Khamis, Fawzy A. El Yazbi and Mohamed M.A. Hamdy
DOI: 10.7324/JAPS.2014.40605Pages: 033-039

Gastroprotective and anti-secretory effect of Pep-Up Tablet on pyloric ligation-induced gastric ulcer model in rats
Hardik Soni, Sweta Patel, Arindam Paul, Ghanshyam Patel
DOI: 10.7324/JAPS.2014.40916Pages: 089-092

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Formulation of Desloratadine Oral Disintegrating Tablets
Mohamed A. Etman, Mona Gamal, Aly H. Nada, Mohamed A. Shams-Eldeen
DOI: 10.7324/JAPS.2014.41110Pages: 054-061

Noveon AA1 as enhancer of HPMC as a direct compression matrix for controlled release
Sara Laguna-López, Leopoldo Villafuerte-Robles
DOI: 10.7324/JAPS.2014.41111Pages: 062-068

Comparative Evaluation of the Disintegrant Properties of Rice Husk Cellulose, Corn Starch and Avicel in Metronidazole Tablet Formulation
Onyinye Jennifer Uwaezuoke, Oluyemisi A. Bamiro, Ndidi C. Ngwuluka, Omolola Tolulope Ajalla, Aderonke O. Okinbaloye
DOI: 10.7324/JAPS.2014.41219Pages: 112-117

Formulation and Evaluation of Tablet using Latex Powder of Jatropha curcas as a Natural Binder
Sangramsinh Ghatage, Shitalkumar Patil, Ramling Patrakar, Sachinkumar Patil
DOI: 10.7324/JAPS.2015.50114Pages: 077-081

The Compaction, Mechanical and Disintegration Properties of Modified Pennisetum glaucum (Poaceae) Starch in Directly Compressed Chloroquine Tablet Formulations
Mbang N. Femi-Oyewo, Tolulope O. Ajala, Damilola Babs-Awolowo
DOI: 10.7324/JAPS.2015.50207Pages: 043-050

Novel chewable tablet-in-tablet dosage form of Orlistat and Venlafaxine hydrochloride: development and evaluation
Abdul Mannan, K. Purushotham Rao
DOI: 10.7324/JAPS.2015.50315Pages: 091-097

Fabrication of Bucco-matrix tablets of Amoxicillin trihydrate on the basis of release and permeation kinetics
Gopa Roy Biswas, Subhasis Chakraborty, Nabarun Ghosh, Sutapa Biswas Majee
DOI: 10.7324/JAPS.2015.50408Pages: 048-052

Development of Enteric Coated Sustained Release Matrix Tablets of Sertraline Hydrochloride
Pravallika Uppala, Salma shaik, Saisri Anusha Valluru, Buchi N Nalluri
DOI: 10.7324/JAPS.2015.50410Pages: 058-064

A Five-Year Stability Study of Controlled-Release Diltiazem Hydrochloride Tablets Based on Poly(Ethylene Oxide)
Laila H. Emara, Ahmed A. El-Ashmawy, Nesrin F. Taha
DOI: 10.7324/JAPS.2015.50703Pages: 012-022

Formulation, in vitro Characterization and Stability Studies of Fast Dispersing Tablets of Diclofenac Sodium
Ranjit Prasad Swain, R. Nagamani, Satyajit Panda
DOI: 10.7324/JAPS.2015.50715Pages: 094-102

Optimized gastroretentive floating carvedilol tablets: an approach for prolonged gastric residence time and enhanced absorption
Khalid El-Say
DOI: 10.7324/JAPS.2016.60603Pages: 012-019

Simultaneous estimation of Aliskiren hemifumarate and Hydrochlorothiazide in combined Tablet Formulation by Simultaneous equation, Absorbance ratio and First derivative Spectroscopic Methods
Ashim Kumar Sen, Dhanya B Sen, Rajesh A Maheshwari, Ramachandran Balaraman, Avinash K Seth
DOI: 10.7324/JAPS.2016.60724Pages: 164-170

In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia
Ahmed F. Hanafy
DOI: 10.7324/JAPS.2016.60909Pages: 063-068

Formulation and evaluation of lamivudine sustained release tablet using okra mucilage
Narahari N. Palei, Santhosh K. Mamidi, Jayaraman Rajangam
DOI: 10.7324/JAPS.2016.60910Pages: 069-075

Analytical method development and validation for simultaneous estimation of Teneligliptin hydrobromide hydrate and Metformin hydrochloride from it’s pharmaceutical dosage form by three different UV spectrophotometric methods
Ashim Kumar Sen, Denish N. Hinsu, Dhanya B. Sen, Aarti S. Zanwar, Rajesh A. Maheshwari, Vikas R. Chandrakar
DOI: 10.7324/JAPS.2016.60924Pages: 157-165

Non-Starch Plant Polysaccharides: Physicochemical Modifications and Pharmaceutical Applications
Sutapa Biswas Majee, Dhruti Avlani, Gopa Roy Biswas
DOI: 10.7324/JAPS.2016.601033Pages: 231-241

Development and Validation of Content Uniformity Analytical Procedure of Glipizide Extended Release Tablet
Ilma Nugrahani, Indhah Fatmawati, Slamet Ibrahim
DOI: 10.7324/JAPS.2016.601228Pages: 192-196

Fast disintegrating tablets of amiodarone for intra-oral administration
Ebtessam Essa, Marwa Negm, Esmat Zin Eldin, Gamal El Maghraby
DOI: 10.7324/JAPS.2017.70109Pages: 064-072

Validated Ultraviolet-Spectrometric Method for Determination of Sofosbuvir in Tablets Formulation
Mohamed A. El Hamd, Ramadan Ali, Adel A. Marzouk, Osama H. Abdelmageed
DOI: 10.7324/JAPS.2017.70214Pages: 114-119

In vitro and In vivo Evaluation of Tablets Containing Meloxicam- PEG 6000 Ball-Milled Co-Ground Mixture
Mohamed Etman, Mustafa Shekedef, Aly Nada, Assem Ismail
DOI: 10.7324/JAPS.2017.70306Pages: 031-039

Design of buccal mucoadhesive tablets: understanding and development
Laisa Lis Fontinele de Sá, Naiane Carvalho Nogueira, Edson Cavalcanti Da Silva Filho, Ana Figueiras, Francisco Veiga, Lívio César Cunha Nunes, José Lamartine Soares-Sobrinho
DOI: 10.7324/JAPS.2018.8223Pages: 150-163

A Review on Various Formulation Methods in preparing Colon targeted mini-tablets for Chronotherapy
Mohd Abdul Hadi, N. G. Raghavendra Rao, A. Srinivasa Rao, Tayyaba Mahtab, Sayeeda Tabassum
DOI: 10.7324/JAPS.2018.8321Pages: 158-164

Treated Plasmodium berghei infected pregnant mice by Andrographis paniculata tablet (AS201-01) decreasing the TLR-4 expression and apoptosis index of placental tissue
Budi Prasetyo, Diantri Nari Ratih, Yustinasari, Hilkatul Ilmi, Lidya Tumewu, Aty Widyawaruyanti
DOI: 10.7324/JAPS.2018.8415Pages: 105-108

Formulation and Evaluation of Fast Dissolving Tablet Containing Vilazodone Nanocrystals for Solubility and Dissolution Enhancement Using Soluplus: In vitro-In vivo Study
S. G. Gattani, R. S. Moon
DOI: 10.7324/JAPS.2018.8506Pages: 045-054

Design and Evaluation of Zolpidem Tartrate Matrix Tablets for Extended Release Using Natural Gums and HPMC K100M
Siramsetti Dhanalakshmi, Srinivasa Rao Baratam
DOI: 10.7324/JAPS.2018.8712Pages: 072-077

Application of Fourier transform infrared (FTIR) spectroscopy coupled with multivariate calibration for quantitative analysis of curcuminoid in tablet dosage form
Chairany Siregar, Sudibyo Martono, Abdul Rohman
DOI: 10.7324/JAPS.2018.8821Pages: 151-156

Evaluation of Cissus populnea gum as a directly compressible matrix system for tramadol hydrochloride extended-release tablet
Olutayo Ademola Adeleye, Mbang N. Femi-Oyewo, Michael A. Odeniyi, Tolulope O. Ajala
DOI: 10.7324/JAPS.2019.90214Pages: 105-111

Design and characterization of intra-oral fast dissolving tablets containing diacerein-solid dispersion
Azza Hasan, Abd El hakim Ramadan, Mahmoud Abd Elghany, Shereen Sabry
DOI: 10.7324/JAPS.2020.10607Pages: 044-053

Validation of a high-performance liquid chromatographic method for the assay and dissolution of captopril in mucoadhesive tablet formulation
Ratna Budhi Pebriana, Olivia Damayanti, Yunda Dewi Agustin, Endang Lukitaningsih, Angi Nadya Bestari
DOI: 10.7324/JAPS.2021.110209Pages: 066-074

Formulation of somatostatin analog tablets using quality by design approach
Zoya Shprakh
DOI: 10.7324/JAPS.2021.110412Pages: 096-105

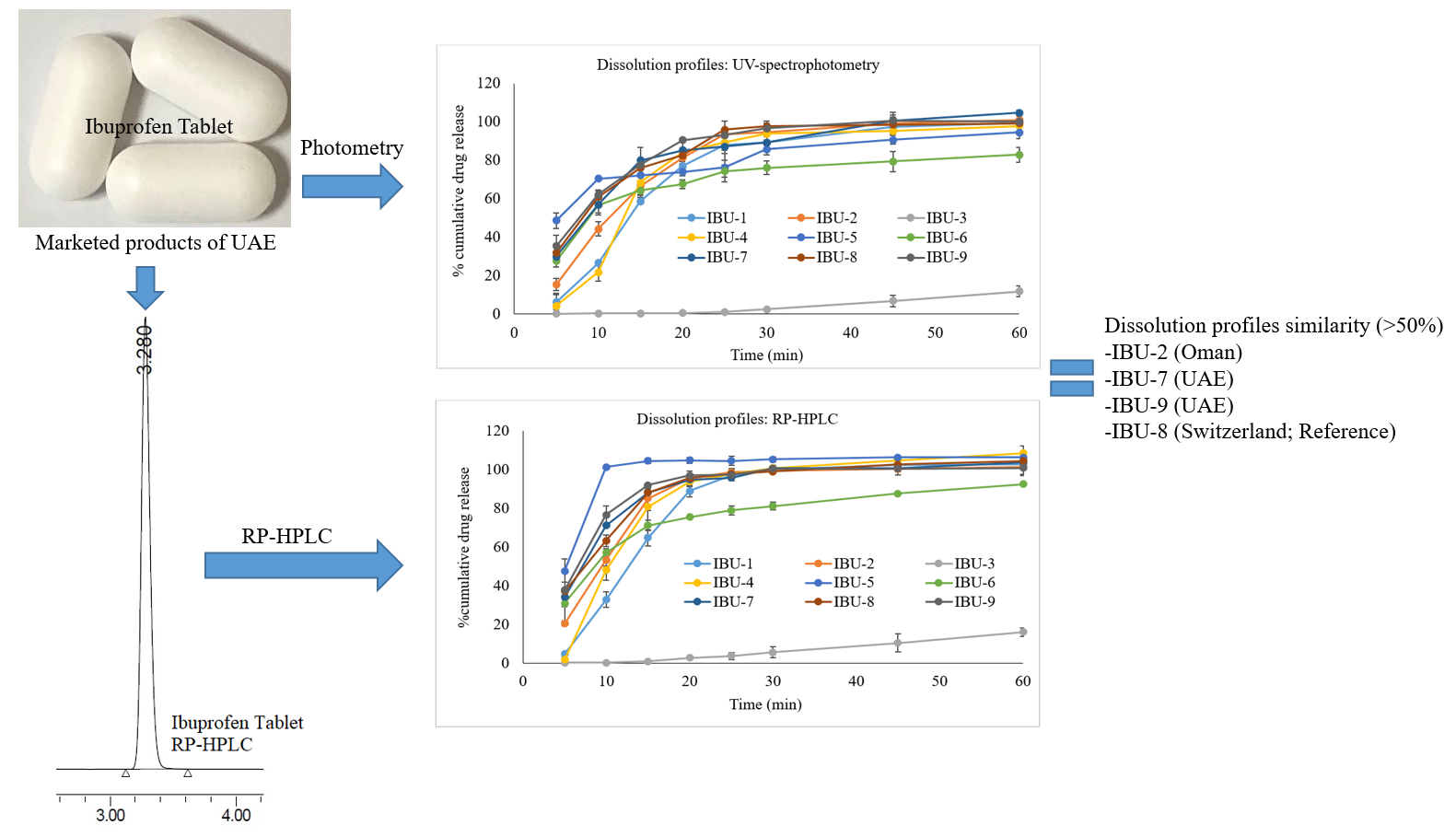
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
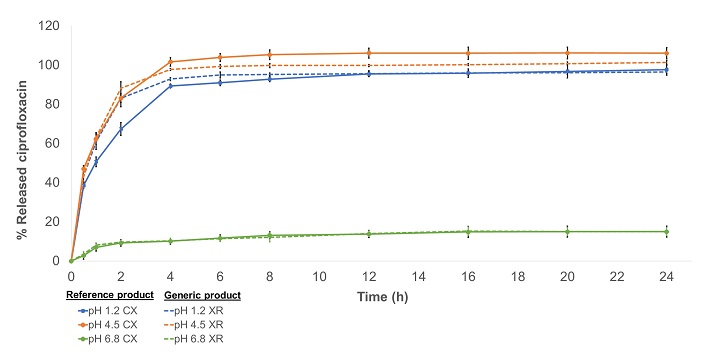
Comparison between the dissolution profiles of prolonged-release ciprofloxacin tablets available in the Colombian market
Andrés Vicente De la Cruz Gómez, Raynni Marcela Ramos Iglesias, Tatiana Sugey Ruiz Afanador, Indira Beatriz Pájaro Bolívar, Gina Paola Domínguez Moré
DOI: 10.7324/JAPS.2022.120322Pages: 209–217
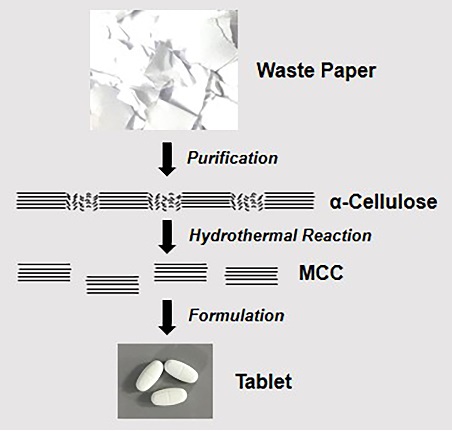
Characterization and tableting properties of microcrystalline cellulose derived from waste paper via hydrothermal method
Riadh Hasan Rana, Md. Sohel Rana, Sabiha Tasnim, Mohammad Rashedul Haque, Shaila Kabir, Md. Shah Amran, Abu Asad Chowdhury
DOI: 10.7324/JAPS.2022.120613Pages: 140-147
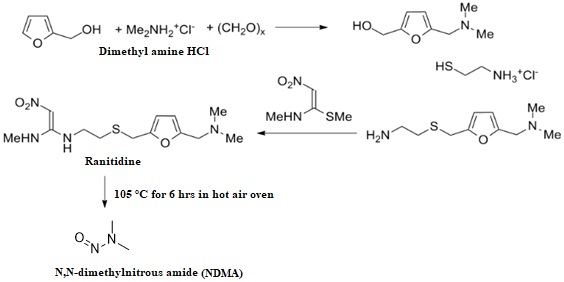
Novel stability indicating LC-MS method for N-Nitroso dimethyl amine genotoxic impurity quantification in ranitidine drug substance and drug product
Ganpisetti Srinivasa Rao, Dharamasoth Ramadevi, B. M. Rao, Nagaraju Rajana, K. Basavaiah
DOI: 10.7324/JAPS.2022.120711Pages: 106-114
Development and in-vitro/in-vivo evaluation of film-coated tablets containing Azadirachta indica A. Juss leaf extracts for diabetes treatment
Ngoc Nha Thao Nguyen, Xuan Chu Duong, Kim Nguyet Nguyen, Thi Ngoc Van Nguyen, Thi Trang Dai Nguyen, Thi Thanh Yen Le, Thi Cam Tu Le, Thi Thu Tram Nguyen, Duy Toan Pham
DOI: 10.7324/JAPS.2023.130119Pages: 193-200

Floating tablets incorporating curcumin solid dispersion as a potential pharmaceutical dosage form for stomach cancer treatment
Duyen Thi My Huynh, Viet-Hung Tran, Minh-Ngoc T. Le, Van-Hoa Huynh, Duy Toan Pham
DOI: 10.7324/JAPS.2023.114417Pages: 240-250

Various innovative UV spectroscopic methodologies for concurrent estimation of dapagliflozin and vildagliptin in combined tablet
Ashim Kumar Sen, Satish B. Khatariya, Dhanya B. Sen, Rajesh A. Maheshwar, Aarti S. Zanwar, Ramaswamy Velmurugan
DOI: 10.7324/JAPS.2023.151424Pages: 213-223
_.jpg)
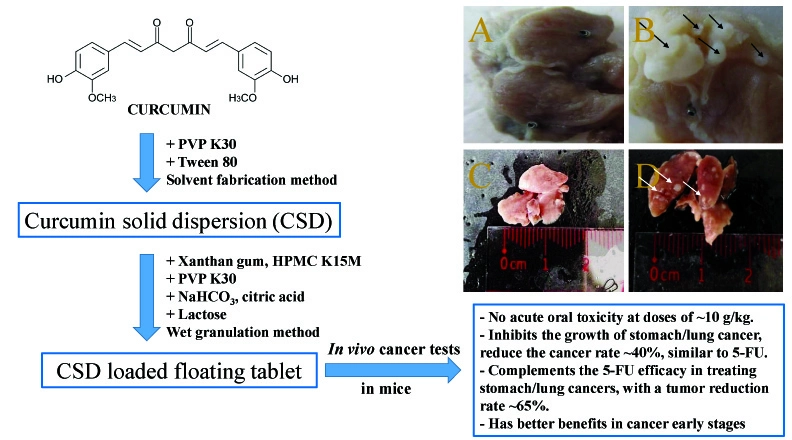
In-vivo anti-tumor activities of curcumin-solid-dispersion loaded floating tablets on stomach and lung cancers
Duyen Thi My Huynh, Van De Tran, Suchiwa Pan-On, Duy Toan Pham
DOI: 10.7324/JAPS.2024.162227Pages: 081-087
Quality by design approach for the formulation of bilayer tablets of domperidone and itopride in gastro-esophageal reflux disease
Roshani Prajapati, Bhavna Kumar, Jagannath Sahoo, Shailendra Shakya, Diwya Kumar Lal
DOI: 10.7324/JAPS.2024.168489Pages: 169-181

Development of a validated RP-HPLC/PDA method for the quantitative estimation of tepotinib in tablet dosage form
Sumalatha Chepyala, Srinivas Medidi, Jitender Kumar Malik
DOI: 10.7324/JAPS.2024.184366Pages: 064-071
_.jpg)
Development and in-vitro evaluation of multilayer mucoadhesive buccal tablets of metoprolol tartrate with chitosan extracted from crustacean shells
V. V. Siva Krishna Pushadapu, Srinivasa Babu Puttagunta, Javed Ali
DOI: 10.7324/JAPS.2024.197009Pages: 224-233
.jpg)