INTRODUCTION
It has become widely recognized throughout the years that there is a strong connection between health benefits and nutrition [1]. As a result, consumers have increasingly been inclined to select food products that are filled with bioactive compounds, as these substances have been found to have a good impact on human health [2]. Plant materials have garnered significant attention due to their high concentration of bioactive chemicals [3]. Typically, most bioactive chemicals are situated within the cellular environment and necessitate liberation into the extracting solvent during extraction [4,5]. The conventional extraction methods, namely maceration, distillation, and soxhlet extraction, are frequently employed to extract bioactive compounds from plant materials [6].
Nevertheless, these methods are associated with drawbacks, including low extraction efficiency, excessive use of extracting solvents, high energy consumption, and prolonged extraction duration [7]. Hence, the concept of green extraction has garnered considerable attention and has been put out by numerous scholars in the field [8]. Green extraction refers to a type of extraction that involves using solvents with reduced extraction capabilities, resulting in lower energy consumption and shorter process durations while increasing the yield of the extraction process [9]. The development of green extraction methods has been motivated by producing environmentally friendly and economically viable solutions in response to industrial issues. These approaches aim to minimize ecological and environmental impacts throughout the extraction process [10].
Microwave-assisted extraction (MAE) is a widely utilized sophisticated technique to extract phytochemicals from plant sources. This technique involves the utilization of microwaves to induce thermal energy, establishing a pressure gradient within the sample [11]. Consequently, phytochemicals present in the plant are released via diffusion, tissue bursting, or cell wall rupturing. The increase in temperature additionally facilitates the softening of plant tissue, enhances mass transfer, heat transfer, and solvent penetration within the sample, disrupts the chemical structure, and aids in the extraction of polyphenols into the solvent [12]. Multiple factors contribute to the phenomenon of MAE, potentially influencing both the quantity and quality of phytochemicals extracted from botanical substances [13]. The utilization of MAE in the extraction process has predominantly been documented for obtaining a flavonoid compound [14]. However, optimizing several factors, such as solvent concentration, microwave power level, and irradiation period, is necessary to achieve improved extraction outcomes [15].
Piper scrotum Ruiz and Pav, generally known as red betel, is frequently observed in several Southeast Asian nations, with a notable presence in Indonesia. The plant in question is a member of the Piperaceae family and is indigenous to the region [16]. In Indonesian society, the leaves of this plant have been traditionally used for medicinal purposes. Numerous research has documented the pharmacological properties exhibited by this particular plant, including antioxidant [17], antibacterial [18], anti-inflammatory [19], and anticancer activity [20]. Piper crocatum has been shown to possess many secondary metabolites, primarily steroids, tannins, saponins, alkaloids, and flavonoids. The P. crocatum plant possesses pharmacological properties attributed explicitly to its components, notably flavonoids [21]. As documented in scientific literature, there is a significant correlation between flavonoids and their antioxidant qualities and antibacterial activity [22]. Nevertheless, the significant accumulation of flavonoids might be a health advantage due to their antioxidant and antibacterial properties, making them suitable for utilization in the pharmaceutical and food sectors [23,24].
The food business has encountered challenges in improving process efficiency without a concurrent investment and resource use increase. Investigating to determine the optimal conditions for the system or the food optimization process is crucial in resolving this predicament [25]. Historically, the optimization process involved examining the impact of altering one parameter at a time on a given output while keeping other parameters constant. However, this approach failed to consider the interactive effects between specific parameters, resulting in a lack of comprehensive understanding of the combined effects of all factors on the response [26]. Moreover, this methodology necessitates further experimentation, escalating expenses, and time consumption. Hence, multivariate statistical methods have been employed to improve the process parameters in food applications [27]. Response surface methodology (RSM) is widely recognized as a prominent approach for analyzing multivariate statistical approaches [28]. The RSM is a statistical and mathematical technique that uses polynomial models to assess data compatibility [29]. Its primary objective is to reveal the underlying patterns and behaviors within the data, ultimately leading to the development of mathematical models to make predictions [30].
Currently, there is a lack of research investigating optimizing flavonoid content extraction from P. cronatum leaf utilizing MAE and RSM as well as the correlation with antioxidant and antibacterial activities. This approach establishes the ideal extraction conditions by considering independent factors such as ethanol concentration, microwave power, and extraction duration. The investigation of various parameters on the efficacy of MAE involved the determination of total flavonoid content (TFC) and the assessment of antioxidant and antibacterial activities. In addition, the relationship between TFC, and antioxidant and antibacterial activities will be examined to elucidate the extract’s mechanism as an antioxidant and antibacterial agent.
MATERIALS AND METHODS
Plant material
The leaf of P. crocatum was cultivated inside the botanical areas of the Faculty of Pharmacy at Universitas Sumatera Utara, located in Indonesia. The leaf was identified as P. crocatum Ruiz and Pav by the Herbarium Medanese, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, with a voucher ID of 512/UN.5.1.1-HM/2023. The collected leaves were subjected to a drying process and stored at an ambient temperature. The dried leaves were pulverized using a household blender, and the resulting material’s average particle size (0.450 mm) was determined using a set of sieves. The dried powder was stored at room temperature before its utilization in the extraction process.
Chemicals and media
Chemicals such as ethanol, methanol, 1,1-diphenyl-2-picryhydrazyl (DPPH), aluminum chloride, and quercetin were used in this research and are analytical and certified by Sigma-Aldrich, United Kingdom. The Staphylococcus aureus (ATCC 29737), deMann Rogosa and Sharpe Agar (MRSA), and then Nutrient Agar (NA) were obtained from the Microbiology Laboratory, Faculty of Pharmacy, Universitas Sumatera Utara, Indonesia.
MAE procedure
Single conditions of MAE were performed in a homemade setup consisting of the microwave with modification (Samsung ME731K, Seoul, South Korea). Seventeen experimental MAE runs on different ethanol concentrations (X1), microwave power (X2), and extraction time (X3), were performed according to the results of Design-expert v.13. In every iteration of the experiment, a mass of 10 g of the sample was combined with a volume of 100 ml of the extraction solvent. The flasks were subsequently positioned within the MAE device, and extractions were conducted at a predetermined frequency. Following the extraction process, the crude extracts were promptly subjected to filtration using filter paper with a pore size ranging from 4 to 12 μm, employing a vacuum system. The collected samples were placed into glass flasks and thereafter stored at a temperature of 4ºC until they were ready for additional examination [31].
Selection of factors
A significant number of characteristics may influence the researched extraction system. Consequently, it is crucial to choose the carefully most substantial impact elements. Several factors can potentially affect a given study’s MAE. These factors include the nature of the solvent used, the ratio of solvent to solid material, the duration of the extraction process, the power level of the microwave used, the temperature at which the extraction is conducted, the properties of the sample being analyzed, and the number of extraction cycles performed [32]. Understanding the effects and combinations of these elements on the MAE process holds significant importance. The initial phase was the selection of the most appropriate solvent. Numerous findings indicate that ethanol is suitable for extracting diverse polyphenolic components from plant sources [33]. The selection of condition ranges for the MAE was informed by existing literature. The X2 exhibited an influence ranging from 180 to 400 W, while the X3 ranged from 3 to 14 minutes. The analytical findings are provided in Table 1.
Total flavonoids content
The TFC of extracts was obtained using spectrophotometry methods. Briefly, 2 ml of extracts in methanol was mixed with 0.10 ml of 10% AlCl3, 0.10 ml of NaC2H3O2·3H2O (1 M), and 2.80 ml of distilled water. The samples were incubated for 40 minutes, and after that, the absorbance of samples was measured at 432 nm. To determine the TFC of the samples, a calibration curve was built utilizing quercetin as the standard compound. The flavonoid concentration is expressed as mg QE/g sample. The equation to determine TFC as can see below (Eq. 1) [34]
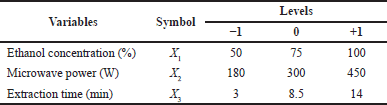 | Table 1. The experimental domain used in BBD. [Click here to view] |
C (QE): Concentration of flavonoid as quercetin equivalent
C: Concentration determined from standard curve (μg/ml)
V: Volume used in the assay (ml)
M: Mass of the sample which used in the assay (g)
F: Dilution factor
Antioxidant activity
The measurement of free radical scavenging activity was conducted using the DPPH technique. A 0.2 mM solution of DPPH in methanol was produced. Subsequently, 100 μl of this solution was added to a solution containing extracts at a concentration of 100 μg/ml. After 60 minutes, the measurement of absorbance was conducted at a wavelength of 516 nm. The calculation of the percentage of inhibition was performed by comparing the absorbance values obtained from the control group with those obtained from the samples, as illustrated below (Eq. 2) [35].
Antibacterial activity
To determine the antibacterial activity of extracts, a standard disc diffusion method was performed. Briefly, the 100 mg/ml of each extract was used as a sample test against S. aureus. Sterile discs with a diameter of 6 mm were saturated with 25 μl of extract solution. Following a 15-minutes incubation period for optimal extract diffusion, the discs were placed onto a NA surface coated with 0.1 ml of a bacterial culture. The bacterial culture had been standardized to a concentration of 0.5 McFarland standards (106 CFU/ml). The plates were subjected to incubation at a temperature of 37ºC for 12–14 hours. The outcomes were documented by measuring the area of growth inhibition surrounding the discs [34].
Statistical analysis and experimental planning
The RSM was employed to investigate the extraction parameters’ effects and optimize the conditions for different responses. The response pattern was established first using a Box–Behnken design (BBD) with three variables. The design, which had 17 combinations and five replicates at the central point, was carried out randomly. Ethanol concentration (X1, 50%–100%), microwave power (X2, 180–450 W), and extraction duration (X3, 3–14 minutes) were the three independent variables employed in this investigation. Each coded variable was made to have a range from –1 to 1 to equalize the parameters. This made the answer more evenly affected, and the units of the parameters were, therefore, unimportant. The following second-order polynomial model, which can typically explain the relationship between the responses and the independent factors, was fitted to the response variables (Eq. 3) [36]:
where Y represents the response variable; Xi and Xj are the independent variables affecting the response; and A0, Ai, Aii, and Aij are the regression coefficients for intercept, linear, quadratic, and interaction terms, respectively.
Optimal extraction parameters were discovered for three separate responses: TFC, antioxidant, and antibacterial activity. Various responses were handled based on the desirability function, and the best conditions were chosen. Using Design-Expert v.13 (Stat-Ease, Minneapolis, MN, USA), multiple linear regression analysis and the experimental design were carried out. ANOVA was used to assess the results statistically, using 0.05 as the significance level. The coefficient of determination (R2), coefficient of variance (CV), and p-values for the model and lack of fit testing were used to assess the models’ suitability [36].
RESULTS AND DISCUSSION
Model fitting analysis
The response surface approach was used to successfully optimize the MAE of flavonoid content from the P. crocatum leaf. Seventeen runs of the experimental design comprised streamlined experimental sets with five independent variable central values. Table 2 lists the BBD configuration and the observed responses for TFC, DPPH scavenging, and antibacterial activity. Using the second-order equation (Eq. 3), the R2 of the model’s intercept, linear, quadratic, and interaction terms were determined. ANOVA was used to determine the significance of the influence of the linear, quadratic, or interaction coefficients on the answer (Table 3). Each component’s p-value indicates its significance (Table 3). The fitted model accurately represents the experimental data, which has high correlation values (R2) ranging from 0.9339 to 0.9976 (Table 3). Table 3’s ANOVA demonstrates that the regression models for TFC, DPPH scavenging activity, and antibacterial activity were statistically significant, with significance levels ranging from p = 0.0001, p = 0.0023, and p = 0.0002, respectively. The models also showed no statistically significant lack of fit with p > 0.05. This led to the establishment of successful well-fitting models for TFC, DPPH scavenging activity, and antibacterial activity [29].
The effect of independent variables on TFC
The TFC of P. crocatum leaf extracts varied from 152.94 to 231.57 mg QE/g DW, depending on different investigated parameter levels. The lowest yield of TFC was obtained on the lower concentration of ethanol (X1, 50%), higher level of microwave power (X2, 450 W), and middle level of extraction time (X3, 8.5 minutes), while the higher TFC was obtained on the middle of X1, X2, and X3 (75%, 300 W, and 8.5 minutes, respectively). According to p values of regression coefficients (Table 3), the TFC was the most significantly influenced by X1 and quadratic of independent variables (X12, X22, and X32) (p < 0.0001, Table 3). In addition, the other variables namely X3 (p < 0.0038), interaction of ethanol concentration and microwave power (X1X2, p < 0.0023), interaction of ethanol concentration and extraction time (X1X3, p < 0.0067), and interaction of microwave power and extraction time (X2X3, p < 0.0375) statistically significantly affect the total flavonoids content. Linear term of X1 and interaction terms of X1X2 and X1X3 exhibited positive influence. The positive effects of independent variables demonstrated that a rise in the response value can result from their positive modifications [37]. The following graph illustrates the second-order polynomial model that was used to represent the TFC as a function of independent variables:
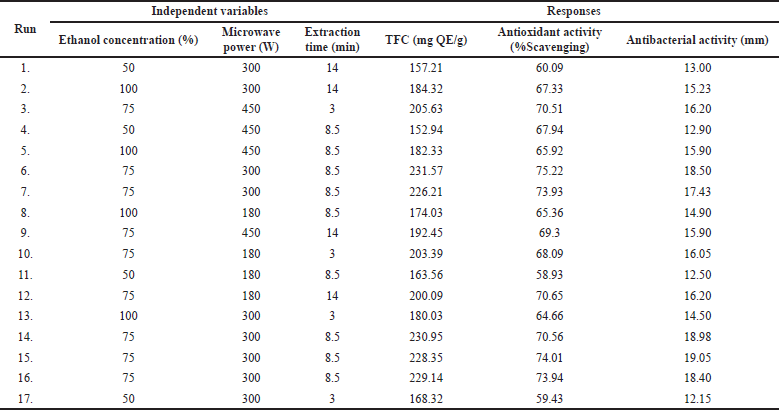 | Table 2. BBD with natural and coded MAE conditions and experimentally obtained values of TPC (mg QE/g), antioxidant activity (% Scavenging activity), and antibacterial activity (mm). [Click here to view] |
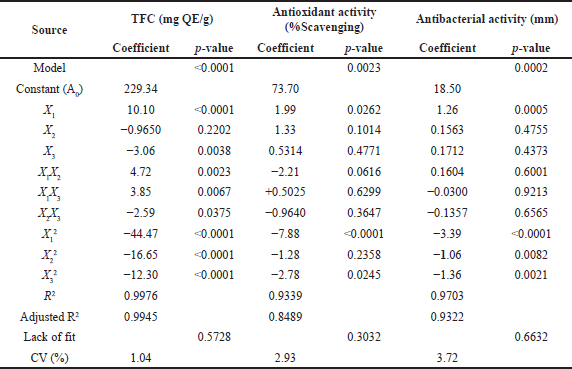 | Table 3. Corresponding p-values of linear, interaction, quadratic, and ANOVA of the fitted second-order polynomial model for TFC, antioxidant, and antibacterial activities. [Click here to view] |
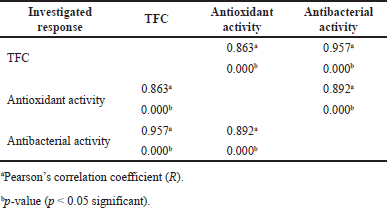 | Table 4. Pearson’s correlation coefficient (R) and p-values for TFC, antioxidant and antibacterial activities. [Click here to view] |
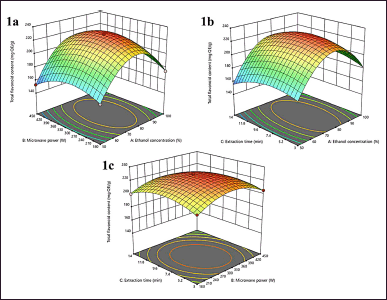 | Figure 1. 3D plots of total flavonoids content (1a: interaction of X1X2, 1b: interaction of X1X3, 1c: interaction of X2X3. [Click here to view] |
Total flavonoids content (mg QE/g) = 229.34 + 10.10 X1 – 0.9650 X2 – 3.06 X3 + 4.72 X1X2 + 3.85 X1X3 – 2.59 X2X3 – 44.47 X12 – 16.65 X22 – 12.30 X32
Response surface plots (Fig. 1a and b) show the relation of X1X2 and X1X3 on the TFC of extracts. The TFC of extracts reached the maximum when the X1 was around 75% with X2 of 300 W. The TFC will decrease after the X1 and X2 increase. The interaction of X1X3 was described as a significant effect of X1, the graphs in Figure 1b, also show that the TFC slightly increased with an increase of X1 from 50% to 80%, and decreased after 80% although the X3 in the lowest or highest conditions.
That was described as the effect of X1 is significant on TFC [38]. In addition, the interaction of X1X2 and X1X3 was significantly proven through total flavonoid extraction (p < 0.0023 and p < 0.0067, respectively). In this research, ethanol was used as the solvent due to its high extraction efficacy, compatibility with human consumption, and environmental safety considerations [39]. The efficacy of flavonoid extraction is enhanced when alcohol is combined with water, as compared to using alcohol as a solvent alone [40]. This is attributed to the fact that the extraction and separation of flavonoids are heavily influenced by the polarity of solvents and the chemical properties of the molecules involved [41]. The presence of a specific quantity of water is likely to induce the expansion of plant material, leading to an augmentation in the surface area of contact between the solvent and the plant matrix. This, in turn, directly influences the effectiveness of the extraction process [42]. Furthermore, to achieve optimal extraction of target compounds, it is advisable to conduct the extraction using a solvent mixture with a ratio determined based on the chemical composition and polarity of the target compound(s) [43]. The selection of a suitable solvent for MAE is contingent upon its capacity to absorb microwave energy and then convert it into heat. The dielectric characteristics of the solvent influence this conversion process [44].
Furthermore, Figure 1c, shows the significant interaction of X2X3 (p < 0.0375). This interaction has not depended on one factor. The X2 and X3 affect TFC and will increase if the X2 and X3 increase to 330 W and 10 minutes. The TFC has slightly decreased after the maximum point of X2 and X3. This phenomenon can be elucidated by the following observation: an elevation in microwave power resulted in a rise in system temperature, facilitating the extraction of nonflavonoid components [45]. Consequently, there was a proportional reduction in the overall concentration of flavonoids in the extracted samples. One notable advantage of utilizing MAE compared to traditional extraction methods is the reduction in the time necessary for extracting phytochemicals from plant sources [46]. The concentration of flavonoids in the extracts exhibited a decline when the duration of the extraction process exceeded 10 minutes, as depicted in Figure 1c. The extended duration of the sample’s exposure to microwave irradiation and the solvent likely resulted in the extraction of chemical compounds from the extract, besides from flavonoids. These chemicals may include minerals and carbohydrates. Our findings were consistent with the outcomes reported in other studies [47].
The effect of independent variables on DPPH scavenging activity
This work aimed to assess the antioxidant activity of the leaf extracts of P. crocatum by in vitro experiments, specifically the DPPH radical scavenging assay. The DPPH radical scavenging assay involves the radicals’ quenching by hydrogen atom transfer by antioxidants [48]. The DPPH scavenging activity of the extracts is quantified as the percentage of scavenging. The percentage of DPPH scavenging exhibited by the extracts obtained using MAE fell within a specific range from 58.93% to 75.22% (Table 2). The lowest % activity was observed on the lower level of X1 (50%) and X2 (180 W), but the middle level of X3 (8.5 min). These results more highest than those reported by Alfarabi et al. [49] of 59.34% in 100 μg/ml conventional extract of P. crocatum leaf and ethanolic extract of P. crocatum leaf (33.00%) in 100 μg/ml reported by Fatmawaty et al. [17]. On the other hand, the antioxidant activity of this work is higher than that reported by Rahardjo et al. [50], and hot water extract of P. crocatum leaf reported by Kamaruzaman et al. [51] of 74.90% in 20 mg/ml. The antioxidant activity of the extract has a strong correlation with TFC which is R = 0.863 (Table 4). The increase in the total content of flavonoid extract has a significant impact on increasing its antioxidant activity. This result is similar to other studies that were reported by Do et al. [32] and Alide et al. [52].
Response surface plots (Fig. 2a) show the interaction of X1 and X2 is not significantly affected by % DPPH scavenging activity, but X1 has significantly (p < 0.0262) affected the % DPPH scavenging activity. The increasing X1 up to 85% was identified, leading to the % DPPH scavenging activity increase, and above 85%, the % DPPH scavenging activity will decrease. This phenomenon was identified in a range X2 of 180–450 W. Similar to the previous factor, the interaction of X1 X3 and X2 X3 was described not significantly. The response of % DPPH scavenging activity with these factors’ interaction can be seen in Figure 2b and c. The second-order polynomial model used to express the % DPPH scavenging activity as a function of independent variables (in terms of coded values) is shown below:
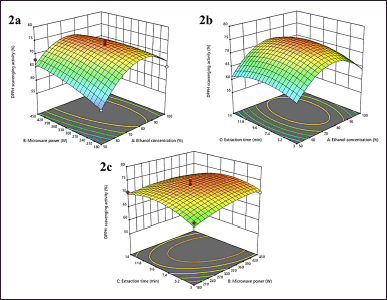 | Figure 2. 3D plots of antioxidant activity (2a: interaction of X1X2, 2b: interaction of X1X3, 2c: interaction of X2X3. [Click here to view] |
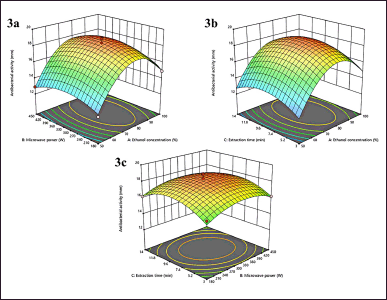 | Figure 3. 3D plots of antibacterial activity (3a: interaction of X1X2, 3b: interaction of X1X3, 3c: interaction of X2X3. [Click here to view] |
% DPPH scavenging activity = 73.70 + 1.99 X1 + 1.33 X2 + 0.5314 X3 – 2.21 X1X2 + 0.5025 X1X3 – 0.9640 X2X3 – 7.88 X12 – 1.28 X22 – 2.78 X32
The X1 has a factor with a significant impact against % DPPH scavenging activity with a positive coefficient. Many studies have reported the impact of changing X1 led % DPPH scavenging activity. Kaneria et al. [53] reported that the 75% methanolic extract is the best solvent for the highest % DPPH scavenging activity from A. indica and M. zapota. Mannoubi et al. [54] reported that 80% ethanol is the best solvent compared to 80% methanol and acetone to get the highest antioxidant activity using the DPPH method. In addition, Gonfa et al. [55] showed that the 80% ethanolic extract has a better DPPH scavenging activity than absolute ethanol and absolute methanol, although 80% methanol is the best solvent. Furthermore, ethanol is a solvent with a low toxicity risk, so it is better used in the extraction [56]. These reports described the significant impact of X1 through % DPPH scavenging activity and all results are similar to this study.
The effect of independent variables on antibacterial activity
The antibacterial effect of the extract was carried out using the diffusion disc method against Staphylococcus aureus (ATCC 29737). The 100 μg/ml extracts were used to observe antibacterial activity through the inhibition zone (mm). The inhibition zone after the extracts treated can be seen in Table 2. The inhibition zone after treated extracts varies from 12.15 to 19.05 mm. The lowest inhibition zone was obtained from lower X1 (50%), middle X2 (300 W), and lower X3 (3 minutes), while the highest inhibition zone from middle X1, X2, and X3 of 75%, 300 W, and 8.5 minutes, respectively. This result shows better antibacterial activity against S. aureus than previous reports by Kusuma et al. [57]. Based on our observation, only X1 has significantly affected the antibacterial effect from three linear variables with a positive coefficient (p < 0.0005). In addition, the quadratic variables X12, X22, and X32 have significantly impacted the antibacterial activity with p < 0.0001, p < 0.0082, and p < 0.0021, respectively. In more detail, the second-order polynomial model to express the antibacterial activity as a function of independent variables (in terms of coded values) is shown below:
Antibacterial activity (mm) = 18.50 + 1.26 X1 + 0.1563 X2 + 0.1712 X3 + 0.1604 X1X2 – 0.0300 X1X3 – 0.1357 X2X3 – 3.39 X12 – 1.06 X22 – 1.36 X32
Response surface plots (Fig. 3a and b) show the effect of X1 against antibacterial activity in interaction with X2 and X3. The graphs describe the X1 significantly impacts the antibacterial activity of extracts. The superior effect was identified in specific conditions of X1, which is 75% to 85%, with increasing or decreasing X2 and X3. These are correct with the ANOVA analysis that is shown in Table 3. Figure 2c gives a different impact on antibacterial activity. It is not identified which one is more affected by the interaction between X2 and X3. The maximum antibacterial activity is described at a certain point from X2 and X3 of 300 W to 380 W and 7 to 10 minutes, respectively. This study shows that antibacterial activity has a strong correlation with TFC which is R = 0.957 (Table 4) and it is similar to previous studies by Yuan et al. [58] who mention that flavonoid compounds have antibacterial activity with correlation coefficients above 0.93. As reported by Jawhari et al. [59], the Anacyclus pyrethrum capitula extract has the highest flavonoid content than seeds extract and the strongest antibacterial activity against S. Aureus. Similar to reports by Bouchelaghem et al. [60], about the antibacterial activity of Hungarian propolis ethanol extract lead increase and with the TFC increase and Sartini et al. [61] described the phenolic content as correlated with antibacterial activity. Furthermore, the activity of flavonoid compounds as antibacterial was identified in many reports. Various processes explain flavones’ antibacterial properties. Chen’s study found that baicalein at 32 and 64 μg/ml reduced quorum-sensing system regulators agrA, RNAIII, and sarA and gene expression of intercellular adhesin (ica) in S. aureus biofilm producer cells [62]. The most effective antibacterial flavonoids are quercetin, myricetin, morin, galangin, entadanin, rutin, piliostigmol, and their derivatives. For instance, quercetin and its derivatives inhibited S. aureus and other germs [63]. Morin is reported to be efficient against Gram-positive bacteria [64]. Combining plant-derived flavonol with β-lactam antibiotics significantly increased MRSA sensitivity to oxacillin [58]. Conversely, flavonoid-rich plants can affect bacterial surface and cellular leakages [65].
Optimization of MAE and validation of the models
The primary aim of this study was to determine the optimal conditions for generating extracts with elevated levels of flavonoids, as well as enhanced antioxidant and antibacterial properties. Based on the analysis of the maximum content of extracted total flavonoids, the percentage of DPPH scavenging, and the inhibitory zone, it can be concluded that the ideal conditions for all three examined responses were as follows: X1 at a level of 78.48%, X2 at a level of 327.96 W, and X3 at a level of 8.60 minutes. The TFC, % DPPH scavenging activity, and antibacterial activity are displayed in Table 5. The determination of optimal conditions, predicted value, and observed value are achieved by the utilization of a desirability function, which yielded a value of 0.944 for multiresponse optimization [66]. To validate the predictive mathematical model of the researched process, MAE was conducted on the estimated ideal conditions for all three examined responses.
This experiment showed the observed results in optimum conditions which are mentioned in Table 5 are not significantly different from the predicted results by RSM. The observed values of TFC, % DPPH scavenging activity, and antibacterial activity were 232.532 ± 1.05 mg GAE/g, 75.352% ± 0.85%, and 17.863 ± 0.92 mm, respectively. While, the predicted values of 229.647 mg GAE/g, 73.915%, and 18.621 mm, respectively. If we compared between observed and predicted values, the entire results were not significantly different even though the observed values of TFC and % DPPH scavenging activity showed a slight rise and the antibacterial activity showed a slight decrease than predicted values. The comparison between the observed experimental findings and the expected values revealed that all response variables fell within the 95% confidence interval of the predicted model. The strong connection seen in the results provides evidence for the appropriateness of the employed model and the effectiveness of RSM in optimizing the studied conditions for minimizing MAE.
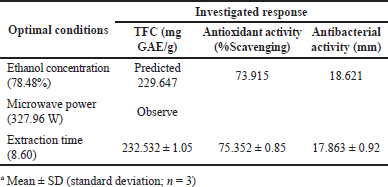 | Table 5. Predicted and observed values of each response at optimal conditions. [Click here to view] |
CONCLUSION
RSM was used to find the best options for MAE of TFC, percentage of DPPH scavenging, and antibacterial activity of the extracts. The ANOVA showed that the second-order polynomial model was a good mathematical representation of the MAE linked with flavonoids with strong antioxidant and antibacterial properties. Taking into account all of the factors, it is clear that X1 had a big effect on the MAE. In the end, the best settings for the three factors that were looked at were for X1 to be 78.48%, X2 to be 327.96 W, and X3 to be 8.60 minutes. The results of this study show that a very effective natural extract can be made when all of the factors are met. Furthermore, the utilization of MAE as an environmentally friendly way to make flavonoid-rich extracts from P. crocatum has provided better antioxidant and antibacterial properties.
ACKNOWLEDGMENTS
The authors are grateful to Universitas Sumatera Utara for their support in providing the chemical reagents and facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This article does not contain any studies with human or animal subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Alvin A, Miller KI, Neilan BA. Exploring the potential of endophytes from medicinal plants as sources of antimycobacterial compounds. Microbiol Res, 2014;169(7–8):483–95. CrossRef
2. Licea-Dominguez S, Estevez-Rioja A, Hernández-Lozano LC, Alvarado-Ponce GE, Asemota H, González-Cordova AF, et al. Market orientation and cuisine innovation as a driven sensory methodology to develop a sweet potato snack as an added-value food product. Int J Gastron Food Sci. 2023;32, CrossRef
3. Jam N, Hajimohammadi R, Gharbani P, Mehrizad A, Antibacterial activity of Punica granatum L. and Areca nut (P.A) combined extracts against some food born pathogenic bacteria. Saudi J Biol Sci. 2022;29(3):1730–36. CrossRef.
4. Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117(4):426−36. CrossRef
5. Jam N, Hajimohammadi R, Gharbani P, Mehrizad A, Evaluation of antibacterial activity of aqueous, ethanolic and methanolic extracts of areca nut fruit on selected bacteria. Biomed Res Int. 2021;6663399. CrossRef.
6. Bitwell C, Indra SS, Luke C, Kakoma MK, A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci Afr. 2023;19(e01585). CrossRef
7. Wu C, Wang F, Liu J, Zou Y, Chen X. A comparison of volatile fractions obtained from Lonicera macranthoides via different extraction processes: ultrasound, microwave, Soxhlet extraction, hydrodistillation, and cold maceration. Integr Med Res. 2015;4(3):171−77. CrossRef
8. Janicka P, P?otka-Wasylka J, Jatkowska N, Chabowska A, Fares MY, Andruch V, et al. Trends in the new generation of green solvents in extraction processes. Curr Opin Green Sustain. 2022;37:100670. CrossRef.
9. Chemat F, Abert-Vian M, Fabiano-Tixier AS, Strube J, Uhlenbrock L, Gunjevic V, et al. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal Chem, 2019;118:248−63. CrossRef
10. Armenta S, Garrigues S, de la Guardia M. The role of green extraction techniques in green analytical chemistry. Trends Analyt Chem. 2015;71:2−8. CrossRef
11. Rizwan M, Gilani SR, Durrani AI, Naseem S. Low temperature green extraction of Acer platanoides cellulose using nitrogen protected microwave-assisted extraction (NPMAE) technique. Carbohydr Polym. 2021;272:118465. CrossRef
12. Kaderides K, Papaoikonomou L, Serafim M, Goula AM. Microwave-assisted extraction of phenolics from pomegranate peels: optimization, kinetics, and comparison with ultrasounds extraction. Chem Eng Process. 2019;137:1–11. CrossRef
13. Alvi T, Asif Z, Khan MKI. Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique-A review. Food Biosci, 2022;46:101580. CrossRef
14. Alara OR, Abdurahman NH, Olalere OA, Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod. 2018;107:36–48. CrossRef
15. Nana O, Momeni J, Boyom FF, Njintang NY, Ngassoum, MB. Microwave-assisted extraction as an advanced technique for optimization of limonoid yields and antioxidant potential from Trichilia roka (Meliaceae). Curr Res Green Sustain Chem. 2021;4:100147. CrossRef
16. Setyawati A, Wahyuningsih MSH, Nugrahaningsih DAA, Effendy C, Ibeneme S, Piper Crocatum Ruiz and Pav as a commonly used typically medicinal plant from Indonesia: What do we know about it? Scoping Review. ICON J. 2023;7(2):61–78. CrossRef
17. Fatmawaty, Anggreni NGM, Fadhil N, Prasasty VD, Potential in vitro and in vivo antioxidant activities from Piper crocatum and Persea americana Leaf Extracts. Biomed Pharmacol J. 2019;12(2):661–7. https://dx.doi.org/10.13005/bpj/1686
18. Astuti P, Wahyono, Nababan OA, Antimicrobial and cytotoxic activities of endophytic fungi isolated from Piper crocatum Ruiz and Pav. Asian Pac J Trop Biomed. 2014;4(2):S592–6. CrossRef
19. Lister INE, Ginting CN, Girsang E, Nataya ED, Azizah AM, Widowati W, Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, anti necrotic, antioxidant potency. SPJ. 2020; 28(10):1182–89. CrossRef
20. Wulandari N, Meiftasari A, Fadliyah H, Jenie RI, Red betel leaves methanolic extract (Piper crocatum Ruiz and Pav.) increases cytotoxic effect of doxorubicin on WiDr colon cancer cells through apoptosis induction. ISCC, 2018;9(1):1–8. http://dx.doi.org/10.14499/indonesianjcanchemoprev9iss1pp1-8
21. Heliawati L, Lestari S, Hasanah U, Ajiati D, Kurnia D. Phytochemical profile of antibacterial agents from red betel leaf (Piper crocatum Ruiz and Pav) against Bacteria in Dental Caries. Molecules. 2022;27(9):2861. CrossRef
22. Gharbani P, Javazi H, The antioxidant, general toxicity and insecticidal activities of Nepeta scrophularioides rech. f. extracts in different developmental stages. Pak J Pharm Sci, 2015;28(5 suppl):1905–9. PMID: 26525034.
23. Nayaka NMDMW, Fidrianny I, Sukrasno, Hartati R, Singgih M, Antioxidant and antibacterial activities of multiflora honey extracts from the Indonesian Apis cerana bee. JTUMED. 2020;15(3):211–17. CrossRef
24. Vaquero MJR, Serravalle LRT, de Nadra MCM, de Saad AMS, Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control, 2010;21(5):779–85. CrossRef
25. Hadiyat MA, Sopha BM, Wibowo BS, Response surface methodology using observational data: a systematic literature review. Appl Sci. 2022;12:10663. CrossRef
26. Brownlee AEI, Epitropakis MG, Mulder J, Paelinck M, Burke EK, A systematic approach to parameter optimization and its application to flight schedule simulation software. J Heuristics. 2022;28:509–38. CrossRef
27. Kumari M and Gupta SK, Response surface methodological (RSM) approach for optimizing the removal of trihalomethanes (THMs) and its precursor’s by surfactant modifed magnetic nano adsorbents (sMNP)—an endeavor to diminish probable cancer risk. Sci Rep. 2019;9:18339. CrossRef
28. Mehrizad A, Gharbani P. Removal of methylene blue from aqueous solution using nano-TiO2/UV process: optimization by response surface methodology. PCCC. 2016;9(2):135–43. CrossRef
29. Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA, Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–77. CrossRef
30. Chen S, Zhang H, Yang L, Zhang S, Jiang H. Optimization of ultrasonic-assisted extraction conditions for bioactive components and antioxidant activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods. 2022;12(3):619. CrossRef
31. Zekovic Z, Vladic J, Vidovic S, Adamovic D, Pavlic B. Optimization of microwave-assisted extraction (MAE) of coriander phenolic antioxidants—response surface methodology approach. J Sci Food Agric. 2016;96(13):4613–22. CrossRef
32. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22(3):296–302. CrossRef
33. Vidovic SS, Zekovic ZP, Lepojevic ZD, Radojkovic MM, Jokic SD, Anackov GT, Optimization of the Ocimum Basilicum L. extraction process regarding the antioxidant activity. Acta Period Technol. 2012; 43:315–23.
34. Lubis MF, Kaban VE, Gurning K, Parhan P, Syahputra H, Juwita NA, et al. Phytochemicals and biological activities of ethanolic extract of garcinia atroviridis leaf grown in Indonesia. J Med Chem Sci, 2023;6(10):2456–69. CrossRef
35. Lubis MF, Syahputra H, Illian DN, Kaban VE, Antioxidant activity and nephroprotective effect of Lansium parasiticum leaves in doxorubicin-induced rats. J Pharm Res. 2022;26(3):565–73. https://dx.doi.org/10.29228/jrp.154
36. Wang X, Wu Y, Chen G, Yue W, Liang Q, Wu Q, Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason Sonochem. 2013;20(3):846–54. CrossRef
37. Yang L, Cao Y, Jiang J, Lin Q, Chen J, Zhu L, Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J Sep Sci. 2010;33(9):1349–55. CrossRef
38. Alara OR, Nour AH, Mudalip SKA, Screening of microwave-assisted-batch extraction parameters for recovering total phenolic and flavonoid contents from Chromolaena odorata leaves through two-level factorial design, Indones J Chem. 2019;19(2):511–21. CrossRef
39. Gil-Martín E, Forbes-Hernández T, Romero A, Cianciosi D, Giampieri F, Battino M, Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem, 2022;378:131918. CrossRef
40. Liu X, Liu Y, Shan C, Yang X, Zhang Q, Xu N et al. Effects of five extraction methods on total content, composition, and stability of flavonoids in jujube. Food Chem. X. 2022;14:100287. CrossRef
41. Dairi S, Dahmoune F, Belbahi A, Remini H, Kadri N, Aoun O et al. Optimization of microwave extraction method of phenolic compounds from red onion using response surface methodology and inhibition of lipoprotein low-density oxidation. J Appl Res Med Aromat Plants. 2021;22:100301. CrossRef
42. Simi? VM, Rajkovi? KM, Stoji?evi? SS, Veli?kovi? DT, Nikoli? NC, Lazi? ML et al. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep Purif Technol, 2016;160:89–97. CrossRef
43. Ranic M, Nikolic M, Pavlovic M, Buntic A, Siler-Marinkovic S, Dimitrijevic-Brankovic S, Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J Clean Prod. 2014;80:69–79. CrossRef
44. Ahmad I, Yanuar A, Mulia K, Mun’im A, Optimization of ionic liquid-based microwave-assisted extraction of polyphenolic content from Peperomia pellucida (L) kunth using response surface methodology. Asian Pac J Trop Biomed. 2017;7(7):660–65. CrossRef
45. Akbari S, Abdurahman NH, Yunus RM, Optimization of saponins, phenolics, and antioxidants extracted from fenugreek seeds using microwave-assisted extraction and response surface methodology as an optimizing tool. C R Chim. 2019; 22(11–12):714–27. CrossRef
46. Jha AK, and Sit N. Comparison of response surface methodology (RSM) and artificial neural network (ANN) modelling for supercritical fluid extraction of phytochemicals from Terminalia chebula pulp and optimization using RSM coupled with desirability function (DF) and genetic algorithm (GA) and ANN with GA. Ind Crops Prod. 2021;170(113769). CrossRef
47. Kaanin-Boudraa G, Brahmi F, Wrona M, Nerín C, Moudache M, Mouhoubi K et al. Response surface methodology and UPLC-QTOF-MSE analysis of phenolic compounds from grapefruit (Citrus× paradisi) by-products as novel ingredients for new antioxidant packaging. LWT, 2021;151:112158. CrossRef
48. Zhao Y, Du S, Wang H, Cai M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014;152:462–66. CrossRef
49. Alfarabi M, Bintang M, Suryani MS. The comparative ability of antioxidant activity of Piper crocatum in inhibiting fatty acid oxidation and free radical scavenging. Hayati J Biosci. 2010;17(4):201–04. CrossRef
50. Rahardjo M, Mangalik G, Sihombing M, da Costa JF. Effect of the extraction solvent polarity and the ratio of feed and solvent on the phytochemical content and antioxidant activity of red betel leaves (Piper crocatum). Indones J Agricultural Res. 2018;1(1):71–7. CrossRef
51. Kamaruzaman SRS, Kasim KF, Jaafar MN. The effect of harvesting time on the antioxidant and antidiabetic activity of Piper Crocatum (Sirih Merah) extract. IOP Conf Ser Mater Sci. 2020;864:012211. CrossRef
52. Alide T, Wangila P, Kiprop A. Effect of cooking temperature and time on total phenolic content, total flavonoid content and total in vitro antioxidant activity of garlic. BMC Res Notes. 2020;13(564):1–7. CrossRef
53. Kaneria M, Kanani B, Chanda S, Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants, Asian Pac J Trop Biomed. 2012;2(3):195–202. CrossRef
54. Mannoubi IE, Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia Ficus Indica fruits. J Food Meas Charact. 2021;15:643–51. CrossRef
55. Gonfa T, Teketle S, Kiros T. Effect of extraction solvent on qualitative and quantitative analysis of major phyto-constituents and in-vitro antioxidant activity evaluation of Cadaba rotundifolia Forssk leaf extracts. Cogent Food Agric. 2020;6:1853867. CrossRef
56. Neto OSZ, Batista EAC, Meirelles AJA. The employment of ethanol as solvent to extract Brazil nut oil. J Clean Prod. 2018;180:866–75. CrossRef
57. Kusuma SAF, Hendriani R, Genta A. Antimicrobial spectrum of red piper betel leaf extract (Piper crocatum Ruiz and Pav) as natural antiseptics against airborne pathogens. J Pharm Sci Res. 2017;9(5):583–87.
58. Yuan G, Guan Y, Yi H, Lai S, Sun Y, Cao S, Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci Rep. 2021;11(10471). CrossRef
59. Jawhari FZ, Moussaoui AEL, Bourhia M, Imtara H, Saghrouchi H, Ammor K et al. Anacyclus pyrethrum var. pyrethrum (L.) and Anacyclus pyrethrum var. depressus (Ball) Maire: correlation between total phenolic and flavonoid contents with antioxidant and antimicrobial activities of chemically characterized extracts. Plants. 2021;10(1):149. CrossRef
60. Bouchelaghem S, Das S, Naorem RS, Czuni L, Papp G, Kocsis M. Evaluation of total phenolic and flavonoid contents, antibacterial and antibiofilm activities of Hungarian propolis ethanolic extract against Staphylococcus aureus. Molecules. 2022;27(2):574. CrossRef
61. Sartini S, Djide MN, Amir MN, Permana AD. Phenolic-rich green tea extract increases the antibacterial activity of amoxicillin against Staphylococcus aureus by in vitro and ex vivo studies. J Pharm Pharmacogn Res. 2020;8(6):491– 500.
62. Chen Y, Liu T, Wang K, Hou C, Cai S, Huang Y et al. Baicalein inhibits Staphylococcus aureus biofilm formation and the Quorum sensing system in vitro. Plos One. 2016;11(4):e0153468. CrossRef
63. Musini A, Singh HN, Vulise J, Pammi SSS, Giri A. Quercetin’s antibiofilm effectiveness against drug resistant Staphylococcus aureus and its validation by in silico modelling. Res Microbiol, 2023;104091. CrossRef
64. Nag D, Dastidar DG, Chakrabarti G, Natural flavonoid morin showed anti-bacterial activity against Vibrio cholera after binding with cell division protein FtsA near ATP binding site. Biochim Biophys Acta Bioenerg. 2021;1865(8):129931. CrossRef
65. Dias MC, Pinto DCGA, Silva AS, Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26(17):5377. CrossRef
66. Gharbani P, Modeling and optimization of reactive yellow 145 dye removal process onto synthesized MnOX-CeO2 using response surface methodology. Colloids Surf. A: Physicochem. Eng. 2018;548:191–197. CrossRef