INTRODUCTION
Due to its potential effects on health, oxidative stress (OS) has recently attracted a lot of attention. Reactive oxygen species (ROS) concentrations perpetually and continuously rise in oxidative stress (OS) [1]. Many diseases, including cancer, neurological disorders, hypertension, arteriosclerosis, diabetes, and infertility, are associated with OS [2,3]. Male infertility has been identified as one of the end products of OS in about 50% of the identified cases [4]. The reproductive system in males, especially the tissues in the testes, is prone to attacks from the invasion of OS because of the increased level of cell division that occurs as well as the demand placed on oxygen demand from the mitochondria [5–7]. The exposure of the sperm cells and the tissues in the testes to oxidative attack is made possible because of the increased content of unsaturated fatty acid, especially when the integrity of the antioxidant is been put at risk [8–11]. Excessive Fe2+ in the system leads to testicular damage and hence reduced fertility rate [12]. Numerous animal studies have been reported involving elevated Fe2+ levels and testicular toxicity which has led to OS in the testes of the animals [12–15]. The morphological metamorphosis that occurs in the epididymis and sperm cells arises from the interaction that occurs between the iron and proteins, lipids as well as molecules in the DNA which, most times, results in infertility [16,17].
Plants have been identified and proven to be rich sources of phytochemicals, which are rich sources of antioxidants, preventing OS produced from exposure to Fe2+ [12]. The interest in herbal remedies has unexpectedly resurfaced as a result of the drive to comprehend and record the knowledge of ancient therapeutic systems [18,19]. Recently, due to some circumstances, interest in the study of natural product chemistry has increased. Only through studying the pharmacology of secondary metabolites from medicinal plants can the many therapeutic demands for bioactive molecules with few or no side effects be addressed [20]. Therefore, there is a pressing need to improve the methods for detecting biologically active natural products as well as those for isolating, purifying, characterizing, and altering the structural makeup of these active components [18,21].
The principal antioxidants found in medicinal plants are phenolics and flavonoids [3,22,23]. The diphenyl propane moiety, which consists of two distinctive aromatic rings linked by three carbon atoms and typically forms an oxygenated heterocycle, is responsible for their collective structural function [24–26]. Anthocyanidins, isoflavones, flavanones, and flavonols (or catechins) are the different types of flavonoids depending on the type of heterocycle involved [27,28]. Benzoic acid and cinnamic acid analogs are examples of phenolic acids [29–31]. Cinnamic acid and its derivatives are known for their various benefits because of their application in the management of different forms of diseases [32]. There are various derivatives of cinnamic acid, having a chemical composition of an acrylic acid group on an aromatic carboxylic acid [33–36]. Sodium 3-phenylpropanoate (KAD 1) a derivative of cinnamic acid has also been synthesized and it is important to investigate its effects in iron-induced testicular injury in an ex vivo study.
MATERIALS AND METHODS
Materials and reagents chemicals
KAD 1 was obtained from the derivatization of cinnamic acid (CAS 140-10-3; Merck, Germany) from our previous study [37], while quercetin (CAS 117-39-5) was obtained from Santa Cruz Biotechnology, Heidelberg, Germany. All substances, including solvents, were of analytical grade. A spectrophotometer (Spectra Max Plus, Molecular Devices, CA, USA) was used to measure all absorbances.
In vitro antioxidant activity
KAD 1’s iron chelating activity was assessed using the standard procedure [38], and antioxidant potential via l, l-diphenyl-2-picrylhydrazyl (DPPH) was evaluated following the procedure described by Ruan et al. [39]. The ferric-reducing antioxidant power (FRAP) experiment was done following the guidelines provided by Benzie and Strain [40].
Ex vivo studies
Experimental rats and organ preparation
We obtained healthy male Wistar rats (10–12 weeks old) from the Animal House, Bowen University, Iwo, Nigeria, weighing 250–300 g each. At room temperature (20°C–25°C), all animals were kept in cages with a 12/12–hour light-dark schedule. Softwood shavings were used as bedding inside the cages to absorb animal waste, and it was replaced frequently. Throughout this study, they had unrestricted access to water and constant food pellets (provided by Ladokun Feeds Nig. Ltd.). Before beginning the experimental methods, animals underwent a minimum of 1 week of acclimatization. The Guide for the Care and Use of Laboratory Animals’ guidelines were followed when conducting the exercise. All relevant national, institutional, and/or foreign regulations for the handling and use of animals were adhered to. In addition, the experiment was approved by the Institutional animal Ethics Committee at Bowen University, Iwo (BUI/BCH/2022/0002). Ten rats were euthanized with halothane, as described by Slott et al. [41], after being fasted the night before, and their testicles were then excised and homogenized in 1% Triton X-100 in 50 mM phosphate buffer. At a temperature of 40°C, the homogenate was centrifuged at 15,000 rpm. The supernatants were obtained in plain tubes for ex vivo research and were stored at −40°C.
Testicular injury induction
The technique described by Ojo et al. [35,36] was used on injured testicles ex vivo with a few minor modifications. In essence, 100 μl of 0.1 mM FeSO4 and varied KAD 1 concentrations (30, 60, 120, and 240 g/ml) were added to 200 μl of the organ supernatant. The samples were used for biochemical examinations after being incubated for 30 minutes at 37°C. The positive control used reaction mixtures with only the organ supernatant, and the negative control used reaction mixtures with only the tissue supernatant and FeSO4.
Measurement of antioxidant activities
Level of glutathione (GSH)
600 ml of the tissue lysates were deproteinized with 10% trichloroacetic acid, according to Salau et al. [42]. The mixture was then centrifuged for 10 minutes at 3,500 rpm. 100 ml of the Ellman reagent and 500 ml of the sample were put into a clean test tube. After incubation for 5 minutes at 25°C, the absorbance was measured at 415 nm. The GSH was used as a reference.
Catalase (CAT) activity
The CAT activity analysis for KAD 1 was evaluated using the method described by Ojo et al. [35,36] with a minor modification. Several KAD 1 concentrations were present in 20 ml of tissue samples, and 780 μl of 50 mM phosphate buffer was added to those samples. Hence, the absorbance was measured at 240 nm for 3 minutes at intervals of 1 minute after adding 300 μl of 2 M H2O2.
Level of lipid peroxidation
KAD 1’s ability to prevent lipid peroxidation was evaluated using the procedure outlined by Ojo et al. [35,36]. 100 μl of tissue lysates with variable concentrations of KAD 1 were introduced progressively to 375 μl of 20% acetic acid, 1,000 μl of 0.25% thiobarbituric acid, and 100 μl of 8.1% sodium dodecyl sulphate (SDS). The reaction mixture was warmed up for 60 minutes at 95°C (in a water bath). After the mixture had cooled to room temperature, the absorbance at 532 nm was measured.
Purinergic activity
Na/K+ ATPase enzyme activity
To measure the Na+/K+ ATPase activity, the procedure as described in Erukainure et al. [43] was slightly modified. In 1.3 ml of 0.1 M Tris-HCl buffer, 200 μl of the organ lysate with various KAD 1 concentrations, 200 μl of 5 mM KCl, and 40 ml of 50 mM ATP were added. After being vigorously agitated for 30 minutes at 37°C, the reaction mixture was added to 1 μl of distilled water, as well as 1 ml of 1.25% ammonium molybdate. After that, 1 ml of 9% ascorbic acid was added to the solution, which was then left at room temperature for 30 minutes. At 660 nm, the absorbance was measured.
E-NTPDase enzyme activity
Ojo et al. [35,36] procedure, with some minor modifications, was used. 400 μl of a reaction mixture made up of 1.5 mM CaCl2, 5 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 45 mM Tris-HCl, 225 mM sucrose, and 10 mM glucose was added to 40 μl of tissue lysates containing different doses of KAD 1. After that, the mixtures were incubated for a further 10 minutes at 37°C. The solution was then kept at 37°C using an electric-powered shaker after 40 μl of 50 mM adenosine triphposphate (ATP) was added. 400 μl of 10% trichloroacetic acid (TCA) was added to the mixture to halt the process. After an ice-based incubation period of 10 minutes, the absorbance at 600 nm was measured.
Data analysis
Software called Graphpad Prism 9.0.1 was used to analyze the data. The descriptive data were represented by the mean standard deviation (±SD). One-way ANOVA and Tukey’s post hoc analysis, at a significance level of p < 0.05 was used to compare the mean.
RESULTS
Antioxidant activities
Figure 1 shows the DPPH scavenging radical ability of KAD 1. It is seen that the scavenging property increases significantly (p < 0.05) as the concentration increases when compared with the standard quercetin, which also had the same trend. KAD 1 displayed the capacity to mop up DPPH radicals (Figure 1), while Figure 2 displays the FRAP of KAD 1 and it was observed that the compound possesses a certain level of FRAP potential in a dose-dependent manner as compared to the standard that shows a higher quality in a dose-dependent mode significantly. From the highlight of KAD 1 Fe2+ chelating potential displayed in Figure 3, a trend was observed in a concentration-dependent mode significantly when compared to the standard control, quercetin. The reduced GSH level is shown in Figure 4. The antioxidant CAT result is displayed in Figure 5 with the 30 mg/ml group having the highest CAT potential when compared to the other groups. Figure 6 showed that lipid peroxidation was induced in negative control with Fe2+. Furthermore, the level of malondialdehyde (MDA) in the testicular toxicity damage with KAD 1 reduced the lipid peroxidation activity considerably in a dose-dependent mode.
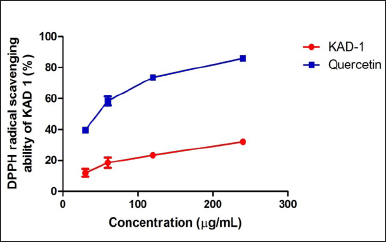 | Figure 1. DPPH scavenging ability of KAD 1. Data are expressed as mean ± SD (n = 3). [Click here to view] |
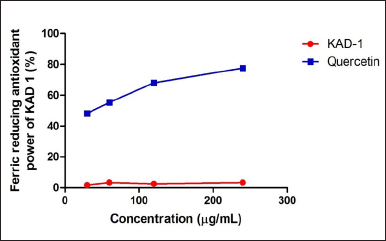 | Figure 2. KAD 1 impact on FRAP. Data are expressed as mean ± SD (n = 3). [Click here to view] |
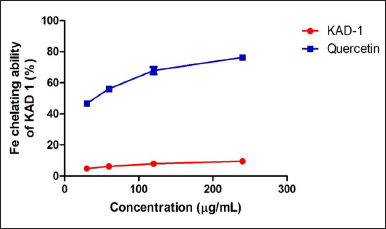 | Figure 3. Iron chelating ability of KAD 1. Data are expressed as mean ± SD (n = 3). [Click here to view] |
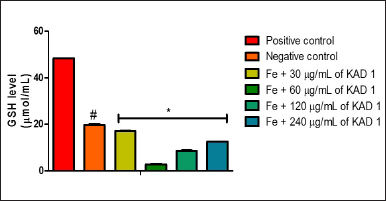 | Figure 4. KAD 1’s impact on GSH levels in iron-mediated oxidative testicular damage. Data are expressed as mean ±SD (n = 3), while * and # are statistically significant (p < 0.05) when compared to positive and negative controls, respectively. [Click here to view] |
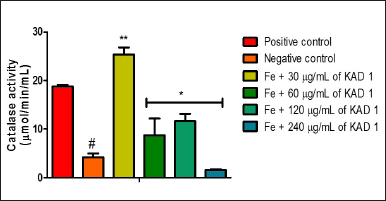 | Figure 5. KAD 1’s impact on CAT enzyme activity in iron-mediated oxidative testicular injury. Data are expressed as mean ±SD (n = 3), while *, **, and # are statistically significant (* # at p < 0.05 and ** at p < 0.01) when compared to positive and negative controls, respectively. [Click here to view] |
Purinergic function
The impact of KAD 1 on ATPase activity is represented in Figure 7. This outcome reveals that the treated groups differ significantly (p < 0.05) from the negative control. The difference observed is the reduction in activity as the concentration of KAD 1 increases. ENTPDase activity is shown in Figure 8. The activity is shown to increase as compared to the negative control. Nevertheless, the group treated with 120 mg/ml shows to have the highest activity compared to other treatment groups.
DISCUSSION
Excessive generation of ROS is a major contributor to man sterility, and prostrate impotence [44]. Previous studies and research reported the importance of phytochemicals that act as antioxidants, which are lethal to the toxic ROS in the male reproductive organ [45–47]. Fe2+ triggers the production of radicals (such as hydroxyl and hydroperoxyl) through the contact it has with H2O2 that is manufactured in the mitochondria of the Fenton reaction respiratory pathway. The occurrence of these dangerous lethal chemicals in the cells could bring about a series of reactions that would then produce more ROS in the system [48]. Thereafter the increased level of ROS tends to endanger the antioxidant capacity of the system which ultimately leads to the production of radicals [49,50].
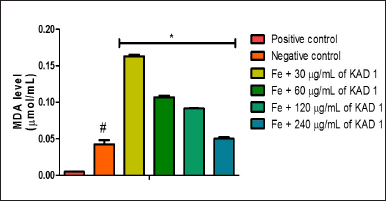 | Figure 6. Impact of KAD 1 on MDA concentration in iron-induced testicular oxidative injury. Data are expressed as mean ±SD (n = 3), while * and # are statistically significant (p < 0.05) when compared to positive and negative controls, respectively. [Click here to view] |
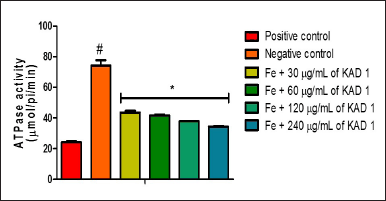 | Figure 7. Impact of KAD 1 on ATPase activity in iron-mediated oxidative testicular injury. Data are expressed as mean ±SD (n = 3), while * and # are statistically significant (p < 0.05) when compared to positive and negative controls, respectively. [Click here to view] |
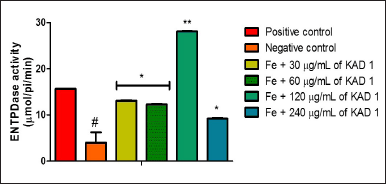 | Figure 8. Impact of KAD 1 on ENDTPase activity in iron-mediated oxidative testicular damage. Data are expressed as mean ±SD (n = 3), while * and # are statistically significant (* # at p < 0.05 and ** at p < 0.01) when compared to positive and negative controls, respectively. [Click here to view] |
KAD 1 showed its antioxidant potential, displaying its potential to scavenge free radicals produced in the system. FRAP as well as Fe chelating was also measured in KAD 1 and found that it can reduce ferric ions. Previous reports have stated the danger observed in the testes after exposure to Fe2+, which has led to the continuous reduction of CAT and GSH. Furthermore, exposure to Fe2+ also leads to lipid peroxidation, causing damage to the testicles [51,52]. The results obtained from this research show a depleted CAT and GSH in the negative control, which suggests that OS has occurred in the testes and this could be ascribed to the iron induction that took place through the Fenton, as well as Haber Weiss reactions [53]. Increased levels of MDA observed in the negative control when compared to the positive control, and treated groups could be a pointer to the fact that lipid peroxidation has occurred in the testicular tissues. The MDA level in treated groups reduces as the concentration of KAD 1 increases, which also suggests the potency of KAD 1 on the testicular tissue. OS in the testes is injurious to fertility in men [35,36].
Purinergic enzymes are vital in the secretion of fluids and protein in the testicles [12,54]. From the result obtained from ATPase activity, the untreated group experienced an increased activity as against the control and treated groups. The elevated levels signify that there could be an imbalance of the acid/base content in the testicular tissue owning to the attack that reduced the ATP levels [35,36]. The ATPases that are present on the surface of the membrane in the testes are responsible for the creation of the ideal pH condition, necessary for smooth sperm cell maturation as well as storage [12]. In cases of a prostrate, an increased level of ATPase is known to boost motility, as well as an invasion [55]. Additionally, the survival of the sperm cells as well as their functionality critically depends on the maintenance of the pH range [56]. Furthermore, the KAD 1 potential to reverse this action could suggest the potential of the KAD 1. The reduction in the activity of ENTDPase following treatment with KAD 1 indicates that there could be increased metabolism of glucose in the tissues of the testes, resulting in an elevated level of ATP in the testes observed in a similar study [12].
Although in antimicrobial studies, metal complexes displayed better activity than the starting materials. Recently, alkali earth metal’s complexes of a polyphenolic compound, cichoric acid, possessed significant antimicrobial activity than free compound [57,58]. However, their biological activity is mostly affected by the chelation process [59]. This is the first time investigating the potential of KAD 1 in ameliorating iron-induced testicular injury. Although we did not investigate the effect of chelation on KAD 1 activity on iron-induced testicular toxicity, the chelating process might be a factor responsible for the poor activity of KAD 1 at 60 mg/ml and above.
CONCLUSION
Overall, the results of this study point to a potential application of KAD 1 in the prevention of OS testis injury. The ability of KAD 1 to regulate nucleotide hydrolysis and reduce OS suggests that it has the potential to protect against oxidative testis toxicity. Thus, KAD 1 may be a suitable potent modality, which can help treat testicular injury.
AUTHOR CONTRIBUTIONS
Each author gave their approval for the final version to be published, agreed to submit the paper to the specified journal, and made substantial contributions to the conceptualization, design, data collecting, analysis, and interpretation of the research. Each author promised to be accountable for every aspect of the work and contributed to its creation or critically revised the book for key intellectual substance. All of the writers are eligible to be authors per the requirements/guidelines of the International Council of Medical Journal Editors (ICMJE).
FINANCIAL SUPPORT
This research received no external funding.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
Rats were cared for in compliance with procedures endorsed by the institutional animal ethics committee at Bowen University, Iwo (BUI). Before the start of the trial, the approval was obtained (approval number: BUI/BCH/2022/0002).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. van Raamsdonk JM, Vega IE, Brundin P. OS in neurodegenerative disease: causation or association? Oncotarget. 2017;8:10777–8. CrossRef
2. Bin-Jumah MN, Nadeem MS, Gilani SJ, Mubeen B, Ullah I, Alzarea SI, et al. Lycopene: a natural arsenal in the war against OS and cardiovascular diseases. Antioxidants. 2022;11(2):232. CrossRef
3. Ogunlakin AD, Sonibare MA, Yeye OE, Gyebi GA, Ayokunle DI, Arigbede OE, et al. Isolation and characterization of novel hydroxyflavone from Kigelia africana (Lam.) Benth. fruit ethyl acetate fraction against CHO 1 and HeLa cancer cell lines: in vitro and in silico studies. J Mol Struct. 2023;2023:135180. CrossRef
4. Berby B, Bichara C, Rives-Feraille A, Jumeau F, Pizio PD, Sétif V, et al. OS is associated with telomere interaction impairment and chromatin condensation defects in spermatozoa of infertile males. Antioxidants. 2021;10:593. CrossRef
5. He Y, Wang J, Ren J, Zhao Y, Chen J, Chen X. Effect of COVID-19 on male reproductive system—a systematic review. Front Endocrinol. 2021;12:677701. CrossRef
6. Unsal V, Cicek M, Sabancilar ?. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Environ Health Rev. 2021;36:279–95. CrossRef
7. Sadeghi N, Tavalaee M, Shahverdi A, Sengupta P, Leisegang K, Saleh R, et al. Vulnerability of the male reproductive system to SARS-CoV-2 invasion: potential role for the endoplasmic reticulum chaperone Grp78/HSPA5/BiP. Cell J. 2022;24:427–33. CrossRef
8. Ijaz MU, Tahir A, Samad A, Anwar H. Nobiletin ameliorates nonylphenol-induced testicular damage by improving biochemical, steroidogenic, hormonal, spermatogenic, apoptotic and histological profile. Hum Exp Toxicol. 2021;40:403–16.
9. Takeshima T, Usui K, Mori K, Asai T, Yasuda K, Kuroda S, et al. OS and male infertility. Reprod Med Biol. 2021;20:41–52. CrossRef
10. De Luca MN, Colone M, Gambioli R, Stringaro A, Unfer V. OS and male fertility: role of antioxidants and inositols. Antioxidants. 2021;10:1283. CrossRef
11. Evans EP, Scholten JT, Mzyk A, Reyes-San-Martin C, Llumbet AE, Hamoh T, et al. Male subfertility and OS. Redox Biol. 2021;46:102071. CrossRef
12. Olofinsan KA, Salau VF, Erukainure OL, Islam MS. Ocimum tenuiflorum mitigates iron-induced testicular toxicity via modulation of redox imbalance, cholinergic and purinergic dysfunctions, and glucose metabolizing enzymes activities. Andrologia. 2021;53:e14179. CrossRef
13. Jurado-Campos A, Soria-Meneses PJ, Sánchez-Rubio F, Niza E, Bravo I, Alonso-Moreno C, et al. Vitamin E delivery systems increase resistance to OS in red deer sperm cells: hydrogel and nanoemulsion carriers. Antioxidants. 2021;10:1780. CrossRef
14. Li Y, Zhu Z, Cui H, Ding K, Zhao Y, Ma X, et al. Effect of zearalenone-induced ferroptosis on mice spermatogenesis. Animals. 2022;12:3026. CrossRef
15. Lin J, Zuo C, Liang T, Huang Y, Kang P, Xiao K, et al. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022;13:11532–42. CrossRef
16. Yesil S, Sungu N, Kilicarslan A, Kuskonmaz SM, Kara H, Kucuk A, et al. Exenatide reduces OS and cell death in testis in iron overload rat model. Exp Ther Med. 2018;16:4349–56. CrossRef
17. Hamilton LE, Oko R, Miranda-Vizuete A, Sutovsky P. Sperm redox system equilibrium: implications for fertilization and male fertility. Adv Exp Med Biol. 2022;1358:345–67. CrossRef
18. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–614. CrossRef
19. Ogunlakin AD, Sonibare MA. Ethnobotanical survey of medicinal plants used as remedy for female infertility and menstrual disorder in southwestern Nigeria. Nig J Pharm Res. 2019;15(2):205–17. CrossRef
20. Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020;19(5):1199–209. CrossRef
21. Barbosa JR, de Carvalho Junior RN. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: biologically active potential that can be exploited against COVID-19. Trends Food Sci Technol. 2021;108:223–35. CrossRef
22. Farzaneh V, Carvalho IS. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind Crops Prod. 2015;65:247–58. CrossRef
23. Thiombiano HM, Bangou MJ, Nacoulma AP, Ouoba B, Sawadogo M, Lema A, et al. Ethnobotanical survey on medicinal plants used in Burkina Faso in the treatment of breast cancer, phytochemistry and antioxidant activities: Euphorbia poissonii Pax and Flueggea virosa (Willd.) Voigt. (Euphorbiaceae). Afr J Pharm Biol Med Sci. 2022;5:1–6. CrossRef
24. Gulcin ?. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94:651–715. CrossRef
25. Sharma A, Sharma S, Kumar A, Kumar V, Sharma AK. Plant secondary metabolites: an introduction of their chemistry and biological significance with physicochemical aspect. In Plant secondary metabolites: physico-chemical properties and therapeutic applications. Singapore: Springer Nature Singapore; 2022. pp 1–45. CrossRef
26. Nishal S, Phaugat P, Bazaad J, Dhaka R, Khatkar S, Khatkar A, et al. A concise review of common plant-derived compounds as a potential therapy for Alzheimer’s disease and Parkinson’s disease: insight into structure-activity-relationship. CNS Neurol Disord Drug Targets. 2022;2022:35702799. CrossRef
27. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, et al. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33:2221–43. CrossRef
28. Dias MC, Pinto DC, Silva AM. Plant flavonoids: chemical characteristics and biological activity. Molecules. 2021;26:5377. CrossRef
29. Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in food: cancer prevention and apoptosis induction. Curr Med Chem. 2018;25(36):4740–57. CrossRef
30. Ogunlakin AD, Sonibare MA, Jabeen A, Shah SF, Shaheen F. Antioxidant and anti-proliferative studies on Kigelia africana (Lam.) Benth. and its constituents. Trop J Nat Prod Res. 2021;5(3):570–5. CrossRef
31. Memete AR, Timar AV, Vuscan AN, Venter AC, Vicas SI. Phytochemical composition of different botanical parts of Morus species, health benefits and application in food industry. Plants. 2022;11(2):152. CrossRef
32. B?aszczyk N, Rosiak A, Ka?u?na-Czapli?ska J. The potential role of cinnamon in human health. Forests. 2021;12(5):648. CrossRef
33. Ruwizhi N, Aderibigbe BA. Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci. 2020;21(16):5712. CrossRef
34. Savych A, Marchyshyn S, Kyryliv M, Bekus I. Cinnamic acid and its derivatives in the herbal mixtures and their antidiabetic activity. Farmacia. 2021;69(3):595–601. CrossRef
35. Ojo AB, Gyebi GA, Alabi O, Iyobhebhe M, Kayode AB, Nwonuma CO, et al. Syzygium aromaticum (L.) Merr. & LM Perry mitigates iron-mediated oxidative brain injury via in vitro, ex vivo, and in silico approaches. J Mol Struct. 2022;1268:133675. CrossRef.
36. Ojo OA, Ogunlakin AD, Iyobhebhe M, Olowosoke CB, Taiwo OA, Akinola A, et al. Computer aided and experimental study of cinnamic acid analog for OS treatment. The therapeutic validations. Inform Med Unlocked. 2022;35:101137. CrossRef
37. Ogunlakin AD, Sonibare MA, Yeye OE, Jabeen A, Shah SF, Ojo OA, et al. Design, synthesis, and characterization of cinnamic acid derivatives with two novel acrylohydrazones on HeLa and CHO-1 cancer cell lines: the experimental and computational perspective. Chem Afr. 2023;2023:1–22. CrossRef
38. Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. CrossRef
39. Ruan ZP, Zhang LL, Lin YM. Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules. 2008;13(10):2545–56. CrossRef
40. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6. CrossRef
41. Slott VL, Linder RE, Dyer CJ. Method of euthanasia does not affect sperm motility in the laboratory rat. Reprod Toxicol. 1994;8:371–4. CrossRef
42. Salau VF, Erukainure OL, Islam M. Caffeic acid protects against iron-induced cardiotoxicity by suppressing angiotensin-converting enzyme activity and modulating lipid spectrum, gluconeogenesis and nucleotide hydrolyzing enzyme activities. Biol Trace Elem Res. 2021;199(3):1052–61. CrossRef
43. Erukainure OL, Mopuri R, Oyebode OA, Koorbanally NA, Islam MS. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed Pharmacother. 2017;96:37–47. CrossRef
44. Ritchie C, Ko EY. OS in the pathophysiology of male infertility. Andrologia. 2021;53(1):e13581. CrossRef
45. Gad FA, Farouk SM, Emam MA. Antiapoptotic and antioxidant capacity of phytochemicals from Roselle (Hibiscus sabdariffa) and their potential effects on monosodium glutamate-induced testicular damage in rat. Environ Sci Pollut Res. 2021;28(2):2379–90. CrossRef
46. Erukainure OL, Atolani O, Banerjee P, Abel R, Pooe OJ, Adeyemi OS, et al. Oxidative testicular injury: effect of l-leucine on redox, cholinergic and purinergic dysfunctions, and dysregulated metabolic pathways. Amino Acids. 2021;53(3):359–80. CrossRef
47. Owumi SE, Irozuru CE, Arunsi UO, Faleke HO, Oyelere AK. Caffeic acid mitigates aflatoxin B1-mediated toxicity in the male rat reproductive system by modulating inflammatory and apoptotic responses, testicular function, and the redox-regulatory systems. J Food Biochem. 2022;46(5):e14090. CrossRef
48. Pignatello JJ, Oliveros E, MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol. 2006;36(1):1–84. CrossRef
49. Pereira SC, Oliveira PF, Oliveira SR, Pereira MD, Alves MG. Impact of environmental and lifestyle use of chromium on male fertility: focus on antioxidant activity and OS. Antioxidants. 2021;10(9):1365. CrossRef
50. Liu Y, Xiao Z, Chen F, Yue L, Zou H, Lyu J, et al. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: trends, meta-analysis, and prospect. Sci Total Environ. 2021;780:146578. CrossRef
51. Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27(3):450–2. CrossRef
52. Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of OS during diabetes mellitus. J Biomark. 2013;2013:378790. CrossRef
53. Das TK, Wati MR, Fatima-Shad K. OS gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch Neurosci. 2015;2(2):e60038. CrossRef
54. Gelain DP, de Souza LF, Bernard EA. Extracellular purines from cells of seminiferous tubules. Mol Cell Biochem. 2003;245(1):1–9. CrossRef
55. Michel V, Licon-Munoz Y, Trujillo K, Bisoffi M, Parra KJ. Inhibitors of vacuolar ATPase proton pumps inhibit human prostate cancer cell invasion and prostate-specific antigen expression and secretion. Int J Cancer. 2013;132(2):E1–10. CrossRef
56. Nishigaki T, José O, González-Cota AL, Romero F, Treviño CL, Darszon A. Intracellular pH in sperm physiology. Biochem Biophys Res Commun. 2014;450(3):1149–58. CrossRef
57. Anitha C, Sumathi S, Tharmaraj P, Sheela CD. Synthesis, characterization, and biological activity of some transition metal complexes derived from novel hydrazone azo schiff base ligand. Int J Inorg Chem. 2011;2011:493942. CrossRef
58. Jablonska-Trypuc A, Wydro U, Wolejko E, Swiderski G, Lewandowski W. Biological activity of new cichoric acid–metal complexes in bacterial strains, yeast-like fungi, and human cell cultures in vitro. Nutrients. 2020;12(1):154. CrossRef
59. Obaleye JA, Adediji JF, Adebayo MA. Synthesis and biological activities on metal complexes of 2, 5-diamino-1, 3, 4-thiadiazole derived from semicarbazide hydrochloride. Molecules. 2011;16(7):5861–74. CrossRef