INTRODUCTION
Herbal medicines, medicines with active ingredients made from plant parts such as roots, leaves, and flowers, have demonstrated their outstanding beneficial activities in various diseases (Huynh et al., 2022; Nguyen et al., 2021b; Pham et al., 2022; Tran et al., 2022). Among numerous herbs, curcumin, a compound commonly found in turmeric (Curcuma longa L., Zingiberaceae), has been extensively researched due to its potential multi-purpose therapeutic actions (Fuloria et al., 2022; Hewlings and Kalman, 2017). Specifically, curcumin is a prospective chemotherapeutic agent for stomach cancer, as it has been proven both effective in inhibiting tumor initiation, promotion, and metastasis and safe, with no dose-limiting toxicity in clinical trials at doses up to 10 g/day (Aggarwal et al., 2003; Pari et al., 2008). Despite demonstrating much potential, no clinical trial has been reported on curcumin for stomach cancer treatment, to the best of our knowledge. Only one related study has been conducted on curcumin’s ability in preventing gastric cancer (Clinical Trials, n.d.). This fact could be attributed to the inherent drawbacks of unmodified curcumin as an oral drug. For instance, curcumin possesses a very low water solubility of 0.6 μg/ml (Patel et al., 2009). Additionally, curcumin in conventional oral dosage forms is generally released in the intestine and is broken down by intestinal enzymes and alkaline pH (Prashar et al., 2011). Moreover, orally absorbed curcumin is rapidly metabolized in the liver by the glucuronidation pathway (Aggarwal and Sung, 2009). Consequently, the curcumin oral bioavailability in conventional dosage forms such as traditional tablets is unacceptable (i.e., for tablet, the oral bioavailability of curcumin is only 1%, and the maximum plasma concentration is 0.06 ± 0.01 μg/ml, compared to the intravenous route, with a concentration of 0.36 ± 0.05 μg/ml) (Liu et al., 2016; Pouton, 2006). Therefore, further research to solve these issues is necessary.
The solubility and dissolution rate of a drug substance are important factors determining the extent and rate of drug release and absorption from the oral administration route (Papich and Martinez, 2015). Thus, to improve curcumin oral bioavailability, these two factors must be critically considered. For the solubility enhancement, formulation methods such as creating a curcumin solid dispersion (CSD) to reduce the drug molecular sizes and alter its polymorph are beneficial (Pan-On et al., 2018, 2022; Phothi et al., 2022). Solid dispersion, defined as a homogeneous mixture of one or more active compounds in an appropriate inert carrier or matrix in a solid state, is one of the most widely recognized strategies to increase the poorly water-soluble drug solubility and release rate (Alshehri et al., 2020; Vo et al., 2013). For absorption enhancement, it is necessary to increase the curcumin residence time in the gastrointestinal tract, as well as to avoid curcumin degradation by the alkaline pH of the small intestine environment. To this end, the gastric drug delivery systems prove their effectiveness. The gastric drug delivery system is a method for sustaining the drug release while the formulations/active ingredients retain/float in the gastrointestinal tract, without being affected by the rate of gastric emptying, thereby preventing curcumin from being broken down by the small intestine’s alkaline pH (Wook Huh et al., 2021). These systems include a mucoadhesive system, floating system, high-density system, magnetic system, and swelling system (Tripathi et al., 2019). Among them, the floating systems (i.e., floating tablets) that possess a density lower than the gastric juice density (1.004 g/cm3) have gained much attention.
With the main aim of increasing the oral bioavailability of curcumin, this work employed both the aforementioned approaches by incorporating the CSD in the floating tablet dosage form for the application of gastric cancer treatment. The CSD was first formulated to enhance curcumin solubility. Then, the CSD was incorporated into the floating tablet formula to bypass the alkaline pH in the intestine. The physicochemical properties of the product were fully evaluated, and its pharmacological effects were tested in the in-vitro cytotoxic model on the stomach cancer cell line N87.
MATERIALS AND METHODS
Materials
Curcumin (95.5%) was imported from India. Xanthan gum, hydroxypropyl methylcellulose (HPMC) K4M, K15M, and 615, lactose, and poly(vinyl pyrrolidone) (PVP) K30 were purchased from the USA. Aerosil, magnesium stearate, CaCO3, NaHCO3, citric acid, KCl, and HCl were bought from China. All the other reagents, chemicals, and solvents were of pharmaceutical grades or higher.
The stomach cancer cell line N87 (ATCC) and the CellTiter 96® non-radioactive cell proliferation assay-3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) (Promega) were imported from the USA. 5-Fluorouracil (5-FU) (Ebewe® 500 mg/10 ml) was bought from Austria. Fetal bovine serum (FBS), Roswell Park Memorial Institute (RPMI) culture media, penicillin-streptomycin (Pen-Strep), trypan blue, trypsin-Ethylenediaminetetraacetic acid (EDTA), L-glutamine, and dimethyl sulfoxide were bought from Sigma-Aldrich, Singapore.
CSD formulation
The CSD was formulated based on our published study (Pan-On et al., 2018), with slight modifications. Briefly, the CSD was fabricated using the simple solvent (with ethanol) or melting solvent method with different polymers including β-cyclodextrin, poly(ethylene glycol) 6000, PVP K30, and HPMC 606, with or without the surfactant Tween 80, at various ratios (data not shown). The best formula, in terms of curcumin solubility and stability, was the formulation with a ratio of curcumin:PVP K30:Tween 80 of 1:4:0.22 w/w/w, fabricated by the solvent method. This condition was then employed to make the CSD.
Floating tablets incorporating CSD formulation
Firstly, the blank floating tablets were fabricated to find the optimal formulation with the fastest floating potential, longest floating time, and highest integrity during the floating duration. For this, the tablets were formulated using the simple wet granulation method, with slight modifications (Shishu and Aggarwal, 2008). The compositions included the SD (without curcumin) as the main ingredient, lactose as a diluent, xanthan gum/HPMC K4M/HPMC K15M/HPMC 615 as matrix generator, PVP K30 as a binder, NaHCO3/CaCO3 and citric acid as gas-generating excipients, and aerosil and magnesium stearate as glidant and lubricant. The process starts with homogeneous mixing of the SD (without curcumin), the matrix generator, lactose, and citric acid. Then, the mixture was moistened and wet-granulated with EtOH:water and PVP K30 through a 2 mm sieve. The granules were dried at 50°C–60°C to a moisture of ≤7%, passed through a 0.5 mm sieve, and mixed with gas-generating excipients, glidant, and lubricant. Finally, the powder was compressed on a rotary tablet press (Rimek, India) with a tablet weight of ~1,000 mg.
To obtain the optimal blank floating tablet, six parameters were varied, namely (1) the granulating solvent (EtOH:water at the ratio of 10:0, 9:1, 7:3, and 5:5 v/v), (2) the binder (PVP K30) concentrations in the formula (1%, 2%, 3%, 4%, and 5% w/w), (3) the type of gas-generating excipients (NaHCO3 and CaCO3), (4) the ratio of NaHCO3/CaCO3 and citric acid (1:0.2, 1:0.5, and 1:0.76 w/w), (5) the type/amount of matrix generator (xanthan gum, HPMC K4M, HPMC K15M, and HPMC 615), and (6) the tablet hardness. The tablet floating potential, floating time, and integrity during the floating duration were determined to select the optimal formula.
Secondly, the optimal blank floating tablet formula was then selected to incorporate the CSD, with a curcumin content of 100 mg/tablet. The process of preparing floating tablets containing CSD was similar to the process of preparing the blank floating tablets; however, in the first step, the blank SD was replaced with the CSD containing 100 mg of curcumin. The matrix generators and gas-generating excipients compositions of the final products were further adjusted to maximize the tablet potency. The complete best formulation is shown in Table 1.
Physicochemical characterizations
Floating potential
The floating ability of the floating tablets was tested in 200 ml of HCl buffer pH 1.2, by determining the time when the tablet fully emerges in the liquid. The tablets should float as quickly as possible (≤180 seconds) (Jaimini et al., 2007; Kanwar et al., 2016). Each experiment was repeated six times.
Floating time
The tablets were subjected to a dissolution tester (Erweka, Germany) using the paddle type I as the mixer. The parameters were set at a paddle speed of 100 rpm, at a temperature of 37°C ± 0.5°C, and a medium consisting of 900 ml HCl buffer pH 1.2. The tablets should float as long as possible (≥8 hours). Each experiment was repeated six times.
Tablet integrity
During and after the floating experiments, the tablet’s integrities were frequently observed and their physical stability was visually assessed by three levels of (1) good (intact tablets), (2) acceptable (partially broken tablets), and (3) poor (completely broken tablets). Each experiment was repeated six times.
In-vivo floating ability
To further confirm the tablet’s floating potential and floating time, an in-vivo gastric X-ray model in dogs has been applied. To this end, barium sulfate was used as a tracer in the formulation of floating tablets. The dogs weighing 11–15 kg were fasted overnight and anesthetized; and after 15 minutes, the floating tablets were subjected orally into the dog digestive system with 150 ml of water. Then, the dog’s stomach X-ray images were taken at different time intervals, namely immediately after taking the drug (0 minute), 30, 90, 150, 210, and 270 minutes, and continued until the tablet signal disappeared in the dog’s stomach. The floating potential and floating time were then assessed accordingly.
Tablet mass uniformity
The tablets (20 units) were randomly selected and individually weighed. The acceptance criteria were those of no more than two units whose weights were outside the ±5% range of the average weight.
Tablet hardness
The tablets’ hardness (20 units) was separately determined by the hardness tester (Erweka, Germany) following the manufacturer’s protocol. The acceptance criteria were set at the hardness of 40–60 N.
Tablet dissolution profile
The dissolution profile of the CSD and the floating tablet incorporating CSD were conducted following the standardized protocol with the dissolution tester (Erweka, Germany) for 8 hours using the paddle apparatus, in 900 ml buffer pH 1.2, at a speed of 100 rpm, and in a temperature of 37°C ± 0.5°C. At each time interval, 10 ml of the dissolution media was withdrawn, diluted with methanol and acetonitrile, filtered through a 0.45 μm membrane, and subjected to the high performance liquid chromatography (HPLC) analysis with the same condition in the Curcumin Quantification section. The percentage of released/dissolved curcumin was calculated based on the following equation:
Where,C0 is the standarld curcumin concentration (μg/ml), Sc, St is the curcumin peak area of the test sample and the reference sample, respectively, and m is the initial curcumin amount (mg).
Curcumin quantification
The curcumin in the floating tablets was extracted using methanol and subjected to the HPLC analysis for quantification. The conditions were set as follows: RP18 column (250 × 4.6 mm, 5 μm), mobile phase: acetonitrile:phosphoric acid pH 3.0 (50:50 v/v), flow rate: 1 ml/minute, injection volume: 10 μl, column temperature: 50°C, and detector: UV-Vis at a wavelength of 428 nm. The method has been validated based on the ICH guideline, including the specificity, linearity/range, accuracy, precision, limit of detection, and limit of quantitation.
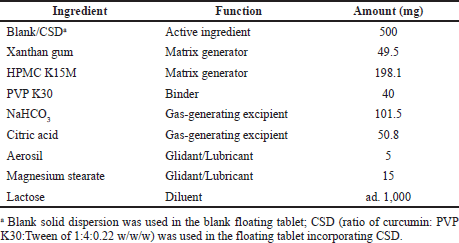 | Table 1. Floating tablet incorporating CSD formulation. [Click here to view] |
Stability study
The long-term and accelerated stabilities of the floating tablets incorporating CSD have been assessed following the guidelines on the stability testing in the ASEAN region, zone IVB condition (i.e., hot and very humid region) (European Medicines Agency, n.d.). For this, the condition for the long-term stability test and for the accelerated stability test is set at the temperature of 30°C ± 2°C with a relative humidity of 75% ± 5% and at the temperature of 40°C ± 2°C with a relative humidity of 75% ± 5%, respectively. After 0, 3, 6, 9, 12, 18, and 24 months for the long-term stability test and 0, 1, 2, 3, 4, 5, and 6 months for the accelerated stability test, the physicochemical properties of the tablets were re-analyzed, including floating potential, floating time, tablet integrity, tablet hardness, tablet dissolution profile, and curcumin quantification.
Then, based on the stability results, the tablet shelf-life (t90) was calculated following Equations (2)–(4). Equations (2) and (3) were used to determine the tablet shelf-life based on the long-term stability results. For the accelerated condition, the tablet shelf-life was similarly determined based on Equations (2) and (3); however, the t90 value was then adjusted by Equation (4), following Van’t Hoff rule as follows:
where, k is the constant calculated based on the remaining drug amount (D), the initial drug amount (D0), and the testing time (t). Moreover,
where is the temperature constant, indicating the difference in two storage temperatures. In our case, the constant was calculated to be 2.
Finally, the optimal storage temperature to reach the determined tablet shelf-life was calculated based on the following equation:
where T0th is the optimal storage temperature, T0bq is the ambient storage temperature (30°C), Cbq is the tablet shelf-life (t90) at ambient temperature (30°C), Cmm is the optimal tablet shelf-life, and A is the temperature constant, which was set at 2.
In-vitro cytotoxicity assay
The stomach cancer N87 cell line was cultured in a 75 cm2 flask using the RPMI culture medium supplemented with 10% FBS and 1% Pen-Strep. The cells were then kept in an incubator at 37°C, 5% CO2, and 70% humidity. Cells were replaced with a culture medium every even day and confluence cells were subcultured with trypsin-EDTA following the standardized protocol.
The cytotoxicity of the products on the cells was assessed using the CellTiter 96® non-radioactive cell proliferation assay (MTT assay) (Gerlier and Thomasset, 1986) following the manufacturer’s protocol. Briefly, prior to the cytotoxicity test, N87 cells were seeded into a 96-well culture plate with a concentration of 10,000 cells/well (each well contains 180 μl of culture medium). The plate was then incubated at 37°C, 5% CO2, and 70% humidity for 24 hours. After that, the samples (20 μl) were added to the cells and incubated for another 48 hours at the same condition. There were four sets of samples, with varying concentrations, including (1) the reference chemotherapeutic drug, 5-FU, at concentrations of 200, 100, 50, 25, 12.5, 6.25, and 3.125 μg/ml; (2) the floating tablet excipients (without curcumin), at concentrations of 450, 225, 112.5, 56.25, 28.125, 14.06, and 7.03 μg/ml; (3) the complete floating tablets incorporating the CSD, at concentrations of 500, 250, 125, 62.5, 31.25, 15.6, and 7.8 μg/ml; (4) the mixtures of the complete floating tablets incorporating the CSD and 5-FU, at concentrations (tablet/5-FU μg/ml) of 250/100, 125/50, 62.5/25, 31.25/12.5, 15.6/6.25, and 7.8/3.13 μg/ml. The sample concentration ranges were selected based on our preliminary work and previous studies (Liu et al., 2014; Tian et al., 2012). After 48 hours incubation, 20 μl of the MTT reagent was subjected to the cells and the plate was further incubated for 4 hours at 37°C. Finally, the supernatants were withdrawn and 100 μl of the dissolving/stopping solution mixture was added to solubilize the formed crystals for 1 hour. The solutions were spectroscopically measured using a microplate reader at a wavelength of 490 nm. All experiments were repeated four times. The percent of cell viability was calculated based on Equation (6). Based on the obtained results, the inhibitory concentrations at 50% (IC50) values were then determined and reported as follows:
where, the OD is the optical density, the blank was the sample without cells, and the negative control was the cells treated with the medium only.
RESULTS
Blank floating tablets formulation
The blank floating tablet was formulated, with numerous parameters, to select the optimal formulation (Table 2). Each factor varied sequentially one by one, with other factors/ingredient values being fixed. Firstly, the best granulating solvent (EtOH:water v/v ratio) was determined. A higher amount of EtOH significantly increases the floating potential from 38 seconds in the 10:0 ratio to 110 seconds in the 5:5 ratio. Furthermore, we noticed that the 7:3 and 5:5 ratios yielded incoherent and coarse granules. Thus, the 9:1 ratio was selected for the next factor variation. Secondly, the PVP K30 concentration of 3% w/w was chosen, since lower concentrations yielded short-floating-time tablets and higher concentrations resulted in long-floating-potential tablets. Thirdly, NaHCO3 was decided to be the main gas-generating excipient, as the CaCO3 made the tablet non-floatable for the first 10 minutes. Fourthly, the higher amount of citric acid significantly decreases the floating potential and, however, reduces the tablet integrity. Thus, the ratio of NaHCO3 and citric acid of 1:0.5 w/w was selected. Fifthly, regarding the matrix generator types, HPMC 615 formed a rigid tablet framework that could not float. Xanthan gum has the outstanding advantage of being able to maintain tablet integrity, but it has the disadvantage of a long floating time. In contrast, formulas using HPMC K4M or HPMC K15M help tablets float quickly, but with poor integrity. Therefore, we combined these polymers to optimize the floating tablets with short floating potential, long floating time, and good integrity. For this (formulae 27–35 in Table 2), the ratio of xanthan gum and HPMC K15M of 33:167 w/w possessed a floating time ≥8 hours with good integrity, and thus, this ratio was selected. Finally, the tablet hardness was investigated, and the floating potential increased with the hardness values. Hence, the hardness of 40–60 N was chosen.
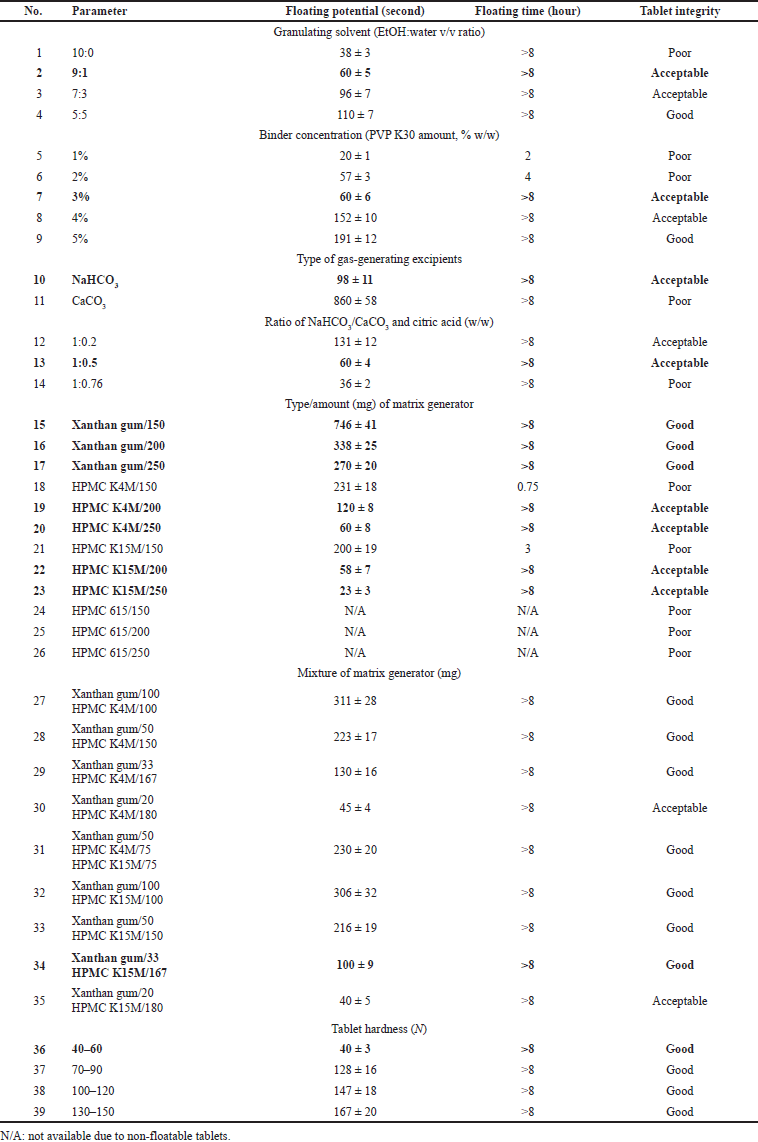 | Table 2. Optimizations of the blank floating tablets with various parameters. For each parameter, the other factors/ingredients were set at constant (n = 3). [Click here to view] |
Floating tablets incorporating CSD formulations
Prior to the floating tablet formulation, the CSD physical stability, as well as the aqueous solubility of curcumin in CSD (formula curcumin:PVP K30:Tween 80 (1:4:0.22) w/w/w), was investigated at the initial timepoint, after 1 year (12 months), and after 2 years (24 months) of storage at room temperature (Fig. 1). Obviously, the curcumin possesses low solubility of 0.6 ± 0.1 μg/ml for the entire 2-year storage. On the other hand, CSD could significantly increase the drug solubility to 50.5 ± 5.7 μg/ml (nearly 100 times enhancement). Moreover, no observable alterations in the CSD’s physical appearance and its solubility were noted after 2 years of storage, indicating the high stability of the CSD.
The optimal blank floating tablet formula was then utilized to load the CSD. For this, to confirm that the best final product was obtained, especially in terms of the floating potential, we re-varied the two main factors, namely the matrix generator (mixture of the xanthan gum and HPMC K15M, with a ratio of 1:5 w/w) and the gas-generating excipient NaHCO3 percentages in the formula (Table 3). The factor values were selected based on the design of the experimental approach, and the results were analyzed by the program BCPharSoft OPT (data not shown). The model was fit and reliable with a predicted R2 of 0.95 and an adjusted R2 of 0.9, and the optimal parameters were the matrix generator percentage of 24.8% and the NaHCO3 percentage of 10.2%. This condition yielded the wet-lab formula with a floating potential of 33 ± 2 seconds, a floating time of >8 hours, and good tablet integrity. These results were in agreement with the model-predicted value, with an insignificant difference between the actual and theoretical values (F = 1.15 < Fcrit = 12.32), indicating that the model was fit and reliable.
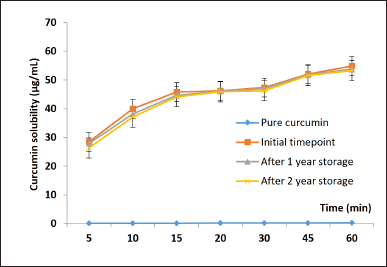 | Figure 1. Aqueous solubility of the pure curcumin and the curcumin in solid dispersion at the initial timepoint, after 1 year, and after 2 years storage at room temperature (n = 3). [Click here to view] |
Floating tablets physicochemical properties
The physicochemical properties of the final product, the floating tablets incorporating CSD, have been investigated in terms of the tablet appearance, hardness, floating potential, floating time, integrity, curcumin determination, curcumin quantification (assay), and curcumin dissolution profile. Additionally, for the stability tests, these properties were also re-investigated on tablets that were kept for 6 months in accelerated conditions (i.e., the temperature of 40°C ± 2°C and relative humidity of 75% ± 5%) and for 24 months in normal conditions (i.e., the temperature of 30°C ± 2°C and relative humidity of 75% ± 5%). Firstly, Figure 2 shows that after 8 hours of testing, the floating tablets significantly enhance the solubility and dissolution of curcumin approximately 241 times higher than that of the floating tablet containing raw curcumin. Secondly, in the in-vivo study, these floating tablets could maintain their floating ability in the dog’s stomach for at least 4.5 hours, since the tablet was X-ray observed at the 4.5 hour time point, but not at the 5.5 hours point (Fig. 3). Thirdly, Table 4 demonstrates that all physicochemical properties of the tablets at the initial time (i.e., after formulation), after 6 months preserved in accelerated condition, and after 24 months preserved in normal condition were in acceptance criteria. These results indicate that the tablets could protect the curcumin and CSD from degradation as well as maintain their properties properly. Thus, the product shelf-life was calculated to be 24 months (2 years), and the recommended storage condition is the normal condition at a temperature of 30°C ± 2°C and relative humidity of 75% ± 5%.
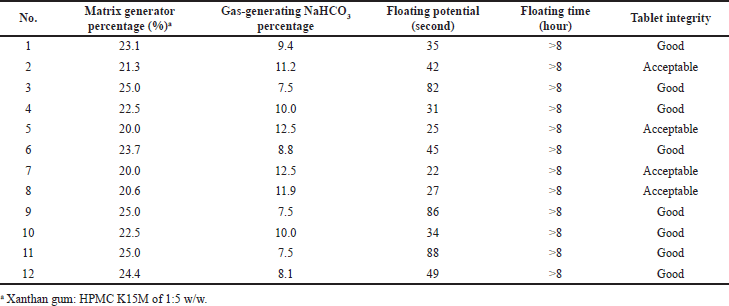 | Table 3. Design of experimental model of two independent variables, the matrix generator percentage (%) and the NaHCO3 percentage (%), and their effects on the three responses, the floating potential, the floating time, and the tablet integrity. [Click here to view] |
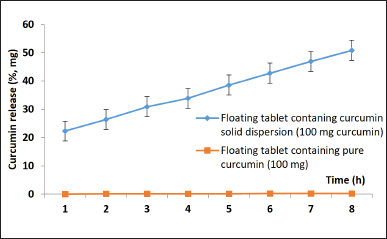 | Figure 2. Dissolution profiles during 8 hours of the floating tablet incorporating the curcumin-solid-dispersion and the corresponding floating tablet incorporating the pure curcumin (n = 3). [Click here to view] |
Floating tablets cytotoxicity on N87 stomach cancer cell line
To investigate the in-vitro ability to treat stomach cancer of the newly formed floating tablets incorporating the CSD, we first evaluate the effects of the tablet and SD excipients on the cells. The results show that the excipient samples did not alter the cell morphology, as well as did not significantly affect the cell viability (data not shown). Regarding the effect of the floating tablet, alone or in conjunction with a well-known stomach cancer treatment (5-FU), our data indicate that the curcumin in the floating tablet possessed an IC50 of 24.01 μM, lower than that of the reference drug 5-FU (46.86 μM), suggesting the effectiveness of the product (Table 5). Interestingly, the IC50 of the combined chemotherapy of the floating tablet and 5-FU was statistically lower than the IC50 of each individual therapy. This fact could be attributed to the synergistic effects of curcumin and 5-FU, which will be discussed in detail in the Discussion section.
DISCUSSION
To protect curcumin from being degraded by the alkaline environment of the small intestine and reduce the rapid metabolism by the liver and prolong its half-life to increase the oral bioavailability of curcumin, this work incorporated the CSD in the floating tablet dosage form for the application of gastric cancer treatment. To this end, the formulation was first optimized by various factors. To prevent the tablet from being pushed out of the stomach quickly, the tablet should possess a short floating potential (i.e., <180 seconds), a long retention time (i.e., >8 hours), and good integrity. The best formula is shown in Table 1, with a floating potential of 35 ± 1 seconds, a retention time of >8 hours, and perfect integrity. Other general tablet parameters have also been satisfied (Table 4).
Regarding the effects of the excipient type/amount on the tablet properties, for the granulating solvent, the formula with ethanol-water (10:0) provided the shortest floating potential; however, the polymer frame structure was not stable, and the rapid ethanol evaporation yielded weak bridges between the particles (Tank et al., 2018). Moreover, without water, the matrix polymer could not expand effectively (Nokhodchi et al., 2012), resulting in poor tablet integrity. With the ethanol-water ratio of 5:5, the integrity of the pellets was the best. Nevertheless, due to a high amount of water, the wet granulation process was very difficult, time-consuming, and unproductive with lots of granules sticking to the sieve. Therefore, the formula using ethanol-water (9:1) was selected.
For the binder, PVP K30 was chosen due to its high solubility in alcohol and has its high binding affinity, even in the dry state, which made it suitable for the wet granulation method (Becker et al., 1997; Nguyen et al., 2021a). Our results showed that a low PVP K30 content (1%, 2%) was not able to bind the granules cohesively, thus making the dissolution medium easy to contact with NaHCO3 and quickly generating CO2. Consequently, the floating potential was shortened. However, because the particles are not well adhered to, they cannot withstand the pressure when the CO2 gas is generated, causing the polymer framework to disintegrate. In contrast, with 5% PVP K30, the polymer frame is well bonded with good tablet integrity, but the floating potential was prolonged. Conclusively, the formulation with 3% PVP-K30 possessed a short floating potential, slow pellets corrosion, and a maintained tablet shape throughout the floating process.
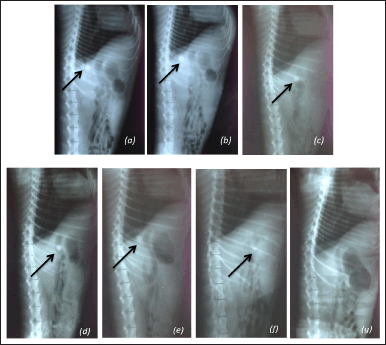 | Figure 3. In-vivo X-ray investigation of the floating time in the dog stomach of the floating tablet incorporating curcumin solid dispersion. The X-ray images demonstrate the dog stomach (a) at the initial time point, (b) after 0.5 hours, (c) after 1.5 hours, (d) after 2.5 hours, (e) after 3.5 hours, (f) after 4.5 hours, and (g) after 5.5 hours. [Click here to view] |
Regarding the gas-generating excipients, NaHCO3 produced CO2 more rapidly than CaCO3, which was consistent with the previous studies (Deb et al., 2010; Nguyen et al., 2016; Tadros, 2010). To further increase the rate of CO2 generation in the early stages of short-floating-potential tablets, it is necessary to add a sufficient amount of acid to the formula. Citric acid was selected in our case due to the preliminary studies and the literature (Jaimini et al., 2007; Nguyen et al., 2016). An increase in the amount of citric acid significantly reduced the tablet’s floating potential of the tablet, yet it destroyed its integrity due to fast CO2 generation. Therefore, the ratio of NaHCO3:citric acid (1:0.5) was selected.
In terms of the matrix generator type/amount, four commonly used excipients were employed, including the xanthan gum, HPMC K4M, HPMC K15M, and HPMC 615. All formulations with HPMC 615 were non-floating and rapidly disintegrated, possibly because the viscosity of HPMC 615 was too low (Perfetti et al., 2012) to form a stable gel framework that traps the gas in contact with the dissolved environment. At the same time, HPMC 615 could not withstand the pressure of CO2 produced, making the pellets not buoyant and rapidly disintegrating. Therefore, HPMC 615 is not a suitable matrix generator, in our case. Among the remaining three polymers, xanthan gum yielded the best tablets with good integrity and long floating time (>8 hours). However, the tablets possessed long floating potential (i.e., >180 seconds), possibly due to the fact that xanthan gum is a natural polymer that takes a long time to expand (Kanwar et al., 2016) and to reduce the tablet density (i.e., the tablets sank first and then resurfaced in the testing media). Therefore, xanthan gum should be combined with other matrix generators with rapid expansion ability such as HPMC K4M and HPMC K15M. To that end, Table 2 shows that HPMC K4M, with an inherent moderate-viscosity property, could not maintain the tablet integrity at a low amount of 150 mg. At higher amounts, HPMC K4M yielded acceptable but not perfect integrity. These results were similar to the previous studies (Chowdary and Hussainy, 2012; Someshwar et al., 2011). The HPMC K15M provides tablets with the shortest floating potential, compared with the xanthan gum and HPMC K4M. These data were in agreement with a previous study (Someshwar et al., 2011). This indicates that HPMC K15M was the suitable candidate for combination with xanthan gum to shorten the floating potential time. Conclusively, the formula of xanthan gum-HPMC K15M with a ratio of 1:5 yielded the best tablets.
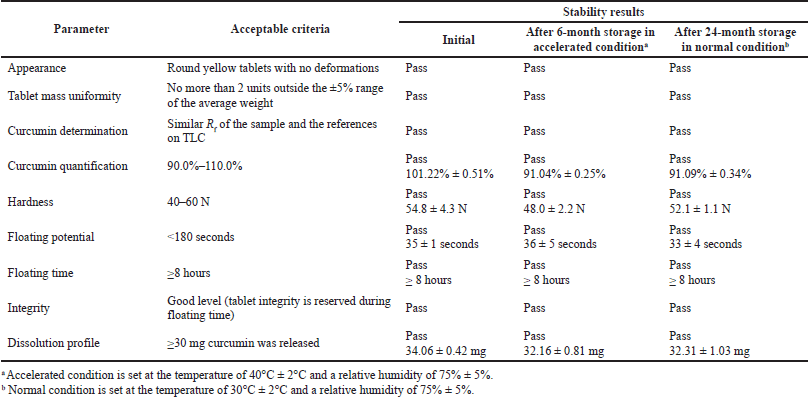 | Table 4. Physicochemical properties of the floating tablets incorporating the CSD at the initial time point, after 6-month storage in accelerated condition, and after 24-month storage in normal condition (n = 3). [Click here to view] |
 | Table 5. The cytotoxicity data on the N87 stomach cancer cell line of the floating tablet incorporating CSD, the reference drug 5-FU, and the combination of the tablet and 5-FU (n = 3). [Click here to view] |
Investigating the influences of the tablets’ hardness on their floating properties, the results showed that the floating potential increased proportionally to the tablet hardness, in accordance with previous works (Jivraj et al., 2000; Someshwar et al., 2011). This could be attributed to two reasons, the polymer swelling matrix generator polymer properties and the tablet porosity. In tablets with high hardness, the porosity of the pellets decreases and the polymer molecules on the tablet surface are compacted; thus, they could not be hydrated quickly when in contact with the medium, leading to a long floating potential time. Overall, all these aforementioned formulation factors significantly affected the floating tablet’s properties, especially the floating potential and floating time. Thus, critical considerations are necessary to formulate optimal products.
Shifting to another issue, the results of X-ray of the dog’s stomach on the floating ability of the tablets demonstrated a good correlation between the in-vitro and in-vivo tests. This re-confirmed that the tablets, when taken into the stomach of biological living systems, are still capable of maintaining their floating properties. The fact that the floating time in the in-vivo study was shorter than that in the in-vitro test (>4 vs. >8 hours) could be due to the effects of the stomach contractions, gastric volume, and enzymatic functions. This result was in agreement with the literature (Chandira et al., 2011).
Another important problem to discuss is the stability of the CSD and the product. One of the most common disadvantages of SD is its poor stability during storage. This phenomenon crucially affects curcumin solubility and oral bioavailability. Hence, we investigated the stability of these floating tablets incorporating CSD under storage conditions at room temperature and accelerated conditions after 1 and 2 years. Interestingly, the results showed that in both conditions the tablet’s physicochemical properties (Table 4) and the curcumin solubility (Fig. 1) were well preserved, with no significant differences compared to the initial values. These data re-confirm that the formula was optimal for the delivery of curcumin.
Finally, the cytotoxicity of the best product was determined on the N87 stomach cancer cell line. After 48 hours of incubation, the excipient samples (i.e., tablets without curcumin) did not alter the cell morphology and reduce its survivability, even at the highest concentration of 450 μM. Thus, the excipients did not affect the cytotoxic potential of curcumin. On the other hand, all test samples containing CSD possessed significant cytotoxicity, corresponding to the sample concentrations. This indicates that the formulations could well preserve the anticancer efficacy of curcumin. Surprisingly, the floating tablet cytotoxic effect on N87 cells was statistically higher than on 5-FU, with an IC50 of 24.01 and 46.86 μM, respectively. This could be due to the unique action of curcumin on stomach cancer cells, possibly by impairing ATP-sensitive potassium channel opening (Li et al., 2014; Liu et al., 2014). Moreover, the fact that the IC50 of both curcumin and 5-FU was lower in the combination treatments compared to that in the individual treatments suggests that curcumin potentiated and enhanced the 5-FU anticancer effect against stomach cancer N87 cell line. Although this phenomenon has been shown in various cancer cell lines (Tian et al., 2012; Yang et al., 2017), we, for the first time, reported it on the N87 cells. Hence, these data could contribute to the literature on the potential effects of curcumin on stomach cancer.
CONCLUSION
This study develops and fully characterizes the floating tablets incorporating the CSD for gastric cancer treatment. The optimal formula possesses a short floating potential of 35 ± 1 seconds, a long floating retention time of >8 hours, good tablet integrity during the floating duration, and perfect physicochemical stability for at least 2 years in the normal storage condition. Furthermore, the tablets could enhance the curcumin solubility and dissolution profiles to >200 times compared to the pure curcumin and reserve the curcumin cytotoxicity on the N87 stomach cell lines with a better efficacy compared to the standard drug 5-FU. Finally, the tablets significantly potentiate and enhance the anticancer effect of 5-FU in a synergistic mechanism. Conclusively, the floating tablet incorporating the CSD could be a potential pharmaceutical product for stomach cancer treatment.
ACKNOWLEDGMENTS
The authors would like to thank Can Tho University and Can Tho University of Medicine and Pharmacy for supporting this research.
AUTHORS’ CONTRIBUTION
Conceptualization was done by D. T. M. H., V.T., and D. T. P.; methodology was done by D. T. M. H., M. T. L., V. H., and V. T.; validation was done by D. T. P.; investigation was done by D. T. M. H., M. T. L., V. H., and V. T.; resources were done by D. T. P.; writing original draft was done by D. T. M. H. and D. T. P.; writing review and editing were done by D. T. M. H., M. T. L., V. T., and D. T. P.
FINANCIAL SUPPORT
The authors declare that they received no funding for this article.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
The research ethics were approved by the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam, code 1454/QD-DHYD-SDH.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci, 2009; 30(2):85–94; doi:10.1016/J.TIPS.2008.11.002. CrossRef
Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res, 2003; 23(1A):363–98.
Alshehri S, Imam SS, Hussain A, Altamimi MA, Alruwaili NB, Alotaibi F, Alanazi A, Shakeel F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: newer formulation techniques, current marketed scenario and patents. Drug Deliv, 2020; 27(1):1625–43; doi:10.1080/10717544.2020.1846638. CrossRef
Becker D, Rigassi T, Bauer-Brandl A. Effectiveness of binders in wet granulation: a comparison using model formulations of different tabletability. Drug Dev Indus Pharm, 1997; 23(8):791–808; doi:10.3109/03639049709150550. CrossRef
Chandira RM, Bhowmik D, Jayakar B. Formulation and evaluation of floating tablets of cefuroxime axetil. Int J Pharm Sci Res, 2011; 4:177–80.
Chowdary K, Hussainy S. Formulation and evaluation of floating tablets of gliclazide employing HPMC and carbopol. Int J Chem Sci, 2012; 10:1213–20.
Clinical Trials. Curcumin in preventing gastric cancer in patients with chronic atrophic gastritis or gastric intestinal metaplasia. n.d. Available via https://clinicaltrials.gov/ct2/show/NCT02782949?term=curcumin&cond=stomach+cancer&draw=2&rank=1 (Accessed 19 July 2022).
Deb J, Ghosh A, Sen K, Paul P, Choudhury A. Formulation and evaluation of metformin HCl floating tablet using pectin as a natural polymer. Int Res J Pharm Sci, 2010; 01:0022.
European Medicines Agency. Stability testing of existing active ingredients and related finished products. n.d. Available via https://www.ema.europa.eu/en/stability-testing-existing-active-ingredients-related-finished-products (Accessed 5 November 2022).
Fuloria S, Mehta J, Chandel A, Sekar M, Rani NNIM, Begum MY, Subramaniyan V, Chidambaram K, Thangavelu L, Nordin R, Wu YS, Sathasivam KV, Lum PT, Meenakshi DU, Kumarasamy V, Azad AK, Fuloria NK. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front Pharmacol, 2022; 13:820806; doi:10.3389/FPHAR.2022.820806/BIBTEX. CrossRef
Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods, 1986; 94(1–2):57–63; doi:10.1016/0022-1759(86)90215-2. CrossRef
Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods, 2017; 6(10); doi:10.3390/FOODS6100092. CrossRef
Huynh DTM, Le MNT, Tran VD, Pham DT. Antibacterial hydrogel containing Piper betel L. extract for acne treatment, an ex vivo investigation. Pharm Sci Asia, 2022; 49(4):372–80; doi:10.29090/PSA.2022.04.22.061. CrossRef
Jaimini M, Rana A, Tanwar Y. Formulation and evaluation of famotidine floating tablets. Curr Drug Deliv, 2007; 4(1):51–5; doi:10.2174/156720107779314730. CrossRef
Jivraj M, Martini LG, Thomson CM. An overview of the different excipients useful for the direct compression of tablets. Pharm Sci Technol Today, 2000; 3(2):58–63; doi:10.1016/S1461-5347(99)00237-0. CrossRef
Kanwar N, Kumar R, Sarwal A, Sinha VR. Preparation and evaluation of floating tablets of pregabalin. Drug Dev Indus Pharm, 2016; 42(4):654–60; doi:10.3109/03639045.2015.1062895. CrossRef
Li X, Chen T, Xu L, Zhang Z, Li L, Chen H. Preparation of curcumin micelles and the in vitro and in vivo evaluation for cancer therapy. J Biomed Nanotechnol, 2014; 10(8):1458–68; doi:10.1166/JBN.2014.1840. CrossRef
Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, Zhai G. Oral bioavailability of curcumin: problems and advancements. J Drug Targeting, 2016; 24(8):694–702; doi:10.3109/1061186X.2016.1157883. CrossRef
Liu X, Sun K, Song A, Zhang X, Zhang X, He X. Curcumin inhibits proliferation of gastric cancer cells by impairing ATP-sensitive potassium channel opening. World J Surg Oncol, 2014; 12(1); doi:10.1186/1477-7819-12-389. CrossRef
Nguyen NNT, Pham DT, Nguyen DT, Trinh TTL. Bilayer tablets with sustained-release metformin and immediate-release sitagliptin: preparation and in vitro/in vivo evaluation. J Pharm Investig, 2021a; 51(5):579–86; doi:10.1007/S40005-021-00533-Z. CrossRef
Nguyen PH, Tran VD, Pham DT, Dao TNP, Dewey RS. Use of and attitudes towards herbal medicine during the COVID-19 pandemic: a cross-sectional study in Vietnam. Eur J Integr Med, 2021b; 44:101328; doi:10.1016/J.EUJIM.2021.101328. CrossRef
Nguyen TNN, Duy TP, Ngoc MT. Formulation and evaluation of low floating lag time metformin hydrochloride 500 mg sustained release floating tablet. Asian J Pharm (AJP), 2016; 10(04):497; doi:10.22377/AJP.V10I04.884. CrossRef
Nokhodchi A, Raja S, Patel P, Asare-Addo K. The role of oral controlled release matrix tablets in drug delivery systems. BioImpacts?: BI, 2012; 2(4):175; doi:10.5681/BI.2012.027.
Pan-On S, Dilokthornsakul P, Tiyaboonchai W. Trends in advanced oral drug delivery system for curcumin: a systematic review. J Control Release, 2022; 348:335–45; doi:10.1016/J.JCONREL.2022.05.048. CrossRef
Pan-On S, Rujivipat S, Ounaroon A, Tiyaboonchai W. Development and characterization of clay facial mask containing turmeric extract solid dispersion. Drug Dev Indus Pharm, 2018; 44(4):590–97; doi:10.1080/03639045.2017.1405434. CrossRef
Papich MG, Martinez MN. Applying Biopharmaceutical classification system (BCS) criteria to predict oral absorption of drugs in dogs: challenges and pitfalls. AAPS J, 2015; 17(4):948; doi:10.1208/S12248-015-9743-7. CrossRef
Pari L, Tewas D, Eckel J. Role of curcumin in health and disease. Arch Physiol Biochem, 2008; 114(2):127–49; doi:10.1080/13813450802033958. CrossRef
Patel RK, Singh SK, Singh SB, Sheth NR, Gendle R. Development and characterization of curcumin loaded transfersome for transdermal delivery. J Pharm Sci Res, 2009; 1:71–80.
Perfetti G, Alphazan T, Wildeboer W, Meesters G. Thermo-physical characterization of pharmacoat((r)) 603, pharmacoat((r)) 615 and mowiol((R)) 4-98. J Therm Anal Calorim, 2012; 109; doi:10.1007/s10973-011-1664-9. CrossRef
Pham DT, Thao NTP, Thuy BTP, Tran VD, Nguyen TQC, Nguyen NNT. Silk fibroin hydrogel containing Sesbania sesban L. extract for rheumatoid arthritis treatment. Drug Deliv, 2022; 29(1):882–8; doi:10.1080/10717544.2022.2050848. CrossRef
Phothi T, Tunsophon S, Tiyaboonchai W, Khongsombat O. Effects of curcumin and γ-oryzanol solid dispersion on the brain of middle-aged rats. Biomed Rep, 2022; 17(1); doi:10.3892/BR.2022.1542. CrossRef
Pouton CW. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci, 2006; 29(3–4):278–87; doi:10.1016/J.EJPS.2006.04.016. CrossRef
Prashar D, Khokra S, Purohit R, Sharma S. Curcumin: a potential bioactive agent. Res J Pharm Biol Chem Sci, 2011; 2:44–52. CrossRef
Shishu NG, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin—‘a technical note.’ AAPS PharmSciTech, 2008; 9(3):810; doi:10.1208/S12249-008-9096-Y. CrossRef
Someshwar K, Chithaluru K, Ramarao T, Kumar KK. Formulation and evaluation of effervescent floating tablets of tizanidine hydrochloride. Acta Pharm (Zagreb, Croatia), 2011; 61(2):217–26; doi:10.2478/V10007-011-0015-5. CrossRef
Tadros MI. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: development, optimization and in vitro-in vivo evaluation in healthy human volunteers. Eur J Pharm Biopharm, 2010; 74(2):332–39; doi:10.1016/J.EJPB.2009.11.010. CrossRef
Tank D, Karan K, Gajera BY, Dave RH. Investigate the effect of solvents on wet granulation of microcrystalline cellulose using hydroxypropyl methylcellulose as a binder and evaluation of rheological and thermal characteristics of granules. Saudi Pharm J, 2018; 26(4):593–602; doi:10.1016/J.JSPS.2018.02.007. CrossRef
Tian F, Fan T, Zhang Y, Jiang Y, Zhang X. Curcumin potentiates the antitumor effects of 5-FU in treatment of esophageal squamous carcinoma cells through downregulating the activation of NF-ΚB signaling pathway in vitro and in vivo. Acta Biochim Biophys Sin, 2012; 44(10):847–55; doi:10.1093/ABBS/GMS074. CrossRef
Tripathi J, Thapa P, Maharjan R, Jeong SH. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics, 2019; 11:193; doi:10.3390/PHARMACEUTICS11040193. CrossRef
Tran VD, Tran VD, Pham DT, Cao TTN, Bahlol M, Dewey RS, Le MH, Nguyen VA. Perspectives on COVID-19 prevention and treatment using herbal medicine in Vietnam: a cross-sectional study. Ann Ig, 2022; 34(5); doi:10.7416/AI.2021.2484.
Vo CLN, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm, 2013; 85(3 Pt B):799–813; doi:10.1016/J.EJPB.2013.09.007. CrossRef
Wook Huh H, Na YG, Kang HC, Kim M, Han M, Pham TMA, Lee H, Baek JS, Lee HK, Cho CW. Novel self-floating tablet for enhanced oral bioavailability of metformin based on cellulose. Int J Pharm, 2021; 592:120113; doi:10.1016/J.IJPHARM.2020.120113. CrossRef
Yang H, Huang S, Wei Y, Cao S, Pi C, Feng T, Liang J, Zhao L, Ren G. Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF- ΚB signaling pathways. J Cancer, 2017; 8(18):3697; doi:10.7150/JCA.20196. CrossRef