INTRODUCTION
Lung cancer and stomach cancer are two of the most common cancers in the world with the first [1] and the fourth [2] leading cause of cancer death, respectively. Recently, the applications of surgery, chemotherapy, and radiation therapy in the treatment of stomach/lung cancer are necessary. However, synthetic chemicals with high toxicity attack nonspecifically both cancer cells and normal ones, thus possessing numerous side effects and significantly affecting patients’ health [3,4]. Therefore, a novel therapeutic approach is necessary to circumvent the mentioned issues.
Curcumin, the main component of the rhizome of turmeric (Curcuma longa L., Zingiberaceae), has been well studied and considered as an anti-cancer agent for various kinds of cancers such as stomach, liver, lung, colon, breast, and skin cancer, as well as an active ingredient for anti-inflammation, antioxidation, antibacterial, antiviral, and antifungal [1,2,5–8]. Moreover, biological and clinical trials have proven that curcumin could safely and effectively treat cancers, especially stomach cancer [1,9,10]. Nevertheless, curcumin has not been widely used in oral drugs due to its low solubility in water and acidic pH, and its instability in alkaline environments [2,8,11–13]. Therefore, in our previous report, curcumin was employed in the curcumin-solid-dispersion (CSD) loaded floating tablets, which demonstrated perfect physicochemical properties such as short floating potential of 35 ± 1 seconds, long floating retention time of more than 8 hours, enhanced curcumin solubility to more than 200 times, outstanding stability for at least 2 years at room temperature, and reserved curcumin cytotoxicity on the N87 stomach cell lines with a better efficacy than the standard drug 5-fluorouracil (5-FU) [14].
Herein, this present study further evaluated the anti-cancer effects of the CSD-loaded floating tablets in an in-vivo setting in stomach and lung cancer mice models. First, the acute toxicity of the tablets was tested in healthy mice. Then, the cytotoxicity effects of the tablets were determined in tumor-bearing mice, induced by the 7,12-dimethyl benz[a]anthracene (DMBA) chemical. The mice were monitored for their vital signs, weights, and tumor characteristics (i.e., size, positions, macroscopic and microscopic images).
MATERIALS AND METHODS
Materials
Healthy Swiss albino mice (weight 30 to 60 g), 7–8 weeks old, were obtained from the Pasteur Institute in Ho Chi Minh City, Vietnam, and settled in appropriate laboratory conditions for a week before experimentations. Mice from different groups were housed in separate cages (max. 10 mice/cage) under room temperature and natural lighting conditions. Mice were fed a standard diet of 2.5/10 g body weight per day with free water intake. The cages were regularly cleaned throughout the experiment period.
Curcumin (purity of 95.5%) was imported from India. Xanthan gum, hydroxypropyl methylcellulose (HPMC) K15M, and poly(vinyl pyrrolidone) (PVP) K30 were purchased from the USA. Aerosil, magnesium stearate, NaHCO3, and citric acid were bought from China. Corn oil imported from Argentina. DMBA was purchased from the USA, and 5-FU (Ebewe® 500 mg/10 ml) was bought from Austria.
Preparations of CSD-loaded floating tablets
The CSD-loaded floating tablets were formulated following our previous report [14], with the optimal formula presented in Table 1. Briefly, the CSD was first fabricated by incorporating curcumin in a mixture of PVP K30 and tween 80, with a ratio of curcumin: PVP K30: Tween 80 of 1:4:0.22 w/w/w, by the solvent method [15,16]. Then, the CSD floating tablets were formulated using the simple wet granulation method, with slight modifications [17]. The tablets are composed of the CSD (active ingredient), xanthan gum and HPMC K15M (matrix generators), PVP K30 (binder), NaHCO3 and citric acid (gas-generating excipients), aerosil and magnesium stearate (glidant/lubricant), and lactose (diluent) (Table 1). The process began by homogeneous mixing the ingredients, followed by moistening and wet-granulating with PVP K30 ethanolic solution through a 2-mm sieve, drying at 50°C–60°C to moisture of ≤7%, and mixing with gas-generating excipients and glidant/lubricant. Finally, the granules were compressed on a rotary tablet press (Rimek, India) with a tablet weight of ~1,000 mg. The blank floating tablets were similarly prepared, without the addition of CSD.
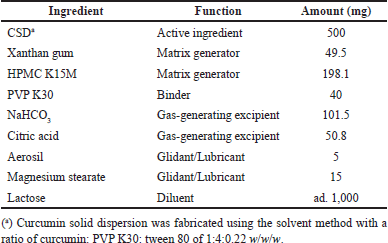 | Table 1. CSD loaded floating tablet formulation. [Click here to view] |
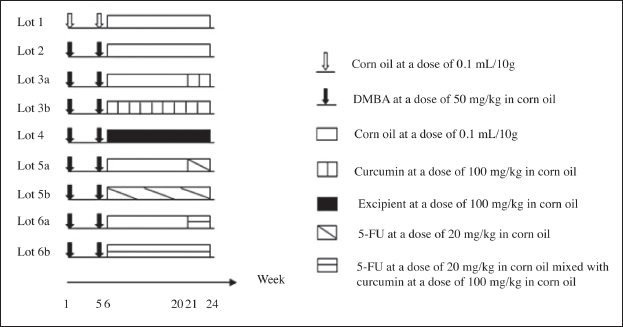 | Figure 1. In-vivo cytotoxicity tests in lung/stomach cancer mice models timeline. [Click here to view] |
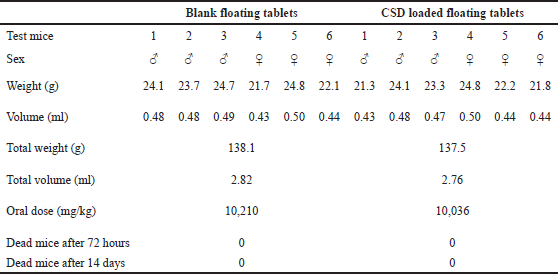 | Table 2. Acute toxicity test of the blank and CSD loaded floating tablets on experimental mice. [Click here to view] |
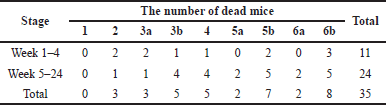 | Table 3. The number of dead mice in each experimental group during the anti-cancer testing period. [Click here to view] |
In-vivo acute toxicity test
Six Swiss albino mice (50% male, 50% female) were fasted for at least 12 hours before being given the maximum oral doses of the test samples (the blank floating tablets and the CSD-loaded floating tablets). The mice parameters of general movement, behavior, hair state, eating, urination, and the number of dead mice were monitored and recorded within 72 hours. After 72 hours, the mice with no abnormal signs were monitored for the next 14 days, followed by dissection to observe the macroscopic changes (if any) in vital organs, including the heart, lungs, intestines, liver, and kidneys.
After the test, the lethal dose (LD) was determined based on the number of dead mice. If 0% of mice died, the maximum dose (Dmax) would be determined. If the mortality rate was 100% or lower, the dose would be repetitively reduced to half-level of the initial dose until an LD50 was reached (i.e., a dose of which 50% of the mice died).
In-vivo cytotoxicity tests in lung/stomach cancer mice models
The experiments were carried out on 85 Swiss albino mice, which were randomly divided into 9 groups. Each group included 50% male and 50% female mice. The experimental process was conducted for 24 weeks following the timeline described in Figure 1 [18–20]. Briefly, mice in group 1 (physiologic control group) were taken corn oil, a medium that helps increase the DMBA absorption in the intestine, throughout the experimental process. Group 2 (disease control group) was fed with DMBA for the initial 5 weeks to induce cancerous tumors in the mice’s stomach and lungs. Groups 3a and b were designed to test the effects of CSD-loaded floating tablets from week 21 to week 6 of this study, respectively, which evaluated the products’ efficacy on these cancers at the early and late stages. The blank floating tablets were used in group 4 to assess whether excipients had any anti-cancer activity. Groups 5a and b (positive/reference control groups) were mice treated with 5-FU, a clinically used chemotherapy to treat stomach and lung cancers, from week 21 to 6, respectively. Finally, a combination of the experimental floating tablets and 5-FU was used in groups 6a and b from week 21 to 6, respectively, to evaluate the complementary anti-cancer effects of curcumin in the disease’s early and late stages.
At each predetermined time interval of the testing duration, mice were monitored for their vital signs, weights, and tumor characteristics (i.e., size, positions, macroscopic and microscopic images).
Weights
Mice were weighed at a fixed hour each week from the beginning to week 24. The mean weight of different groups was calculated and compared.
Tumor macroscopic evaluation
After the experimental duration (24 weeks), mice were killed after being anesthetized with iced CO2, and their abdominal cavities were observed macroscopically. The mice’s stomachs and lungs were separated and washed with 0.9% NaCl. The characteristics of color, surface states, and lesions/tumors appearance/numbers were noted. The newly formed tumors, if any, were fixed in a 10% formalin solution. After 48 hours, the number and volume of tumors in the lungs and stomachs were calculated in the following equations (1) to (4) [17,20–22].
(1)
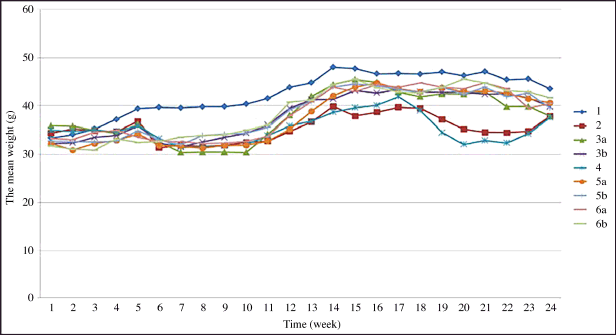 | Figure 2. Mice weight changes of different experimental groups during the anti-cancer testing period. [Click here to view] |
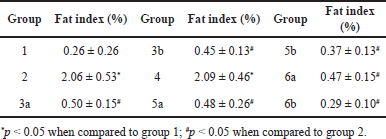 | Table 4. Fat index of different groups of mice from week 5 to 24. Results are expressed as mean ± SD. [Click here to view] |
(2)
The tumor volume (mm3) =1/2 × The largest tumor diameter ×The smallest tumor diameter2 (3)
(4)
Tumor microscopic evaluation
For the microscopic analysis, the mice’s stomachs and lungs were soaked in the 10% formalin solution, followed by staining with hematoxylin-eosin. Microscopic tumors were observed and assessed with optical microscopes in terms of cell morphology, dysplasia, and carcinoma [17,20–22].
Statistical analysis
The results were processed by Excel software, presented as mean ± SD, and evaluated for statistical significance using SPSS version 22.
RESULTS AND DISCUSSIONS
In-vivo acute toxicity test
To check the toxicity of the formulas and to determine the test dose, the acute toxicity test of the blank and CSD-loaded floating tablets was conducted on six mice (Table 2). The maximum dose levels of the blank and CSD-loaded floating tablets were 10.21 and 10.04 g/kg, respectively. At these doses, no dead mice were observed, and all mice were healthy with normal eating, drinking, urinating, and movement patterns, and had no abnormal signs within 72 hours after the test. Furthermore, 14 days after the test, mice dissections demonstrated no abnormalities in the heart, lungs, intestines, livers, and kidneys. Conclusively, the tablets, at a dose of as high as 10 g/kg, equivalent to curcumin amount, did not possess acute toxicity in the experimental mice.
In-vivo cytotoxicity tests in lung/stomach cancer mice models
Dead mice evaluation
First, the number of dead mice during the experiment was assessed and is shown in Table 3. For this, the initial number of mice was 85 and the total number of dead mice was 35, accounting for 41.18%. Regarding the timeline, the number of dead mice from week 1 to week 4 was 11 (12.94%), which was because some mice had not adapted to environmental conditions and were immunocompromised due to DMBA, and from week 5 to week 24 was 24 (28.24%), which was because of the suffocation when being taken the drug and impaired immunity with reduced weight.
Mice weight investigation
Second, the mice weight changes during the testing period were recorded (Fig. 2). In the beginning, the mean weights of these groups were not significantly different from each other (p > 0.05). After the testing period, the overall weights of all groups increased. From week 6 to week 8, the mice’s weight of groups 2, 3a, 4, 5a, 5b, and 6a decreased and was significantly less than that of group 1 (p < 0.05) because mice in these groups got immunocompromised by DMBA. In addition, although mice in groups 3b, 5b, and 6b were taking CSD-loaded floating tablets and 5-FU from week 6, the experimental time was short, thus, there were no significant differences in mice’s weight between these groups, similar to the report of Da et al. [23]. From week 12 to week 24, the weight gain in groups 3a, 3b, 5a, 5b, 6a, and 6b was not significantly different from that in group 1 because mice adapted to the high-fat diet in corn oil, and the immune system gradually recovered. Moreover, the mice’s weights increased nonlinearly between weeks, which could be due to the biological fluctuations of the mice. Lastly, a weak/inadequate correlation between the mice weight changes and tumor formation was noted (0 < r < 0.2), suggesting that the tumor/cancer formation and progression were not well connected to the mice weights.
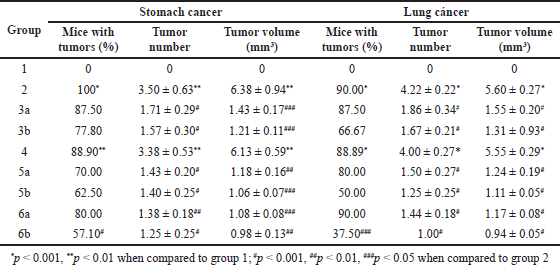 | Table 5. Macroscopic results of the mice’s stomach and lung from week 5 to 24. Results are expressed as mean ± SD. [Click here to view] |
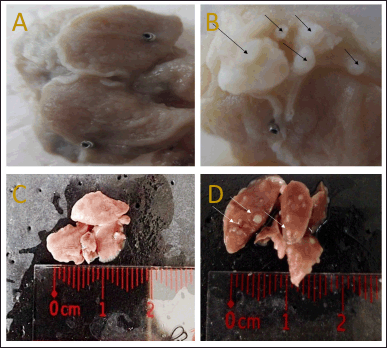 | Figure 3. Macroscopic analyses of (A) normal stomach, (B) cancerous stomach, (C) normal lung, and (D) cancerous lung. [Click here to view] |
Fat accumulation determination
Third, the fat accumulation of mice is expressed through the fat index (Table 4). Fat accumulation is the phenomenon of increased triglycerides and lipids because tumor cells synthesize these molecules by themselves and partly obtain them from their host [24]. Compared with group 1, mice in group 2 and group 4 possessed significantly higher fat accumulation (p < 0.05), indicating the tumor’s hyperactivity after using DMBA, in agreement with a previous study [24]. There was no statistical difference between group 2 and 4 (p > 0.05), suggesting that the floating tablet excipients did not affect the mice fat accumulation. Interestingly, when being treated with CSD-loaded floating tablets and/or 5-FU, the mice’s lipid levels reduced significantly (groups 3a, 3b, 5a, 5b, 6a, and 6b) compared with the disease control group (group 2) (p < 0.05), indicating the treatment efficacy. Curcumin has an anti-inflammatory effect on mice tumors, which helps reduce fat accumulation.
Macroscopic analyses
Fourthly, the macroscopic analyses were conducted and reported (Table 5 and Fig. 3). For both cancer types of stomach and lung, none of the mice in group 1 had tumors, and the percentages of tumors in group 2 and 4 were >85%. The results were similar to the study of Azuine and Bhide [18] and Huang et al. [20], which demonstrated that corn oil did not cause tumors and DMBA initiates tumor formations in mice with a percentage of approximately 100%. The difference between group 4 and 2 was not statistically significant (p > 0.05), showing that the floating tablet excipients had no effects on tumor formation and progression. Compared to group 2, in terms of the tumor percentage, the mean number of tumors, and the mean tumor volume, the treatment groups (3a, 3b, 5a, 5b, 6a, and 6b) possessed significantly lower values, indicating that the CSD loaded floating tablets and/or 5-FU could treat the stomach/lung cancer in mice, in agreement with previous studies [17,21,23,25]. Interestingly, as compared with groups 3 (CSD-loaded floating tablet treatment only) and 5 (5-FU treatment only), the combination treatment (group 6) yielded a better outcome, suggesting the complementary action of curcumin with a synthetic drug (5-FU) in stomach/lung cancer treatment. Finally, concerning two groups that were being treated from week 6 to week 21 (subgroups a and b), the tumor percentage, the mean number of tumors, and the mean tumor volume in the group being treated from week 21 were higher than that in the group being treated from week 6 (3a > 3b, 5a > 5b, and 6a > 6b). Conclusively, using curcumin and/or 5-FU at the early stage would bring better anti-tumor effects than treating at a late stage.
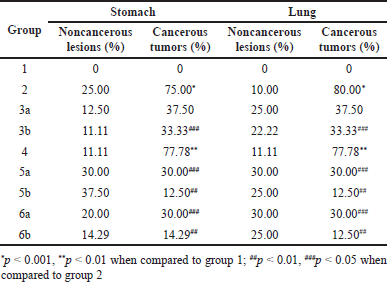 | Table 6. Microscopic results of the mice’s stomach and lung from week 5 to 24. Results are expressed as mean ± SD. [Click here to view] |
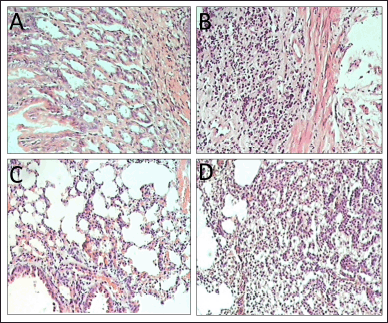 | Figure 4. Microscopic analyses of (A) normal stomach, (B) cancerous stomach, (C) normal lung, and (D) cancerous lung. [Click here to view] |
Microscopic analyses
Finally, the microscopic analyses were reported in Table 6 and Figure 4. For both stomach and lung cancers, all mice in group 1 did not have any lesions. On the other hand, group 2 possessed lesions, of which more than 70% of the lesions were cancerous, suggesting the DMBA could trigger cancers in mice. The noncancerous lesions were due to acute inflammation and congestion. The cancer lesions in group 4 were not significantly different compared to those in group 2 (p > 0.05), indicating the blank floating tablets had no cytotoxicity on the growth of cancer cells. Notably, the cancerous tumor numbers in group 3a were not different from that in group 2 (p > 0.05), whereas these numbers were significantly decreased in groups 3b, 5a, 5b, 6a, and 6b. This confirms the fact that curcumin could potentially inhibit the growth of cancer cells when used in the early stage, but might not in the late stage. Our results were similar to that of a previous report by Sintara et al. [26], which used a curcumin dose of 200 mg/kg in mice for 20 weeks in their study. The results determined that the cancer rate in the curcumin-treated group was reduced by 50% compared to that in the untreated group (100%). Furthermore, although the anti-cancer effects of curcumin (group 3) were lower than that of the standard drug 5-FU (group 5), it could well complement 5-FU to significantly enhance the drug efficacy (group 6).
CONCLUSION
This study investigated the stomach/lung anti-cancer effects of curcumin in a complete dosage form, the CSD-loaded floating tablets, in the in-vivo setting in mice. The results showed that the tablets could significantly inhibit the growth of stomach/lung cancer cells and reduce the cancer rate. In addition, the CSD-loaded floating tablets could potentiate the standard chemotherapeutic drug 5-FU efficacy in treating stomach/lung cancers, helping reduce the dose of 5-FU and limiting its toxicity in patients. Moreover, the study also proved that using curcumin in the early stages would help reduce the cancer rate better than using it in the late stages. In summary, the CSD-loaded floating tablets could be a novel dosage form for stomach/lung cancer treatments in the future.
ACKNOWLEDGMENT
The authors would like to thank Can Tho University and Can Tho University of Medicine and Pharmacy for supporting this research. The authors acknowledge the English editing service of Mr. Peter Barton, Naresuan University, Phitsanulok, Thailand.
AUTHOR CONTRIBUTIONS
Concept: DTMH, DTP; design: DTP; literature search: DTMH, DTP; experimental studies: DTMH, VDT, SP, DTP; data analysis: DTMH, SP, DTP; statistical analysis: VDT; manuscript preparation, manuscript editing and manuscript review: DTMH, VDT, SP, DTP.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work
ETHICAL APPROVALS
The research ethics were approved by the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam, code 1454/QD-DHYD-SDH.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. CrossRef
2. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–53.
3. Pham DT, Saelim N, Tiyaboonchai W. Paclitaxel loaded EDC-crosslinked fibroin nanoparticles: a potential approach for colon cancer treatment. Drug Deliv Transl Res. 2020;10(2):413–24. CrossRef
4. Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces. 2019;181:705–13. CrossRef
5. Lee WH, Loo CY, Bebawy M, Luk F, Mason R, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11(4):338–78. CrossRef
6. Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22(6C):4179–81.
7. Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. CrossRef
8. Prashar D, Khokra S, Purohit R, Sharma S. Curcumin: a potential bioactive agent. Res J Pharm Biol Chem Sci. 2011;2(4):44–52.
9. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98.
10. Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem. 2008;114(2):127–49. CrossRef
11. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. CrossRef
12. Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. CrossRef
13. Rajasekaran SA. Therapeutic potential of curcumin in gastrointestinal diseases. World J Gastrointest Pathophysiol. 2011;2(1):1–14. CrossRef
14. Huynh DTM, Tran VH, Le MNT, Huynh VH, Pham DT. Floating tablets incorporating curcumin solid dispersion as a potential pharmaceutical dosage form for stomach cancer treatment. J Appl Pharm Sci. 2023;13(4):240–50. CrossRef
15. Pan-On S, Dilokthornsakul P, Tiyaboonchai W. Trends in advanced oral drug delivery system for curcumin: a systematic review. J Control Release. 2022;348:335–45. CrossRef
16. Pan-On S, Rujivipat S, Ounaroon A, Tiyaboonchai W. Development and characterization of clay facial mask containing turmeric extract solid dispersion. Drug Dev Ind Pharm. 2018;44(4):590–7. CrossRef
17. Shishu, Gupta N, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin—“a technical note.” AAPS PharmSciTech. 2008;9(3):810–3. CrossRef
18. Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992;17(1):77–83. CrossRef
19. Lu Q, Huang Y, Li M, Zuo B, Lu S, Wang J, et al. Silk fibroin electrogelation mechanisms. Acta Biomater. 2011;7(6):2394–400. CrossRef
20. Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54(22):5841–7.
21. Goindi S, Mann K, Aggarwal N. Gastro-retentive floating beads of curcumin β-cyclodextrin complex to treat stomach tumors. Altern Med Stud. 2011;1(1):12. CrossRef
22. Singh SV, Hu X, Srivastava SK, Singh M, Xia H, Orchard JL, et al. Mechanism of inhibition of benzo[a]pyrene-induced forestomach cancer in mice by dietary curcumin. Carcinogenesis. 1998;19(8):1357–60. CrossRef
23. Da W, Zhu J, Wang L, Sun Q. Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumour Biol. 2015;36(7):5215–23. CrossRef
24. Spector AA. The importance of free fatty acid in tumor nutrition. Cancer Res. 1967;27(9):1580–6.
25. Liu X, Sun K, Song A, Zhang X, Zhang X, He X. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J Surg Oncol. 2014;12:389. CrossRef
26. Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N. Curcumin attenuates gastric cancer induced by N-methyl-N-nitrosourea and saturated sodium chloride in rats. J Biomed Biotechnol. 2012;2012:915380. CrossRef