INTRODUCTION
Among the worldwide population, 15%–20% of people suffer from gastroesophageal reflux disease (GERD). The reason is the production of hydrochloric acid (HCl), which drives from the stomach’s lower portion to the esophagus. It is a condition that changes when stomach acid reflux causes bothersome symptoms like heartburn and regurgitation [1,2]. Esophagitis, esophageal stenosis, cancer, or Barrett’s esophagus can all develop as a result of persistent stomach acid reflux. The capacity of refluxed materials to be removed, delayed stomach emptying, and impaired esophageal mucosa resistance to gastric acid are all factors in the pathophysiology of GERD. Pharmaceuticals for GERD include antacids, H2 receptor antagonists, proton pump inhibitors, and prokinetic drugs result in significant benefits for quality of life, such as decreased physical discomfort, enhanced vitality, increased social and physical activity, and mental wellbeing, have been associated with the treatment of GERD symptoms [3,4].
The medication domperidone (6-chloro-3-[1-[3-(2-oxo-3H-benzimidazol-1-yl) propyl] piperidin-4-yl]-1H benzimidazol-2-one) has a chemical formula that is structurally related to butyrophenones and molecular weight of C22H24ClN5O2 is 425.91 g/mol [5]. This drug is a BCS class-II medication whose solubility is pH-dependent. Domperidone is prescribed in divided doses of 10–40 mg. Itopride is a prokinetic substance with the chemical name N-[[4-(2-dimethylaminoethoxy) phenyl] methyl]-3, 4- dimethoxy-benzamide HCl. According to Gupta et al. [6] and Ma et al. [7], it is a substituted benzamide with the chemical formula C20H26N2O4. Itopride is prescribed in divided doses of 150 mg/day for adult patients and is from BCS Class I. GERD is treated with the prescription medications of domperidone and itopride. They regulate gastric motility antagonizing the inhibitory effect of dopamine at D2 receptors. Domperidone was made an immediate release for quick action. A novel prokinetic drug called itopride functions as an acetylcholine esterase inhibitor as well as an antagonist of the dopamine D2 receptor. It has an antiemetic effect, speeds up gastric emptying, and enhances stomach tension and sensitivity. Itopride gastro-retentive tablets were created to increased bioavailability and for local action. Treatment with itopride and domperidone produces superior results [8].
Bilayer tablets are single-unit dosage forms with formulation for sustained and immediate release in separate layers. These are made to prevent incompatibilities through physical separation and enable the distribution of a broad pharmacological profile; bilayer tablets typically include two or more active medicinal components. In the current study, a new delivery approach has been used by formulating bilayer tablet one layer being immediate release and other being gastroretentive sustained release. Gastro-Retentive Drug Delivery System is the system that prolongs gastric swelling time and directing locate-exact drug discharge in the higher gastric tract for local or general properties. Gastric retention time increases bioavailability, mends solubility, and lessens drug depletion for drugs that are less solvable in an elevated pH atmosphere. Gastric evacuating drug quantity affects the dwelling time of the stomach which varies with the physiology of gastrointestinal tract (GIT) [9]. The substance density inferior to gastric liquids and therefore float in the stomach for an elongated time, depriving upsetting the gastric evacuating rate is known as floating drug delivery systems (FDDSs) [10]. Before floating it may be possible that the delivery system may get evacuated, and the drug released. So, to overcome this limitation, a low-density system plays a vital role, which shows immediate floating and release of the drug on the gastric content surface. A system consisting of low-density materials which entrap oil or air. The swell able polymers and effervescent components, having compartments of liquids that gasify at body temperature are known as the gas-generating system. The matrixes when getting interaction with gastric liquid, carbon dioxide gas is produced which gets entangled within the jelly field hydrocolloid which raises the dosage form and floats known as the low-density FDDS are gas-generating systems [11].
Quality by design (QbD) is characterized as a “systematic approach to development that begins with established objectives and focuses on product and process understanding and process control, as well as quality risk management” [12]. The essential premise of QbD is also described in International Conference on Harmonization (ICH) Q8 [13], Q9 [14], and Q10 [15]. It is a major shift from the traditional approach to ensure quality control of products. Products and processes are designed using innovative risk-based techniques to meet predefined quality. QbD contains quality target product profile (QTTP) and critical quality attributes (CQAs) [16,17].
In the present study, bilayer tablet consisting immediate release layer of domperidone and gastroretentive layer of itopride was formulated using the QbD approach. The formulation composition of domperidone was formulated through the hit and trial method and itopride was optimized by Box Behnken design. Box Behnken design was selected to optimize the concentration of hydroxypropyl methyl cellulose K (HPMC K) 100 M, xanthum gum and Carbomer 974 [18]. Analytical method validation was done for assay and dissolution test. The assay was done through the high performance liquid chromatography (HPLC) method by using the same column whereas dissolution was done through ultraviolet-visible spectroscopy method using United States Pharmacopoeia (USP) apparatus I (Paddle).
MATERIALS AND METHOD
Materials
All the raw material and reagents were procured and provided by Everest Pharmaceuticals Pvt. Ltd., Bhaktapur, Nepal. The active pharmaceutical ingredients (APIs) i.e., itopride hydrochloride (Amilife Science, Gujarat-India) and domperidone maleate (Vasundhara, Hyderabad-India) were used. The excipients including polyvinyl pyrolidone (PVPk-30) (Boeinky Pharmaceutical, Jiaozuo-China), barium sulfate (Lobachemie, Mumbai-India), sunset yellow (Roha, Mumbai-India), xanthum gum (Deason Biochemical, Shandong-China) Carbomer 974 (Shreechem, Mumbai-India), talcum (Neelkanth, Delhi-India), microcrystalline cellulose (MCC P102) grade, magnesium stearate and sodium starch glycolate (Prachin Chemical, Ahmedabad-India), sodium saccharine (Blue Jet Healthcare, Maharashtra-India), HPMC K 100 M (Nitika Pharmaceutical, Nagpur-India), lactose anhydrous (Modern Diaries, Haryana-India). Additionally, HPLC water (Sartorius, HPLC Water System, Germany), acetonitrile (Thermolab Fischer Scientific, India), sodium bicarbonate, sodium potassium dihydrogen phosphate, dipotassium dihydrogen orthophosphate (Merck Life Science Pvt. Ltd., Mumbai-India), sodium phosphate dibasic anhydrous, HCl, dimethyl formamide (Loba Chemie Pvt. Ltd., Mumbai-India). All the chemicals used are of lab or HPLC grade materials.
Instruments
HPLC (Agilent 1260 Infinity II, Mumbai-India), UV spectrophotometer (Agilent Cary 600, Mumbai-India), disintegration apparatus (LAB India, Thane-India), friability apparatus (Roche, India), hardness tester (Thermonik, Mumbai-India), Vernier caliper (Mitutoyo, Japan), dissolution apparatus (LAB India, Thane-India), moisture balance (ADAM, AMB50), four-digit analytical balances (Sartorius, Germany), Fourier transform infrared (Cary-630 FTIR Agilent, Germany), double cone blender (R&D Multipurpose Equipment GMP Lab Model) and double hopper double station compression machine DRTM27STN GMP-Chamunda, Mumbai-India), real-time stability chamber (Newtronics Walk-In Chamber 20,000L, Gujarat-India), accelerated stability chamber (Newtronics 600L, Gujarat-India) were the instruments used.
Methods
QbD approch for optimization of bilayer tablet
Identifying, elucidating, and managing all sources of variability that affect a process are the objectives of QbD. CQAs and the QTTP are essential parts of QbD. The QTPP is a summary of the quality attributes of the finished drug product that will guarantee the desired quality was attained while taking the drug product’s safety and efficacy into account. Early on in the formulation-development process, the QTPP is specified as the design for the product’s development. It includes the dosage strength, delivery method, dosage form, and administration route. Physical, chemical, biological, and microbiological qualities or properties that may be consistently tested and quantified are known as critical quality features. The critical parameters that affect the final formulation such as process variables (mixing time), mileu (relative humidity, temperature) raw materials (APIs and excipients), product/independent variables (polymer type and polymer concentration), measurement [size analysis through different seives, drug release, and floating time (FT)], compression machine (pressure, hardness, revolution per time, and thickness) [19]. The detailed description of QTPP and CQA are presented in Tables 1 and 2.
Pre-compression studies
Performing a pre-compression study is crucial in the context of direct tablet compression. Its purpose is to evaluate flow properties, assess compressibility, and identify and address potential problems associated with tablet formation. These studies should be done before compression as the parameters help in determining the approach to be taken for tablet formation. Given our preliminary plan to utilize the direct compression technique for tablet preparation, conducting a pre-compression study assumes significant importance. The study includes loose bulk density, tapped bulk density, Carr’s index (CI) and Hausner’s ratio (HR), angle of repose (?), and loss on drying (LOD) [20].
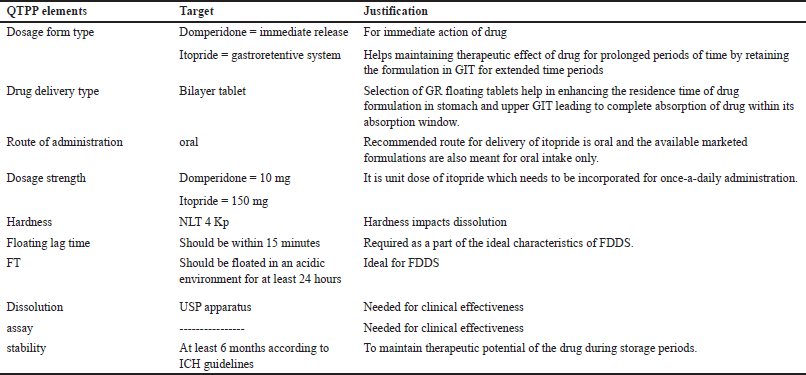 | Table 1. Quality by target product profile of domperidone and itopride. [Click here to view] |
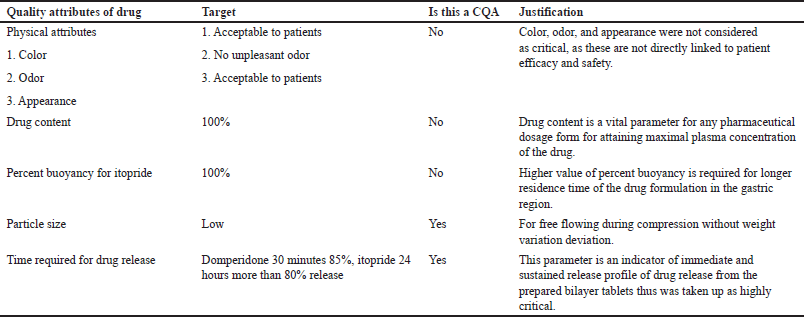 | Table 2. CQAs for formulation. [Click here to view] |
Loose bulk density
Loose bulk density was found by placing 50 g powder in 250 ml graduated glass cylinder without tapping or disturbing the beaker [21]. Bulk density was calculated using the following formula:
Bulk density = Mass / bulk volume.
Tapped bulk density
Tapped bulk density was measured in a bulk density apparatus (Hiccon). The weight of the powder is filled into a graduated glass cylinder (250 ml) height of drop 14 ± 2 mm. Number of taps per minute = 300 ± 15 taps/minute. The total number of taps was 1,250 were repeatedly tapped in bulk density apparatus for a known duration.
Carr’s index
Carr’s compressibility index is a widely recognized method used to determine the compressibility of powders. Compressibility is a measure of a powder or material’s ability to flow freely when subjected to external forces [22]. It was calculated using the formula given below:
CI % = (Tapped density – Bulk density) × 100 /Tapped density.
Hausner’s ratio
It is the ratio of tapped density to bulk density. It indirectly measures the index of flow of powders and is related to interparticle friction [22]. It was calculated using the formula:
HR = Tapped density / Bulk density.
Angle of repose
To determine the (?) the sample is poured onto the horizontal surface through the funnel and the angle of resulting cone-like structure is calculated using the formula:
? = tan−1 (Height of cone/radius of base of cone).
Loss on drying
A back weighing method is used to estimate how much volatile matter is present in raw materials. The volatile substances can be either water, fatty acids, or some volatile liquids. Two gram powder was placed in the plate of moisture balance where the temperature was raised to 105°C. Moisture present in the powder was automatically determined and displayed in the machine [23].
Preparation of domperidone immediate release layer
Domperidone immediate release layer was prepared through the hit and trial method. Five formulations were formulated as shown in Table 3. Domperidone immediate layer was made by direct compression method which was possible due to the good flow property. All the excipients as shown in Table 3 were dried in a tray drier for 1 hour at 50°C to have better flow properties. Domperidone maleate was passed through 60 mesh and other materials through 40 mesh. Domperidone maleate was geometrically mixed with MCC P 102 in a polybag for 5 minutes. All materials were mixed in a double cone blender 5 kg capacity for 10 minutes clockwise and 10 minutes anticlockwise. Tablets were compressed in a 6 mm circular flat die punch set. The total compression weight of the domperidone layer was 100 mg.
Optimization of itopride gastro-retentive layer
Box Behnken design was selected for the optimization of polymers utilized in the formulation of gastroretentive layer of itopride. Three independent variables HPMC K 100 M, xanthum gum, and Carbomer 974 were selected whose limits are shown in Table 4. Floating lag time, swellable index, and cumulative drug release at 24 hours were selected as a dependent variable. Three independent variables with three center points gave 15 runs for the optimization of formulation [11,24,25]. Amounts of other excipients were fixed for Box Behnken studies which are shown in Table 5.
Preparation of itopride gastro-retentive layer
Itopride and citric acid were passed through 60 mesh while other excipients were through 40 mesh as shown in Table 5. After passing all the material, they were mixed in a double cone blender 5 kg capacity for 10 minutes clockwise and 10 minutes anticlockwise. The final compression weight of itopride hydrochloride was 700 mg.
Preparation of bilayer tablets
The bilayer tablet was formulated according to Duredas Trademark technology. The compression force was 5 tons that provides immediate release from one layer and sustained release from another layer within one tablet. Domperidone was placed in hopper A and Itopride was placed in hopper B. The temperature was maintained 25°C–29°C and relative humidity 45%–55%, while the pressure difference was 5–15 Pa between room and corridor. Immediate compression was done after the assay determination of lubricated granules.
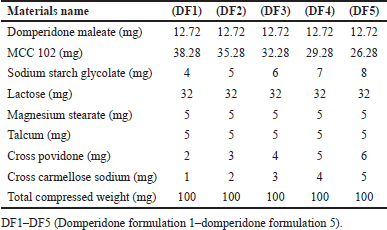 | Table 3. Formulation design for domperidone. [Click here to view] |
Post-compression studies
Different parameters for the evaluation and characterization of prepared tablets like weight variation, hardness, thickness, friability, lag time, FT, and swelling index were assessed [26].
Weight variation test
Twenty tablets were selected, and their average weight was determined. Then, the tablets were individually weighed and the deviation of individual weight from the average weight. Deviation should not exceed the limits set by Indian Pharmacopoeia (IP) which states that if the weight of the tablet is more than 80 mg and less than 250 mg is the weight variation should be in within the limit of ±7.5% as in the case of the domperidone layer. For itopride and bilayer tablets limit was ±5% as the total weight exceeded 250 mg [27]. The deviation from average weight was determined using the following formula:
% Deviation = (Average weight – Individual weight) × 100 / Average weight.
Thickness
Thickness was measured by Vernier calipers (Mitutoyo) [22]. This falls under nonpharmacopoeial tests so limit was set up in-house.
 | Table 4. Box Behnken design for optimization of itopride formulation. [Click here to view] |
Hardness
The tablet’s hardness determines how resistant it is to break, chipping, or abrasion when handled, transported, and stored in advance of use. A hardness test evaluates the force required to shatter a tablet using a diametric compression test. [22]. Hardness was measured by hardness tester (Thermonik) in kilograms per cm2.
Friability
Friability was measured by friability apparatus (Roche) which should be less than 1%. For the determination of friability, 10 tablets are weighed (pinitial) and were run at 25 rpm for 4 minutes in Roche friability apparatus (pfinal). The amount lost after running was examined and calculated from the formula provided below [28].
% Friability = (pinitial − pfinal)/pinitial × 100.
Disintegration
A disintegration test was conducted using disintegration apparatus which includes a basket rack assembly with six USP-specified open-ended clear tubes held vertically on a 10-mesh stainless steel wire screen. A constant 37°C ± 2°C was maintained for the water’s temperature and the device was run until there was no longer any residue on the screen or stuck to the disc’s inner surface, and the disintegration time was recorded [29].
Gastro retentive characteristics (floating lag time, FT, swellable index)
Further, the gastroretentive characteristics of tablets were estimated by determining its floating properties. The time gap before the tablet starts to float in the dissolution medium is known as lag time. The time for which tablets remain floating in the dissolution medium is known as FT. The time of lag time and FT of tablets were observed and recorded. The swelling index of itopride was determined by placing tablets in a dissolution medium after 24 hours it was taken out, blotted with paper towels to remove excess water, and weighed using a four-digit analytical balance [30,31].
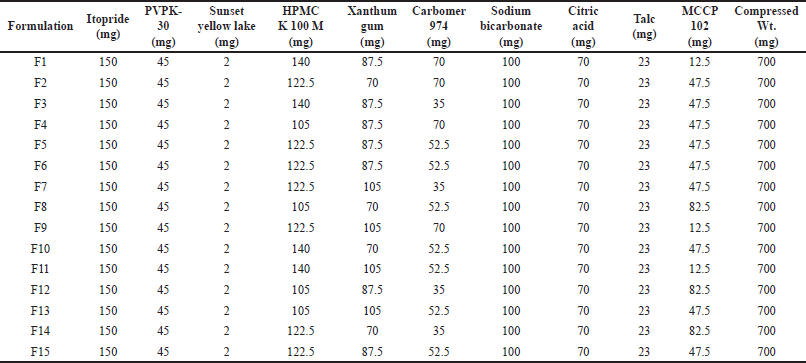 | Table 5. Formulation design for itopride layer by Box Behnken design. [Click here to view] |
Swelling index = [(Wet weight of tablet – Dry weight of tablet) × 100] / Dry weight of tablet.
Determination of domperidone and itopride content (assay)
The assay of domperidone and itopride was done utilizing HPLC. The column (C18, 250 × 4. 6 mm 5 μm) were selected with a flow rate of 1.0 ml per minute. The wavelength 284 nm and temperature 35°C with load 20 μl were selected. The run time was about 15 minutes, and the sample temperature was 15°C. Firstly, the solvent mixture of water and acetonitrile in the ratio of 60:40 was taken which was filtered and degassed. The standard was prepared by weighing 25 mg of itopride hydrochloride and 20 mg of domperidone maleate and they were transferred to a 100 ml volumetric flask where about 80 ml of methanol was added and sonicated to dissolve. Then the volume was made up to the mark with methanol and mixed. 2 ml of the above solution was transferred to a 20 ml volumetric flask and the volume was made up with solvent mixture. For sample preparation of domperidone a quantity of powder equivalent to 20 mg were weighed by crushing tablets of domperidone then added to 100 ml volumetric flask and 70 ml of methanol were added which were sonicated for 10 minutes to dissolve and make volume to 100 ml with methanol then 2 ml of this solution was diluted to 20 ml with the same solvent mixture. For itopride hydrochloride, the quantity of powder equivalent to 150 mg of itopride hydrochloride in 100 ml volumetric flask was added after crushing itopride tablets then 70 ml of methanol were added after that sonicated for 10 minutes to dissolve and make volume to 100 ml with methanol then 1 ml of that solution was diluted to 50 ml with solvent mixture. The mobile phase was prepared using mixtures of buffers and acetonitrile in the ratio of 60:40 was filtered and degassed. Both the standard and the sample were separately injected into the liquid chromatography and the area due to major peaks was recorded.
Content uniformity
According to IP for tablets containing more than one active ingredient this test is applicable to the active ingredients which contain 10 mg or less than 10 mg or less than 10% w/w of active ingredient. Following IP only domperidone content of uniformity was determined. Ten tablets of bilayer tablets were placed in 100 ml volumetric flask which were proceeded for testing the same as the assay mentioned above in assay procedure of domperidone.
In-vitro drug release study
USP paddle-type apparatus with a stirring rate of 50 rpm at 37°C ± 0.5°C was used for a dissolution of domperidone. 900 ml of 0.1 N HCl was used as a dissolution medium. The sampling was done in 30 minutes. Accurately weighed 25 mg of domperidone maleate reference standard was transferred to a 50 ml volumetric flask then added 5 ml of dimethyl formamide and dissolved with the aid of ultrasonicator for 5 minutes. Added sufficient 0.1 M HCl to produce 50 ml and mixed well. Pipette out 1 ml of the solution in a 50 ml volumetric f?lask and diluted to 50 ml with 0.1 M HCl. For blank solution 5 ml of dimethyl formamide in a 50 ml volumetric flask were added with sufficient 0.1 M HCl to produce 50 ml. Pipetted 1 ml of the solution in a 50 ml volumetric flask and were diluted to 50 ml with 0.1 M HCl. The absorbance’s were taken at the wavelength of 286 mm at 30 minutes sampling point from the dissolution medium following IP 2018 Vol 3A page no- 2850-2852 and different research article [32,33].
The dissolution of itopride hydrochloride was done using the same set up but stirring at 75 rpm. 8.5 ml of HCl were dissolved to 1,000 ml with water and about 30 mg of working standard of itopride HCl was added in 100 ml volumetric flask. Added 70 ml of dissolution medium and sonicated for 15 minutes and made up volume with the same medium. 2 ml of this solution to 50 ml with the same diluents for standard preparation was diluted. For sample preparation one tablet in each dissolution vessel was placed and the apparatus was run as per the above condition, finally collecting the sample solution from each vessel at a specified time. The absorbance on the UV spectrophotometer at 257 nm of the standard solution following IP 2018 Vol 3A page no- 2850-2852 and different research article then sample solution was recorded. Sampling was done in each hour with the calculation of cumulative drug release [34,35].
In vitro release pattern was analyzed using kinetic models to explain the kinetics of drug release from the formulation; viz. zero-order [36], first order [37] Higuchi [38] Korsmeyer-Pappas [39,40].
Stability study
The USP defines pharmaceutical product stability as the degree to which a product maintains within predetermined limits and throughout its storage and use times. India and Nepal are categorized to Zone IVb according to ICH climatic zone. Criteria for relative humidity and temperature for accelerated stability study are 40°C ± 2°C 75% RH ± 5% for 6 months and long term 30°C ± 2°C 75% RH ± 5% till its claimed shelf life. Optimized formulation stability study was conducted. Sampling was done in initial month, 3 months and 6 months [41] for accelerated stability study and till its shelf life for real time stability study.
RESULT AND DISCUSSION
QbD approch for optimization of bilayer tablet
On the basis of prior knowledge and the literature, QTPP and CQA were established, and a preliminary risk assessment was completed. Domperidone was geometrically combined with other excipients due to its low strength and content in the formulation, mesh size, and mixing time were determined from prior knowledge. Designing an experiment is crucial therefore, a planned and organized experimental method known as the QbD element gives enhanced information with high precision regarding the impact of changes in the variables on the response of the product and process. Itopride optimized formulation was selected through Box-Behnken design. For compression of bilayer machine without any deformities like sticking, capping, etc., were formed with an optimum speed of turret and pressure of machine (Fig. 1).
 | Figure 1. Representation image of the prepared bilayer tablet. [Click here to view] |
Pre compression parameters
Loose bulk density tapped density, angle of repose, CI and HR, LOD were calculated and shown in domperidone in Table 6 and itopride shown in Table 7. The flow properties were found to be in the good flow property range allowing for direct compression into tablets.
Preparation of domperidone immediate release layer
There were five formulations made by the empirical hit and trial method. Among the five formulation DF5 was found to be the best optimized formulation due to its high drug release and less disintegration time compared to other formulations.
Preparation of itopride gastro retentive layer
Application of Box-Behnken design for optimization of itopride formulation
Box Behnken design was selected for optimization of itopride formulation with 15 runs were developed. Three independent variables of HPMCK 100 M (A), xanthum gum (B) and Carbomer 974 (C) denoted by A, B, and C, respectively, and in responses for independent variables lag time, swell able index, and drug release for 24 hour. One can visually locate a point of compromise among the various responses using overlaid contour plots. A pair of continuous variables (one for the x-axis and one for the y-axis) make up each superimposed contour map. Any additional continuous variables are kept at a fixed level if there are more than two. The polynomial equation’s positive or negative sign and coefficient’s size can be used to understand how independent variables affect dependent variables. Positive or negative sign and magnitude of co-efficient from the polynomial equation can be helpful to understand the effect of independent variables on dependent variables. Contour plots, response surface design, and overlaid contour plot figure were shown in Figure 2a and b. Final optimized formulation given by Box Behnken design was shown in Table 8.
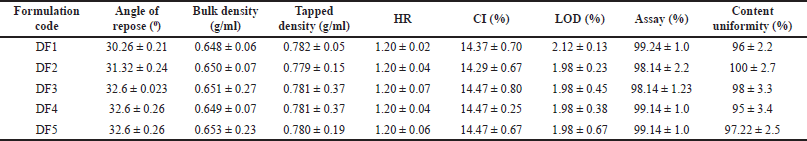 | Table 6. Pre-compression parameters of domperidone formulation. [Click here to view] |
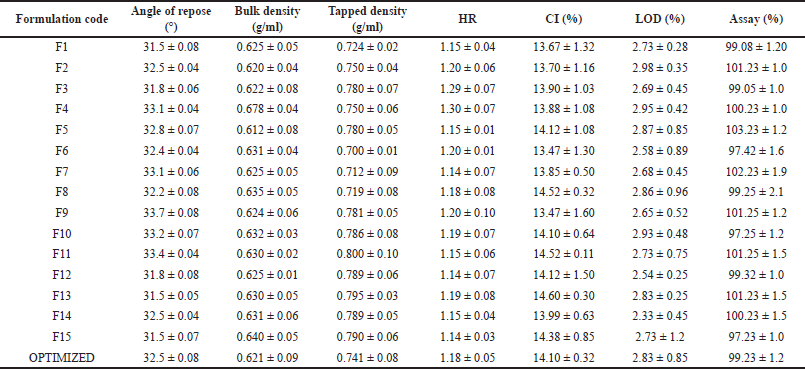 | Table 7. Pre-compression parameters of itopride different formulation, mean ± SD (n = 10 except in assay n = 2). [Click here to view] |
Influence of independent variables on in-vitro lag time
Lag time followed 2FI model according to fit statics. The analysis of variance (ANOVA) analysis suggested that the model was significant with a p-value of 0.0002 and the lack of fit is not significant with a p-value of 0.0686. The difference between the predicted R2 of 0.7284 and the adjusted R2 of 0.8965 is less than 0.2, which is considered to be in a reasonable agreement. Adequate precision of 13.770 indicates an adequate signal as compared to signal signifying that the model can be used to navigate the design space. The lag time of 15 formulations was in the range of 89–103 seconds. The influence of independent variables on the FT equation given by
Lag time = 98.1333 − 4.125 * A − 2.625 * B − 4.75 * C − 1.5 * AB − 2.75 * AC − 3.25 * BC.
From the equation coefficient of A, B, and C, i.e., HPMCK 100 M, xanthum gum, and Carbopol 974 all bear negative sign indicates an increase in their quantity mean decrease in lag time. This was observed in the 2-D contour plot and 3-D response curve shown in Figure 2a.
Influence of independent variables on swellable index
Swellable index followed linear model according to fit statics. The ANOVA analysis suggested that the model was significant with a p-value of 0.0002 and the lack of fit is not significant with a p-value of 0.0707. The difference between the predicted R2 of 0.6401 and the adjusted R2 of 0.7812 is less than 0.2, which is considered to be in a reasonable agreement. Adequate precision of 13.5 indicates an adequate signal as compared to signal signifying that the model can be used to navigate the design space. The swellable index of 15 formulations ranged between 4.21 and 4.32. The influence of independent variables on the swellable index is given by the following equation:
Swellable index = 4.26 − 0.02375 * A − 0.0025 * B − 0.04375 * C.
From the equation coefficient of A, B, and C, i.e., HPMCK 100 M, xanthum gum, and Carbomer 974 all bears negative sign indicates an increase in their quantity mean decrease in swellable index and vice versa. The low value of the coefficients signifies that the polymers show the lower level of influence on the swellable index. This was observed in the 2-D contour plot and 3-D response curve shown in Figure 2a.
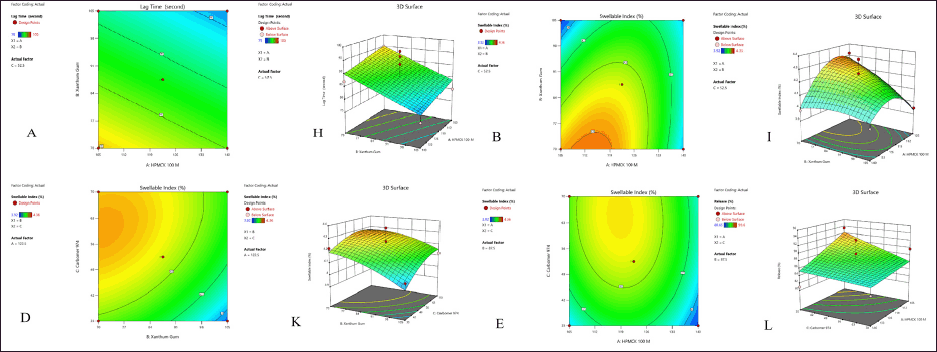 | Figure 2. (a) Contour plot (A, B, D, and E) showing effect in lag time, swellable index at 24 hours (H, I, K, and L) in 3-D surface design. (b) Contour plot (C, F, and G) showing effect of % drug released at 24 hours (J, M, and N) in 3-D surface design. [Click here to view] |
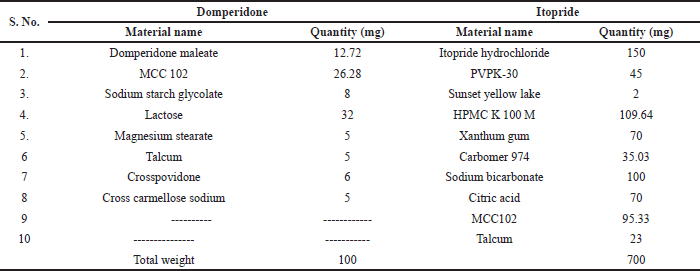 | Table 8. Final optimized formulation for bilayer tablets. [Click here to view] |
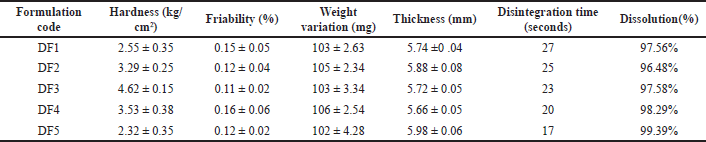 | Table 9. Post-compression parameters of domperiodne formulation. [Click here to view] |
Influence of independent variables on drug release
Drug release followed a quadratic model according to fit statics. The ANOVA analysis suggested that the model was significant with a p-value of 0.0002 and the lack of fit is not significant with a p-value of 0.1248. The difference between the predicted R2 of 0.8368 and the adjusted R2 of 0.9692 is less than 0.2, which is considered to be in a reasonable agreement. Adequate precision of 23.45 indicates an adequate signal as compared to signal signifying that the model can be used to navigate the design space. The drug release at 24 hours of 15 formulations was found to be between 83.64 and 94.60 seconds. The influence of independent variables on the drug release at 24 hours equation given by:
Drug release = 92.8767 − 2.095 * A − 2.11 * B − 1.5775 * C − 0.6125 * AB + 0.5525 * AC + 1.7675 * BC − 2.25208 * A2 − 2.55208 * B2 − 1.45708 * C2.
From the equation coefficient of A, B, and C, i.e., HPMCK 100 M, xanthum gum, and Carbomer 974 bears negative sign indicate an increase in the quantities mean decrease percentage release. This was observed in the 2-D contour plot and 3-D response curve shown in Figure 2b.
Optimization of formula and validation of model
After successful preparation of model and statistically analyzing the effects of independent variables, on selected CQAs viz lag time, swelling index, and drug release after 24 hours, the level of independent variables that will give the optimum response (minimum lag time, maximum swelling index, and drug release) was determined using numerical optimization feature of design expert. The limits for independent variables were set within the original limit and the desired response was provided to the software. Based on this input, the software recommended the optimum values of independent variables with a desirability of 0.568 and 0.528. To ensure the suitability of the model’s predictions, two optimized formulations as suggested by design experience were formulated and subjected to evaluation. Each response was assessed for both the batches. Based on the high degree of concurrence between the experimental values and the predicted values, it can be deduced that the formulation evaluation and optimization design were successful in achieving its objectives.
Preparation of bilayer tablets
From the optimization processes, DF5 and IH2 were selected as optimized composition for the preparation of domperidone and itopride layer of bilayer tablet, respectively. The weight taken for domperidone maleate was 12.72 mg (10/0.786 i.e., factor). The final compression weight of domperidone was 100 mg and itopride hydrochloride was 700 mg. The final compression weight was 800 mg made in 16 × 8.26 mm Oblong plain biconcave die and punch set). Tablets were compressed in double hopper double station compression machine from (DRTM27STN GMP-Chamunda). There was no defect in bilayer tablets like capping, sticking, lamination, etc. (Fig. 1).
 | Table 10. Post compression (IPQA) parameter of itopride formulation. [Click here to view] |
 | Table 11. Dissolution result of itopride formulation [Mean ± SD (n = 6)]. [Click here to view] |
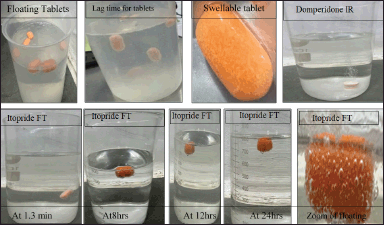 | Figure 3. Images of bilayer tablets representing conditions of FT for itopride and immediate release (IR) for domperidone. [Click here to view] |
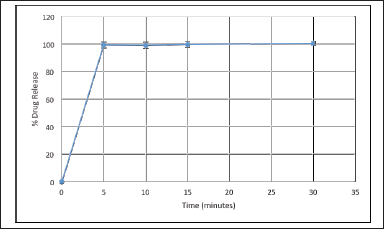 | Figure 4. Dissolution result of domperidone immediate release within 5 minutes. [Click here to view] |
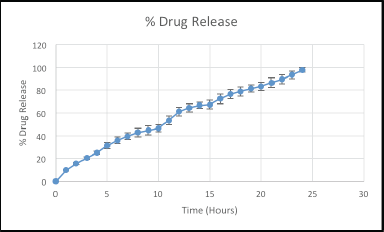 | Figure 5. Dissolution result of itopride hydrochloride in 24 hours. [Click here to view] |
Post compression study
These studies were popularly known as “In Process Quality Assurance” (IPQA Test). These tests added quality to the final products. Lag time, friability, hardness, crushing strength, FT, wt. variation, thickness, swellable index of optimized formulation of itopride with bilayer tablets were done. All the results from post compression tablet were compiled and presented in domperidone in Table 9 and itopride in Table 10. All the lag time swelling index and floating tablets were shown in Figure 3.
Determination of domperidone and itopride content (assay)
The determination of domperidone and itopride were done by HPLC method using the same column and the same method. The assay of domperidone was 99.38% and itopride was 100.66% in the final bilayer tablet formulation.
Content of uniformity
Content of uniformity was determined for domperidone which was found 98.02%–102.34%. Domperidone contains 10 mg of dose, so content of uniformity was done for its following IP.
In-vitro drug release and drug release kinetics
The in-vitro drug release of domperidone from the domperidone layer was 99.39% ± 2.34% in 30 minutes which was well above the limit of not less than (NLT) 85%. An optimized formulation of itopride release meets the specification requirement with IP 2018 Vol 2A page no-1120–1121 where first point: 20%–30%, second point: 45%–55%, third point: more than 80%. Thus, itopride drug releases according to IP are in 4, 16, and 24 hours, respectively. The dissolution releases of optimized formulation of itopride for 4 hours was 27.50%, 16 hours was 53.45%, and 24 hours was 93.65%. Dissolution results of itopride were shown in Table 11. The drug releases were calculated in cumulative drug release. Domperidone and itopride drugs release are shown in Figures 4 and 5. The itopride layer seems to follow the Korsemeyer–Peppas model that involves the drug release from a polymer matrix over an extended period of time. The release exponent (n) is greater than 0.5, it indicates non-Fickian or anomalous transport, suggesting a combination of diffusion, swelling, and erosion as the predominant mechanisms for drug release. Whereas domperidone layer of the bilayer tablet follows a first-order release kinetics, it indicates that the drug release rate from the layer is proportional to the remaining drug amount. This finding suggests that the drug release mechanism primarily involves the dissolution of the drug from the tablet rather than diffusion, swelling, erosion, or relaxation of the polymeric matrix. The response variable and R2-value were shown in Tables 12 and 13.
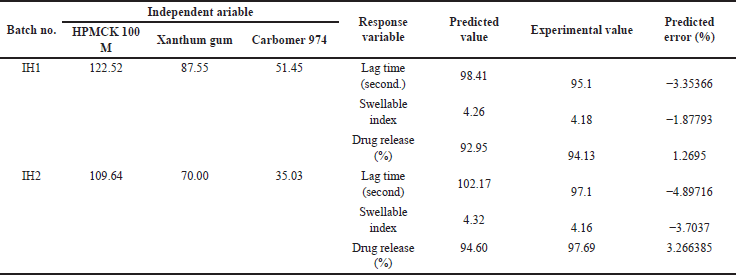 | Table 12. Final optimized formulation with response variable. [Click here to view] |
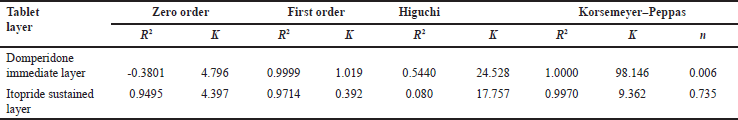 | Table 13. R2 values and rate constants of different release kinetic models. [Click here to view] |
Stability
India & Nepal were categorized to Zone IVb according to the ICH climatic zone. Criteria for relative humidity and temperature for long term 30°C ± 2°C 75% RH ± 5%, accelerated 40°C ± 2°C 75% RH ± 5%. Real-time stability chamber (Newtronics Walkin Chamber 20,000L), Accelerated stability chamber (Newtronics 600L) were used. Different parameters were observed during stability study like hardness, friability, lag time, FT, assay, dissolution results were studied which were studied. There was no significant change in the initial content of API of 5% and the other test met the acceptance criteria.
CONCLUSION
Bilayer tablets consisting of 10 mg domperidone immediate release and 150 mg itopride gastroretentive sustained release with a final compression weight of 800 mg was developed. Domperidone was made immediate release using a super-disintegrated agent in combination for synergistic effect using hit and trial method. They work together by penetration of medium and swelling of the tablet for the release of drug. itopride was optimized using Box Behnken design. There were 15 runs from three independent variables where responses were observed in dependent variables for lag time, swellable index, and drug release up to 24 hours. The selection of the upper layer in gastro-retentive floating tablets should be of low dosage strength with minimal compression weight. This is because of the impact on floating lag time. This formulation will be the best choice for GERD which can be commercialized.
AUTHOR CONTRIBUTIONS
Roshan Prajapati: Writing original draft, data curation. Bhavna Kumar: Conceptualization, editing, reviewing and supervision. Jagannath Sahoo: Review and co-supervision. Shailendra Shakya: Data compilation and editing. Diwya Kumar Lal: Writing draft, software analysis.
FINANCIAL SUPPORT
This research was supported by Everest pharmaceuticals Pvt. Ltd., Narcha, Bhaktapur, Nepal.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Savarino E, Zentilin P, Marabotto E, Bodini G, Della Coletta M, Frazzoni M, et al. A review of pharmacotherapy for treating gastroesophageal reflux disease (GERD). Expert Opin Pharmacother. 2017;18(13):1333–43. CrossRef
2. Chapelle N, Ben Ghezala I, Barkun A, Bardou M. The pharmacotherapeutic management of gastroesophageal reflux disease (GERD). Expert Opin Pharmacother. 2021;22(2):219–27. CrossRef
3. Clarrett DM, Hachem C. Gastroesophageal reflux disease (GERD). Mo Med. 2018;115(3):214–8.
4. Poddar U. Gastroesophageal reflux disease (GERD) in children. Paediatr Int Child Health. 2019;39(1):7–12. CrossRef
5. Silvers D, Kipnes M, Broadstone V, Patterson D, Quigley EM, McCallum R, et al. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. Clin Ther. 1998;20(3):438–53. CrossRef
6. Gupta AM, Belgamwar AV, Wake PS, Rathi TP, Mundhada D. Design and development of hydrodynamically balanced tablet of itopride. J Chem Pharm Res. 2011;3(6):856–64.
7. Ma J, Yuan L-H, Ding M-J, Zhang J, Zhang Q, Xu Q-W, et al. Determination of itopride hydrochloride in human plasma by RP-HPLC with fluorescence detection and its use in bioequivalence study. Pharmacol Res. 2009;59(3):189–93. CrossRef
8. Porwal A, Dwivedi H, Pathak K. Gastroretentive bilayer film for sustained release of atorvastatin calcium and immediate release of amlodipine besylate: pharmaceutical, pharmacokinetic evaluation, and IVIVC. Pharma Dev Technol. 2020;25(4):416–31. CrossRef
9. Tripathi J, Thapa P, Maharjan R, Jeong SH. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics. 2019;11(4):193. CrossRef
10. Iglesias N, Galbis E, Romero-Azogil L, Benito E, Lucas R, García-Martín MG, et al. In-depth study into polymeric materials in low-density gastroretentive formulations. Pharmaceutics. 2020;12(7):636. CrossRef
11. Kim B, Byun Y, Lee EH. DoE-based design of a simple but efficient preparation method for a non-effervescent gastro-retentive floating tablet containing metformin HCl. Pharmaceutics. 2021;13(8):1225. CrossRef
12. Juran JM. Juran on quality by design: the new steps for planning quality into goods and services. New York, NY: Simon and Schuster; 1992.
13. Food and Drug Administration. Pharmaceutical development Q8(R2). Food and Drug Administration; 2009. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development
14. ICH. Validation of analytical procedures: text and methodology Q2(R1). Food and Drug Administration; 2005. Available from: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
15. ICH. Pharmaceutical quality system Q10. Food and Drug Administration; 2010. Available from: https://www.fda.gov/media/71553/download
16. Pramod K, Tahir MA, Charoo NA, Ansari SH, Ali J. Pharmaceutical product development: a quality by design approach. Int J Pharma Investig. 2016;6(3):129–38. CrossRef
17. Dholariya YN, Bansod YB, Vora RM, Mittal SS, Shirsat AE, Bhingare CL. Design and optimization of bilayered tablet of hydrochlorothiazide using the quality-by-design approach. Int J Pharm Investig. 2014;4(2):93. CrossRef
18. Malladi M, Jukanti R. Formulation development and evaluation of a novel bi-dependent clarithromycin gastroretentive drug delivery system using Box-Behnken design. J Drug Deliv Sci Technol. 2016;35:134–45. CrossRef
19. Won DH, Park H, Ha E-S, Kim H-H, Jang SW, Kim M-S. Optimization of bilayer tablet manufacturing process for fixed dose combination of sustained release high-dose drug and immediate release low-dose drug based on quality by design (QbD). Int J Pharm. 2021;605:120838. CrossRef
20. Jadhav SB, Mali A, Rajeghadage S, Bathe R. Formulation and evaluation of immediate release tablets of imipramine hydrochloride. Int J Biomed Adv Res. 2014;5(11):559–65. CrossRef
21. Marshal K. Compression and consolidation of powdered solids. In: Lachman L, Lieberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. Mumbai, India: Varghese Publishing House; 1987.
22. Sarfraz RM, Khan HU, Mahmood A, Ahmad M, Maheen S, Sher M. Formulation and evaluation of mouth disintegrating tablets of atenolol and atorvastatin. Indian J Pharm Sci. 2015;77(1):83–90. CrossRef
23. Behera A, Nayak A, Mohanty B, Barik B. Development and optimization of losartan potassium tablets. Int J Appl Pharma. 2010;2:15–9.
24. Ratnaparkhi MP. Formulation and development of floating drug delivery of itopride HCl. J Drug Deliv Ther. 2013;3(4):222–8. CrossRef
25. Tak JW, Gupta B, Thapa RK, Woo KB, Kim SY, Go TG, et al. Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box–Behnken design. AAPS PharmSciTech. 2017;18:1125–34. CrossRef
26. Remya P, Damodharan N, Sulakshan Kumar C. Formulation and evaluation of bilayered tablets of ibuprofen and methocarbamol. Int J PharmTech Res. 2010;2(2):1250–55.
27. Joshna B, Sirisolla JD. Formulation, evaluation and comparison of mesalamine compression coated tablets by using natural and semi synthetic polymers. J Drug Deliv Therap. 2022;12(4-S):33–9. CrossRef
28. González R, Peña MÁ, Torres NS, Torrado G. Design, development, and characterization of amorphous rosuvastatin calcium tablets. PLoS One. 2022;17(3):e0265263. CrossRef
29. Nagar P, Singh K, Chauhan I, Verma M, Yasir M, Khan A, et al. Orally disintegrating tablets: formulation, preparation techniques and evaluation. J Appl Pharma Sci. 2011;1(4):35–45.
30. Nigusse B, Gebre-Mariam T, Belete A. Design, development and optimization of sustained release floating, bioadhesive and swellable matrix tablet of ranitidine hydrochloride. PLoS One. 2021;16(6):e0253391. CrossRef
31. Jagdale SC, Agavekar AJ, Pandya SV, Kuchekar BS, Chabukswar AR. Formulation and evaluation of gastroretentive drug delivery system of propranolol hydrochloride. AAPS PharmSciTech. 2009;10(3):1071–9. CrossRef
32. Sawant R, Bhangale L, Joshi R, Lanke P. Validated spectrophotometric methods for simultaneous estimation of Paracetamol, domperidone and tramadol HCl in pure and tablet dosage form. J Chem Metrol. 2010;4(1):21.
33. Afzal M, Muddassir M, Alarifi A, Ansari MT. Box-Behnken assisted validation and optimization of an RP-HPLC method for simultaneous determination of domperidone and lansoprazole. Separations. 2021;8(1):5. CrossRef
34. Perumal SS, Ekambaram SP, Raja S. Analytical method development and validation of simultaneous estimation of rabeprazole, pantoprazole, and itopride by reverse-phase high-performance liquid chromatography. J Food Drug Anal. 2014;22(4):520–6. CrossRef
35. Zate SU, Kothawade PI, Gajbe JW, Pramod AS, Boraste SS. Spectrophotometric method development and validation of itopride hydrochloride in bulk and dosage form. Int J Drug Deliv. 2010;2(4):340–3. CrossRef
36. Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
37. Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–33. CrossRef
38. Higuchi T. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52(12):1145–9. CrossRef
39. Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25–35. CrossRef
40. Bohrey S, Chourasiya V, Pandey A. Polymeric nanoparticles containing diazepam: preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016;3(1):3. CrossRef
41. Remington JP. Remington: the science and practice of pharmacy. In: Troy DB, Beringer P, editors. Baltimore, MD: Lippincott Williams & Wilkins; 2006.