INTRODUCTION
Microcrystalline cellulose (MCC) is partially depolymerized cellulose prepared by treating pure α-cellulose, obtained from different plant fibers, with mineral acids. Cellulose molecules with a level-off degree of polymerization (DP) of less than 350 are considered as MCC (United States Pharmacopoeia (USP) 36-NF 31, 2007). It is a crystalline white, odorless, tasteless powder and is widely used as a water retainer in cosmetics and as a suspension stabilizer (Chuayjuljit et al., 2010; E1-Sakhawy and Hassan, 2007). Due to the high compressibility and free flowing characteristics, it creates an immense effect in the tableting process and is used widely as a directly compressible material or diluent in tablet formulation (Bolhuis and Chawhan, 1996; Zhang et al., 2007).
Preparation of MCC from cotton (Chauhan et al., 2009), cotton rags (El-Sakhawy and Hassan, 2007), coconut shells (Shlieout et al., 2002), cotton rags (Chauhan et al., 2009), waste cotton fabrics (Chuayjuljit et al., 2010), sugarcane baggage (Ilindra and Dhake, 2008; Padmadisastra and Gonda, 1989; Paralikar and Bhatawdekar, 1988), jute (Abdullah, 1991; Jahan et al., 2011), rice husk (Ilindra and Dhake, 2008), wheat straw (Monschein et al., 2013), orange mesocarp (Ejikeme, 2008), cotton wool (Rashid et al., 2017), soybean husk (Uesu et al., 2000), and sisal fibers (Bhimte and Tayade, 2007) has been reported in different journals. Among the various preparation methods such as the extrusion, enzyme-mediated process (Monschein et al., 2013), alkali hydrolysis (Owolabi et al., 2017), and steam explosion and acid hydrolysis methods (Chuayjuljit et al., 2010; E1-Sakhawy and Hassan, 2007), the last one is the most preferable due to the shorter duration and efficient production. An isolated pure cellulose chain from the above sources by chemical treatment comprises crystalline and amorphous parts, of which the amorphous part is easily degraded by acid to produce the crystalline powder (Hakansson and Ahlgren, 2005). Various properties of the MCC such as moisture content, particle size and shape, bulk and tapped density, crystallinity, and DP can be controlled by selecting the suitable starting materials and reaction conditions such as temperature, time, acid concentrations, and drying process (Landín et al., 1993; Shlieout et al., 2002; Wu et al., 2001).
Despite many articles published describing methods of preparation of MCC from various natural sources, no study on preparation of MCC from waste paper and its tableting properties has been published yet. As the waste paper contains a certain amount of inorganic salts, pretreatment with a chelating agent was used in this study to reduce a certain amount of ash. Moreover, acid hydrolysis could further reduce the ash content to comply with pharmacopeia requirements. The method has explored a new way to utilize high ash content waste materials such as waste paper, which have never been used in the pharmaceutical field. Moreover, we have confirmed the tableting properties of the derived MCC.
MATERIALS AND METHODS
Materials
Ethylenediaminetetraacetic acid disodium (EDTA-2Na) dihydrate, sodium dodecyl sulfate (SDS), hydrochloric acid (37%), sodium hydroxide, anthraquinone, and hydrogen peroxide (30%) were purchased from Sigma-Aldrich, Germany. Waste paper was collected from a local bookbinding shop where the cutting part of the waste paper was not in the written state.
Preparation of MCC
The waste paper was collected from the local shop, and the visible extraneous materials were removed from the sample by hand. The paper (60 g) was chopped into tiny pieces (1 × 1 cm), soaked in distilled water, and crushed to remove the water-soluble chemicals used in the processing of the paper and then filtered. The filtered material was then treated with a 3.72% EDTA-2Na, 1.0% SDS, and 0.5% sodium hydroxide solution at 40°C–50°C for 2 hours (solid-to-liquid ratio 1:20) with frequent stirring, and the process was repeated twice. After complete removal of the liquid by filtration and washing, the material was treated with a 1% sodium hydroxide and 0.01% anthraquinone solution at higher temperature (160°C) for 2 hours (high pressure reaction vessel, model FcF-1, Zhengzhou Keda, China). Then, it was washed vigorously until complete neutralization. The material was then bleached in a 3% (on dry basis) hydrogen peroxide solution at 60°C–70°C for 1 hour. Then, the bleached material was washed thoroughly and filtered to remove the bleaching materials completely. The bleached material was then subjected to hydrothermal degradation (high pressure reaction vessel, model FCF-1, Zhengzhou Keda, China) at a solid-to-liquid ratio of 1:20, a hydrochloric acid concentration of 0.5?mol/l, and a 160°C temperature for 120?minutes to produce the MCC. The derived MCC product was then washed well until neutralization with distilled water, dried at 60°C–70°C for 3 hours, and ground into powder. The powder was passed through 120- and 200-mesh sieves. MCC grade 1 was defined as powder that passed through a 200-mesh sieve with a particle size of less than 75 microns, while MCC grade 2 was defined as powder that went through a 120-mesh sieve but was kept on a 200-mesh screen with a particle size of 75–125 microns.
Identification of MCC
Chemical identification
The derived MCC was chemically identified by observing the color after treating the 10 mg sample with 2 ml of an iodinated zinc chloride solution.
Identification of MCC by determining DP
The intrinsic viscosity of cellulose in a copper (II) ethylenediamine solution (cuene) was used to evaluate the DP of the MCC sample (Sihtola et al., 1963). MCC solutions with concentrations of 0%, 0.0625%, 0.125%, 0.25%, 0.5%, and 1.0% were prepared in a two-fold diluted cuene solvent. The time it took to move the solution surface between the levels of the Ostwald Viscometer 1831 at 25°C was used to measure the relative viscosity of each MCC solution. After measuring the density of each solution with a densitometer, reduced viscosity was computed using the relative viscosity value. The plot of reduced viscosity versus concentration was used to calculate the intrinsic viscosity. The MCC’s intrinsic viscosity was indicated by the intercept of the y-axis of the plot. The DP was determined using the following equation based on the intrinsic viscosity (η) value:
(DP)0.85 = 1.1 × η.
Identification by Fourier transform-infrared spectroscopy (FT-IR)
The MCC sample was blended with potassium bromide powder (0.1% MCC) and ground properly to convert the mixture into a fine powder, which was compacted to prepare a disc for FT-IR examination. In transmission mode, FT-IR spectra were acquired using an FT-IR 8400S Shimadzu spectrophotometer in the frequency range of 4,000–40 cm−1, with a resolution of 2 cm−1. A total of 30 scans were collected.
Characterization of the derived MCC
Determination of bulk density and tapped density
Ten grams of each powder sample was poured into a 50 ml clean, dry measuring cylinder for bulk density determination, and the bulk volume filled by each of the samples was determined (Azubuike and Okhamafe, 2012; Ohwoavworhua et al., 2004). After 200 taps by the instrument (digital automatic tap/bulk density test apparatus, model VTAP-MATIC, Veego, India), the tapped volumes, V200, were determined for each sample. The bulk density and tap density of each sample were calculated by dividing the weight (10 g) by the bulk volume and tap volume, respectively.
Analysis of flow property by measuring Hausner’s and compressibility index (Carr’s Index)
Hausner’s and Carr’s indexes of the MCC powder samples were derived using the bulk and tapped density values from the equation as described earlier (Aulton, 1996; Bowker and Stahl, 2008; Ohwoavworhua et al., 2004; Podczeck et al., 2007).
Analysis of flow property by measuring angle of repose
For determination of the angle of repose of the MCC samples, a funnel was clamped 3 cm above the horizontal surface where a graph sheet was placed under the funnel. The powder was passed via the tube of the funnel. After determination of the height of the pile of powder (h) and the mean diameter of the base of powder, the angle of repose was computed using the equation tan θ = 2h/D where h is the height of the pile of the MCC powder, D is the diameter of the base of the pile of the powder, and θ is the angle of repose (Train, 1958).
Particle size and shape analysis by scanning electron microscopy (SEM)
An SEM photograph of the derived MCC was taken with the help of JEOL JSM-6490LA, an analytical scanning electron microscope, at 100-fold magnification. The samples were seated upon the specimen’s surface with double-sided adhesive tape followed by the application of electrically conductive coating.
Determination of ash value
The sulfated ash value (%) of the derived MCC and the marketed Avicel PH101 was determined according to the procedures described in United States Pharmacopeia (USP, 2007).
Tableting properties of MCC
Preparation of tablet
For the preparation of the tablet, 100 mg of aceclofenac (active pharmaceutical), 88 mg of MCC, 10 mg of Na-starch glycolate (disintegrating agent), and 2 mg of magnesium stearate (lubricant and glidant) were taken, mixed properly, and subjected to direct compression.
Determination of hardness of the tablets
A tablet hardness tester (Pharmag, India, model TABTEST-101) was used to determine the hardness of the derived tablets (10 tablets from each group). The force necessary to break down the tablet was measured in Newtons.
Determination of friability of the tablets
According to the specifications, all of the tablets (20 tablets from each group) were exposed to the Digital Friability Test Apparatus (Electronics India, model 1902) chamber at 25 rpm for 4 minutes. The weight of the testing tablets was measured once the time period was up, and the percent weight loss was computed as described in BP.
Determination of disintegration time of the tablets
Six tablets were inserted into the tube of the USP disintegration test device (Electronics India, model 2901) for the disintegration test. At 37°C ± 2°C and 50 rpm, the assembly was suspended in the beaker with 900 ml distilled water as the medium. According to British Pharmacopoeia (BP, 2011a; 2011b), the time it took for each tablet to disintegrate completely was measured.
In vitro dissolution study of the tablets
The in vitro dissolution of the aceclofenac tablets was measured using the previously describe method (Islam et al., 2011). Six tablets were evaluated at a speed of 100 rpm in USP dissolving type II equipment (Electronics India, model 1918). The dissolving medium was 900 ml of a pH 6.8 ± 0.05 phosphate buffer solution at 37°C ± 0.5°C. After 30 minutes, 20 ml of solution was collected from each basket of the apparatus, filtered, and diluted, and the absorbance at 274 nm was measured using a UV-VIS spectrophotometer (model UV 1700, Shimadzu). The percentage of the released aceclofenac drug after 30 minutes was computed using the standard curve of aceclofenac prepared by assessing the absorbance of different concentrations of the standard aceclofenac solution.
RESULTS AND DISCUSSION
Identification of the derived MCC
The MCC derived by treating the waste paper by the hydrothermal process was a white, tasteless, and odorless powder material that was chemically identified as MCC by an iodinated zinc chloride solution. In the presence of the reagent, the sample turned into violet-blue color, indicating the MCC nature of the sample.
We used an Ostwald viscometer to measure the reduced viscosity of the produced MCC and conventional Avicel PH101. The intrinsic viscosity of Avicel PH101 and the produced MCC was determined to be 55.476 and 79.034 from the y-intercept of the graph of reduced viscosity versus concentration, respectively (Fig. 1). The straight lines of Avicel PH101 and the derived MCC both had R2 values of 0.9828 and 0.9791, demonstrating a high correlation between concentration and reduced viscosity (Fig. 1). The DP value calculated from the intrinsic viscosity using the equation for Avicel PH101 and the derived MCC was 126 and 191, respectively. Both the marketed and derived MCC comply with the USP specification for the DP for MCC.
FT-IR spectra (Fig. 2) of the MCC samples were noted on an FT-IR 8400S Shimadzu spectrophotometer within the 4,000–400 cm−1 frequency range in transmission mode. The positions of different bands of the derived MCC are very similar to that of Avicel PH101, indicating the similarity of compounds. The absorption peaks at 3,449 cm−1 due to O–H stretching, at around 2,900 ~ 3,000 cm−1 due to C–H stretching, and at around 1,061 and 1,104 cm−1 due to C–O–C stretching are evident in the spectrum of the derived MCC and Avicel PH101, which are similar to those of the standard cellulose backbone (Chen et al., 1997). The invisibility of the absorption band at 1,740 cm−1 in the derived MCC is an indication of the nonexistence of hemicellulose in the derived MCC (Revol et al., 1982). Moreover, the absence of a peak at 1,595 cm−1 (Diana et al., 2011) which is associated with the aromatic C–O stretching mode is an indication of the absence of lignin in the derived MCC sample. All these results clearly indicate the high purity of MCC, i.e., the complete absence of hemicellulose and lignin. Additionally, the broad and high intensity of the band in the 3,600–3,100 cm−1 region due to OH–stretching vibration indicates the presence of hydrogen bonds with higher crystallinity in the derived MCC sample. Shifting of the peak to the higher wavenumber region occurs due to the presence of a high amorphous portion in the MCC structure. The derived MCC shows comparable shifting of the peak and level of broadband at this region with Avicel PH101, indicating similar crystalline characteristics of the derived MCC to Avicel PH101 (Kacurakova et al., 1998).
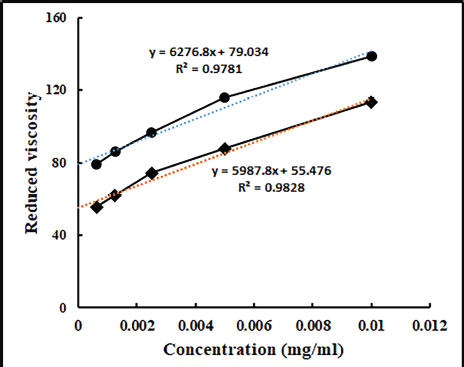 | Figure 1. Determination of DP of Avicel PH101 and prepared MCC. [Click here to view] |
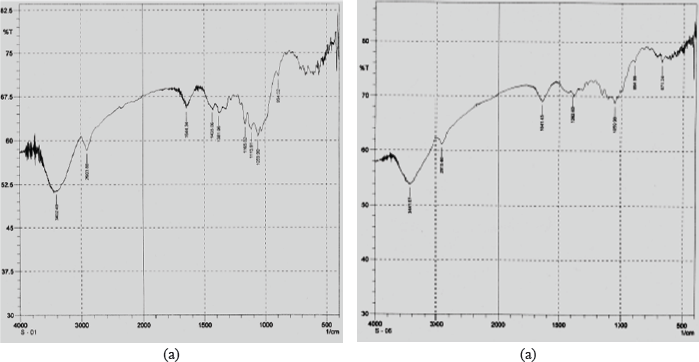 | Figure 2. FT-IR spectra of Avicel PH101 (a) and prepared MCC (b). [Click here to view] |
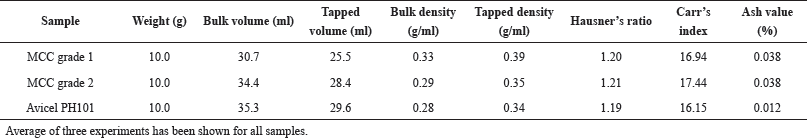 | Table 1. Bulk density, tapped density, Hausner’s ratio, and Carr’s index of prepared MCC and Avicel PH101. [Click here to view] |
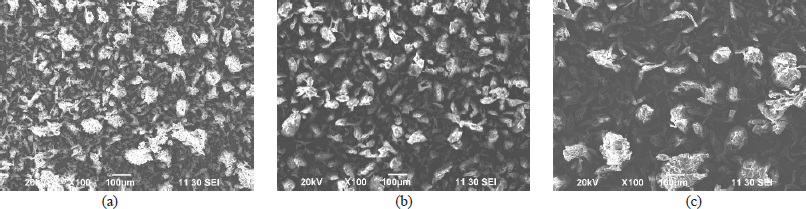 | Figure 3. Scanning electron micrographs of Avicel PH101 (a) and prepared MCC grade 1 (b) and grade 2 (c). [Click here to view] |
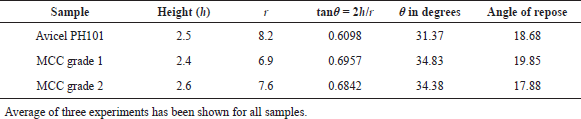 | Table 2. Angle of repose of MCC grade 1, MCC grade 2, and Avicel PH101. [Click here to view] |
Characterization of the derived MCC
Flow property is a vital parameter for a pharmaceutical excipient. The powder should have an optimum flow property to be used as an excipient. In general, the higher the bulk and tapped densities, the better the potential for a material to flow and to rearrange under compression. Bulk density gives an estimate of the flowability of a powder material, while tapped density is a measure of how well a powder can be packed in a confined space on repeated tapping. In the present study, we observed that both the grades of MCC had closer values of bulk and tapped density to that of Avicel PH101 (Table 1). Again, Hausner’s ratio which is related to interparticle friction can be used to predict the powder flow. Hausner’s ratio of the derived MCC is similar to that of Avicel PH101, and the value is lower than 1.25, indicating the good flowability of the derived MCC (Table 1). Carr’s index is another parameter of powder flowability (Okhamafe et al., 1991). Normally, Carr’s index values of 5–15, 16–18, 19–21, 22–35, and 36–40 indicate excellent, good, fairly passable, poor, and very poor powder flowability, respectively (Carr, 1965). In the present study, Carr’s indexes of Avicel PH101, MCC grade 1, and MCC grade 2 were found to be 16.15, 16.94, and 17.44, respectively. The compressibility index of MCC grade 1 and grade 2 was found very close to that of Avicel PH101. The lower value of Carr’s index indicates the good flowability of the derived MCC.
On the other hand, the angle of repose is an indirect method of qualifying powder flowability because of their relationship with interparticle cohesion. According to USP, powders with an angle of repose lower than 30° show excellent, while 31°–35° and 36°–40° show good and passable flow properties, respectively. An angle of repose higher than 65° indicates very poor flowability. The angle of repose of the two grades of the derived MCC is below 35, indicating good flow property of the derived sample. Therefore, taking the values of different parameters to evaluate flow property together, we can conclude that the derived MCC showed good flow property, which is comparable with the marketed Avicel PH101 (Table 2).
The SEM photomicrograph (Fig. 3) illustrated that the particles of Avicel PH101 appeared to be similar in size as compared to MCC grade 1 and smaller in size as compared with MCC grade 2. MCC grade 2 has the biggest particle size. Close examination of the SEM photograph of the derived MCC and Avicel PH101 indicates that all the three samples have a rod to spherical shape particle, which might be responsible for good flow property, which further supports the result of the good flow property observed with the derived MCC. On the other hand, the ash value of the derived MCC is lower than 0.04% (Table 1), which complies with the specifications of USP. Moreover, it further suggests that our established method of using a chelating agent might be highly effective in removing the inorganic metals from the starting materials.
Tableting properties of the derived MCC
To test the tableting capabilities of the produced MCC as a directly compressible excipient, we made an aceclofenac tablet and measured several metrics that must match pharmacopeia specifications for tablets. We mixed the active drug with the derived MCC or Avicel PH101 and other required compounds (Table 3) and compressed the mixture to form tablets. Different physical properties of the produced tablets were examined, including hardness, friability, disintegration, and dissolution (Table 3). The formulated tablets were put through hardness and friability tests to see if they could tolerate the physical force during transport. The hardness of tablets prepared using Avicel PH101 and those prepared with MCC grade 1 and grade 2 was 46.6, 44.9, and 50.8 N, respectively. According to USP guidelines, oral tablets should have minimum hardness of 40 N. Moreover, the tablets from Avicel PH101 and those prepared from MCC grades 1 and 2 showed 0.17%, 0.19%, and 0.28% weight loss during the friability analysis. According to USP, the weight loss of the tablets must be lower than 0.8%, indicating that the derived MCC has the ability to produce sufficiently hard tablets which comply with USP specifications.
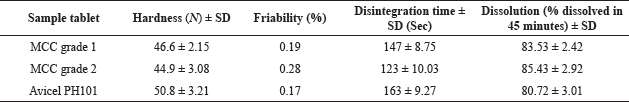 | Table 3. Hardness, friability, disintegration, and dissolution of the tablets from MCC and Avicel PH101. [Click here to view] |
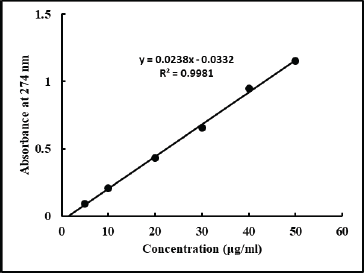 | Figure 4. Standard curve of aceclofenac for the dissolution study of tablets prepared from Avicel PH101 and MCC. [Click here to view] |
The USP disintegration apparatus was used to perform in vitro disintegration of the formulated tablets from MCC. All of the formulated tablets disintegrate in 163 seconds, suggesting that the tablets have an excellent disintegration property. In the present study, we have used the derived MCC from waste paper in the formulation of aceclofenac tablets as the directly compressible agent. In addition to the powder compressibility effect, it also provides a disintegrating effect in tablets which might be due to the high interparticle porosity of the MCC powder (Doelker, 1993). Accordingly, Avicel PH101 and the derived MCC might contribute a disintegrating effect of the prepared tablets along with the super disintegrating agent, sodium starch glycolate. However, the disintegration time of the tablets prepared from the derived MCC is low compared to that prepared from Avicel PH101 (Table 3). This might be due to the lower hardness of the tablets. The higher friability (%) of the prepared tablets from the derived MCC further confirms the lower hardness of the tablets prepared from the derived MCC. Thus, the derived MCC provides lower strength to the prepared tablets resulting in faster disintegration of the tablets.
The USP dissolution equipment II was used to conduct an in vitro dissolution research on the prepared tablets. To assay the released aceclofenac in the dissolution medium after the specified time, we prepared the standard curve of aceclofenac (Fig. 4). The square of the correlation coefficient of the straight line is >0.99, indicating an excellent correlation between the concentration and absorbance value. All the tablets formulations prepared from MCC showed more than 80% drug release in 900 ml of phosphate buffer (pH 6.8) at 100 rpm within 45 minutes. This indicates good dissolution of aceclofenac from the prepared directly compressed tablet dosage forms. Taking the above together, we can conclude that the MCC derived from waste paper is comparable to the marketed Avicel PH101 in terms of different parameters such as identity, particle size (in case of grade 1), and shape and flow property. Moreover, both the grades of MCC are highly suitable to use as a directly compressible excipient in tablets.
CONCLUSION
The present work was undertaken with the motive of utilizing waste paper materials to derive a highly applicable directly compressible excipient, MCC. We were able to derive the MCC from the waste paper by the hydrothermal method. The derived MCC was highly comparable to the marketed Avicel PH101 in terms of identification and different parameters such as bulk density, flow property, and ash value. Moreover, we also confirmed the adequate performance of the derived MCC as a directly compressible agent in tablet formulation by preparing and evaluating aceclofenac tablets.
ACKNOWLEDGMENTS
The authors are thankful to Beximco Pharmaceuticals Ltd., Bangladesh, for giving aceclofenac and Avicel PH101 as a gift.
AUTHORS’ CONTRIBUTIONS
All the authors of this article made significant contributions to generating the concept, design of the experiment, and data analysis. They also took part in drafting the article and critical evaluation during finalizing the report.
CONFLICTS OF INTEREST
The authors report no conflicts of interest in this work.
FUNDING
The research has been financially supported by the Grant for Advanced Research in Education (GARE) from Bangladesh Bureau of Educational Information and Statistics (BANBEIS) under the Ministry of Education, Government of the People’s Republic of Bangladesh (Project ID: PS20191102, No.- 37.20.0000.004.033.020.2016.1053).
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdullah ABM. Production of jute microcrystalline cellulose. J Bangladesh Acad Sci, 1991; 15(2):85–7.
Aulton ME. Tablet. In: Pharmaceutics- the science of dosage form design, Churchill Livingston, New York, 1996.
Azubuike CP, Okhamafe AO. Physicochemical, spectroscopic and thermal properties of microcrystalline cellulose derived from corn cobs. Int J Recycl Org Waste Agric, 2012; 1:1–7. CrossRef
Bowker MJ, Stahl PH. Preparation of water-soluble compounds through salt formation. In: Wermuth CG (ed.). The practice of medical chemistry, Elsevier, Burlington, MA, pp 747–66, 2008. CrossRef
British Pharmacopoeia. Appendix XII A. Disintegration test for tablets and capsules. Ph. Eur. method (2.9.1). London, United Kingdom, 2011a.
British Pharmacopoeia. Appendix XII A. Disintegration test for tablets and capsules. Ph. Eur. method (2.9.1). London, United Kingdom, 2011b.
Bhimte NA, Tayade PT. Evaluation of microcrystalline cellulose prepared from sisal fibers as a tablet excipient: a technical note. AAPS Pharm Sci Technol, 2007; 8(1):E56–62. CrossRef
Bolhuis GH, Chawhan ZT. Materials for direct compaction. In: Alderbon G, Nystrom C, (ed.). Pharmaceutical powder compaction technology, Marcel Dekker, New York, NY, pp 419–501, 1996. CrossRef
Carr RI Jr. Classifying flow properties of solids. Chem Eng, 1965; 1:69–74.
Chauhan YP, Sapkal RS, Sapkal VS, Zamre GS. Microcrystalline cellulose from cotton rags (waste from garment and hosiery industries). Int J Chem Sci, 2009; 7(2):681–8.
Chen LM, Wilson RH, McCann MC. Investigation of macromolecule orientation in dry and hydrated walls of single onion epidermal cells by FTIR microspectroscopy. J Mol Struct, 1997; 408:257–60. CrossRef
Chuayjuljit S, Su-uthai S, Charuchinda S. Poly(vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: properties and biodegradability study. Waste Manag Res, 2010; 28:109–17. CrossRef
Diana C, Florin C, Valentin IP. Amrorphous cellulose—structure and characterization. Cellulose Chem Technol, 2011; 45:13–21.
Doelker E. Comparative compaction properties of various microcrystalline cellulose types and generic products. Drug Dev Ind Pharm, 1993; 19:2399–471. CrossRef
Ejikeme PM. Investigation of the physicochemical properties of microcrystalline cellulose from agricultural wastes I: Orange mesocarp. Cellulose, 2008; 15(1):141–7. CrossRef
El-Sakhawy M, Hassan ML. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr Polym, 2007; 67:1–10. CrossRef
Hakansson H, Ahlgren P. Acid hydrolysis of some industrial pulps, effects of hydrolysis conditions and raw materials. Cellulose, 2005; 12:177–83. CrossRef
Ilindra A, Dhake JD. Microcrystalline cellulose from bagasse and rice straw. Int J Chem Technol, 2008; 15(5):497–9.
Jahan MS, Saeed A, He Z, Ni Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose, 2011; 18(2):451–9. CrossRef
Kacurakova M, Belton PS. Wilson RH, Hirsch J. Hydration properties of xylan-type structures: an FT-IR study of xylooligosaccharides. J Sci Food Agri, 1998; 77:38–44. CrossRef
Landín M, Martínez-Pacheco R, Gómez-Amoza JL, Souto C, Concheiro A, Rowe RC. Effect of batch variation and source of pulp on the properties of microcrystalline cellulose. Int J Pharm, 1993; 91:133–41. CrossRef
Monschein M, Reisinger C, Nidetzky B. Enzymatic hydrolysis of microcrystalline cellulose and pretreated wheat straw: a detailed comparison using convenient kinetic analysis. Bioresour Technol, 2013; 128:679–87. CrossRef
Okhamafe, AO, Igboechi A, Obaseki TO. Celluloses extracted from groundnut shell and rice husk 1: preliminary physicochemical characterization. Pharm World J, 1991; 8 (4):120–30.
Owolabi AF, Hafiz MMK, Hossain MS, Fazita MRN. Influence of alkali hydrogen peroxide prehydrolysis on the isolation of microcrystalline cellulose from oil palm fronds. Int J Biol Macromol, 2017; 95:1228–34. CrossRef
Padmadisastra Y, Gonda I. Preliminary studies of the development of a direct compression cellulose excipient from bagasse. J Pharm Sci, 1989; 78(6):508–21. CrossRef
Paralikar KM, Bhatawdekar SP. Microcrystalline cellulose from bagasse pulp. Biol Wastes, 1988; 24:75–7. CrossRef
Rashid M, Gafur MA, Sharafat MK, Minami H, Miah MAJ, Ahmad H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr Polym, 2017; 170:72–9. CrossRef
Revol JF, Stewart D, Wilson HM, Hendra PJ, Morrison IM. On the cross-sectional shape of cellulose crystallites in Valonia ventricosa. Carbohydr Polym, 1982; 2:123–34. CrossRef
Islam SMA, Islam S, Shahriar M, Dewan I. Comparative in vitro dissolution study of aceclofenac marketed tablets in two different dissolution media by validated analytical method. J Appl Pharm Sci, 2011; 1(09):87–92.
Ohwoavworhua FO, Kunle OO, Ofoefule. Extraction and characterisation of microcrystalline cellusose derived from luffa cylindrica plant. Afr J Pharm Res Develop, 2004; 1(1):1–6.
Podczeck F, Course N, Newton JM, Short MB. Gastrointestinal transit of model mini-tablet controlled release oral dosage forms in fasted human volunteers. J Pharm Pharmacol, 2007; 59: 941–5. CrossRef
Shlieout G, Arnold K, Muller G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS Pharm Sci Tech, 2002; 3:E11. CrossRef
Sihtola H, Kyrklund B, Laamanen L, Palenius I. Comparison and conversion of viscosity and DP-values determined by different methods. Paperi ja Puu, 1963; 45(4a):225–32.
Train D. Some aspects of the property of angle of repose of powders. J Pharm Pharmacol, 1958; 10(1):127T–35T. CrossRef
Uesu NY, Pineda EA, Hechenleitner AA. Microcrystalline cellulose from soybean husk: effects of solvent treatments on its properties as acetylsalicylic acid carrier. Int J Pharm, 2000; 206:85–96. CrossRef
United States Pharmacopeia. 2007. Powder flow. PF 28(2). p 618, 2020
USP 36-NF 31. Monograph: microcrystalline cellulose, p 643. Rockville, MD.
Wu JS, Ho HO, Sheu MT. A statistical design to evaluate the influence of manufacturing factors on the material properties and functionalities of microcrystalline cellulose. Euro J Pharm Sci, 2001; 12:417–25. CrossRef
Zhang C, Li D, Yu H, Zhang B, Jin F. Purification and characterization of piceid-B-D-glucosidase from Aspergillusoryzae. Process Biochem, 2007; 42:83–8. CrossRef