Formulation and evaluation of Salbutamol sulphate microspheres by solvent evaporation method
V.V Prasanth, Akashmoy Chakraborty, Sam T Mathew, Rinku Mathappan, V. Kamalakkannan
Pages: 133-137


Enteric coated tablets of novel proton pump inhibitor with super disintegrants design, in-vitro evaluation and stability studies
Putta Rajesh Kumar, Hiremath Doddayya, S. Rajendra Reddy
Pages: 106-111

New Trends in the Co-crystallization of Active Pharmaceutical Ingredients
Veerendra K. Nanjwade, F. V. Manvi, Shamrez Ali. M, Basavaraj K. Nanjwade, Meenaxi M. Maste
Pages: 01-05

Novel Vesicular System: An Overview
Seema M. Jadhav, Pournima Morey, Mrs. Manisha Karpe, Vilasrao Kadam
Pages: 193-202

Evaluation of Transdermal Formulations: A Technical Note
Mudasir Mohamad, Roheena Jan
Pages: 37-40

Stability Testing of Pharmaceutical Products
Sanjay Bajaj, Dinesh Singla, Neha Sakhuja
DOI: 10.7324/JAPS.2012.2322Pages: 129-138

Studies on directly compressed ondansetron hydrochloride mucoadhesive buccal tablets using gelatin, chitosan and xanthan gum along with HPMC K4M
Syed Amezuddin Azhar, Putta Rajesh Kumar, Vivek Sood, Somashekar Shyale
DOI: 10.7324/JAPS.2012.2517Pages: 100-105

Development, physical-chemical stability and rheological behavior of silicones formulations containing Dimethylaminoethanol (DMAE)
Paula Souza Prestes, Roberta Balansin Rigon, Gustavo Narvaes Guimarães, Maria Luiza Ozores Polacow, Maria Silvia Mariani Pires-de-Campos, Marlus Chorilli, Gislaine Ricci Leonardi
DOI: 10.7324/JAPS.2013.30201Pages: 001-005

Stability Indicating HPLC Method for the Determination of Hydrochlorothiazide in Pharmaceutical Dosage form
Sachin Bhagwate and N. J. Gaikwad
DOI: 10.7324/JAPS.2013.30215Pages: 088-092

Design of dissolution media for in-vitro bioequivalence testing of Lamivudine
Nagiat T Hwisa, Shanta Kumari Adiki, Prakash Katakam, Babu Rao Chandu
DOI: 10.7324/JAPS.2013.3617Pages: 106-110

Stability-indicating HPLC-DAD method for the determination of Granisetron hydrochloride in its pharmaceutical preparations
Mokhtar Mabrouk, Hamed El-Fatatry, Ismail Hewala and Ehab Emam
DOI: 10.7324/JAPS.2013.3633Pages: 189-202

Validation of a Stability-Indicating Assay of Amprolium Hydrochloride in Water Soluble Powder Formulation using Hydrophilic Interaction Liquid Chromatography
Mashhour Ghanem and Saleh Abu-Lafi
DOI: 10.7324/JAPS.2013.31009Pages: 051-058

Stability indicating chromatographic techniques for the determination of pipoxolan HCl
Hala E. Zaazaa, Afaf O. Mohamed, Mohamed Abdelkawy and Maha A.Hawwam
DOI: 10.7324/JAPS.2013.31011Pages: 066-073

Stability indicating spectrophotometric methods for determination of Tiemonium methylsulphate in the presence of its degradation products
Hala E. Zaazaa, Samah S. Abbas, Zeinab A.EL- Sherif, Badr El-Zeany and Dalia A. ELÙ€Haddad
DOI: 10.7324/JAPS.2014.40106Pages: 033-045

Development and Validation of a Stability Indicating Spectrofluorimetric Method for the Determination of Lanzoprazole via its Degradation Product
Ghaleb Oriquat, Afaf Osman, Mohammad Abdul-Azim and Sawsan Abuhamdah
DOI: 10.7324/JAPS.2014.40410Pages: 057-061

Thermal analysis on phase sensitive granisetron in situ forming implants
Evren ALGIN YAPAR, Tamer BAYKARA
DOI: 10.7324/JAPS.2014.40702Pages: 010-013

Evaluation of the quality and stability of amoxicillin oral suspension
Blanca Elena Ortega Markman, Maria Regina Walter Koschtschak, Elizabeth Wu Meihuey, Paulo Cesar Pires Rosa
DOI: 10.7324/JAPS.2014.40706Pages: 038-040

Simple Spectrophotometric and HPTLC-Densitometric Methods for Determination of Cefdinir in Bulk Powder and Pharmaceuticals, and in Presence of its Hydrolytic Degradation Products
Omar Abdel-Aziz, Maha Farouk, Reham Nagi, Laila Abdel-Fattah
DOI: 10.7324/JAPS.2014.40722Pages: 129-136

DoE Approach: A Stability Indicating RP-HPLC Method for Simultaneous Estimation of Methylparaben, Mometasone furoate and Eberconazole nitrate in Topical Formulations
Prakashkumar B. Modi, Nehal J. Shah
DOI: 10.7324/JAPS.2014.41204Pages: 020-025

Stability Indicating RP-HPLC Assay Method for Estimation of Dronedarone Hydrochloride in Tablet
Atul T Hemke, Shivshanker Kukade, Rajesh T Lohiya, Krishna R Gupta
DOI: 10.7324/JAPS.2015.50516Pages: 083-088

A Five-Year Stability Study of Controlled-Release Diltiazem Hydrochloride Tablets Based on Poly(Ethylene Oxide)
Laila H. Emara, Ahmed A. El-Ashmawy, Nesrin F. Taha
DOI: 10.7324/JAPS.2015.50703Pages: 012-022

Antibacterial, antitussive, antioxidant and toxicological evaluation of Joshanda lozenges
Monika Bansal, Monica Gulati, Sachin Kumar Singh, Sanjiv Duggal
DOI: 10.7324/JAPS.2015.50711Pages: 064-070

Formulation and Characterization of Ketoprofen Emulgels
Ramakanth Ambala, Sateesh Kumar Vemula
DOI: 10.7324/JAPS.2015.50717Pages: 112-117

A novel stability indicating HPLC-method for simultaneous determination of atenolol and nifedipine in presence of atenolol pharmacopeoial impurities
Hisham Hashem, Ibrahim Adel Ehab, Elhenawee Magda
DOI: 10.7324/JAPS.2015.50804Pages: 017-025

Fabrication, Physicochemical Characterization and Evaluation of In vitro Anticancer Efficacy of a Novel pH Sensitive Polymeric Nanoparticles for Efficient Delivery of Hydrophobic Drug against Colon Cancer
Manikandan Mahalingam, Kannan Krishnamoorthy
DOI: 10.7324/JAPS.2015.501123Pages: 135-145

Determination of the Chemical Stability of Various Formulations of Tobramycin Eye-Drops by HPLC Method and Data Analysis by R-GUI Stability Software
María Ana Rosasco, Adriana Inés Segall
DOI: 10.7324/JAPS.2015.501202Pages: 008-013

Validated, Ultra High Efficiency RP-HPLC and Stability Indicating Method for Determination of Tranylcypromines Sulphate in Bulk and in Tablet Dosage Forms
Gamal H. Ragab, Hanaa M. Saleh, Magda M. EL-Henawee, Omnia F. Elsayed
DOI: 10.7324/JAPS.2016.60209Pages: 064-071

Development and Validation of a Stability Indicating HPLC Method for the Simultaneous Analysis of Esomeprazole and Itopride in Bulk and In Capsules
M. Nageswara Rao, K. B. M. Krishna, B. Hari Babu
DOI: 10.7324/JAPS.2016.60210Pages: 072-080

Spectroscopic Investigations for Photo Stability of Diclofenac Sodium Complexed with Hydroxypropyl-β-Cyclodextrin
Alagumuthu Manikandan, Shubhada Chandrasekhar Nemani, V. Sadheeshkumar, Sivakumar Arumugam
DOI: 10.7324/JAPS.2016.60414Pages: 098-103

Determination of Benzalkonium Chloride in Ophthalmic Solutions by Stability-Indicating HPLC Method: Application to a Stability Study
Hashem AlAani, Yasmin AlNukkary
DOI: 10.7324/JAPS.2016.60513Pages: 080-089

Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Sumatriptan in Bulk and Pharmaceutical Dosage Form
M. Srinidhi, Md. Mushabbar Basha, V. Raj Kumar, J. Rajendra Kumar
DOI: 10.7324/JAPS.2016.60604Pages: 020-025

Development and validation of a stability indicating HPLC-diode array-fluorescence method for the determination of meclofenoxate hydrochloride and p-chlorophenoxyacetic acid
Marwa Said Moneeb, Feda Elgammal, Suzy Mohamed Sabry
DOI: 10.7324/JAPS.2016.60701Pages: 001-011

A Simple and Sensitive HPLC/UV Method for Determination of Meloxicam in Human Plasma for Bioavailability and Bioequivalence Studies
Laila H. Emara, Maha F. Emam, Nesrin F. Taha, Hala M. Raslan, Ahmed A. El-Ashmawy
DOI: 10.7324/JAPS.2016.60702Pages: 012-019

A Sensitive Ultra-Fast Bioanalytical Method for the Quantification of Rabeprazole in Human Plasma
Shankar S. Velan, Suneetha Vuppu
DOI: 10.7324/JAPS.2016.60726Pages: 178-183

Development of Validated Stability Indicating RP-HPLC-PDA Method for Camptothecin Analysis
Buchi N. Nalluri, Saisrianusha Valluru, Chandrapriyanka Bonthu
DOI: 10.7324/JAPS.2016.60921Pages: 140-146

Physical stabilization of amorphous itraconazole in solid dispersions for improved dissolution profile
Yogesh Vilas Pore, Vikram Ramchandra Shinde, J. Venkateswara Rao
DOI: 10.7324/JAPS.2016.601005Pages: 037-044

A Simple RP-HPLC Method Development and Validation for the Simultaneous Estimation of Naproxen and Rabeprazole
Mohammad Firoz Khan, Shaila Sharmin Zuthi, Md. Shahidulla Kayser, Md. Shariful Islam, Sharmeen Asad, Mohammad A. Rashid
DOI: 10.7324/JAPS.2016.601123Pages: 147-152

Development, characterization and cytotoxicity evaluation of Gingko biloba extract (EGB761) loaded microemulsion for intra-nasal application
Manisha Singh, Surya Pratap Singh, R. Rachana
DOI: 10.7324/JAPS.2017.70104Pages: 024-034

Stability Indicating HPLC Method for the Simultaneous Quantification of Aspirin and Pravastatin in bulk and Tablets: Method Development and Validation
Ravi Varma Athota, Shanmukha Kumar Jagarlapudi, Mutta Reddy Singampalli
DOI: 10.7324/JAPS.2017.70308Pages: 048-056

Development of Natural Preservative from Silene vulgaris Extract in Topical Formulation under a Challenge Test and its Stability Stud
Smahane BOUKHIRA, Mounyr BALOUIRI, Latifa El MANSOURI, Amal El HAMSAS El YOUBI, Mouna BOUARFA, Siham LEBTAR, Ahmed OUHAMMOU, Dalila BOUSTA
DOI: 10.7324/JAPS.2017.70421Pages: 142-148

A Validated Stability Indicating Reversed Phase High Performance Liquid Chromatographic Method of Leflunomide and Characterization of Its Degradation Products through Retro-Synthesis
Tapas Kumar Laha, Subrata Sen
DOI: 10.7324/JAPS.2017.70503Pages: 012-017

Development of Brazil nut oil microemulsion as vehicle for Levamisole
Pricila Castilho Gustmann, Aron Carlos de Melo Cotrim, Evaldo Martins Pires, Carla Regina Andrighetti, Dênia Mendes Sousa Valladão, Elton Brito Ribeiro
DOI: 10.7324/JAPS.2017.70813Pages: 092-098

Hot melt extrusion method for preparation of ibuprofen/sucroester WE15 solid dispersions: evaluation and stability assessment
Laila H. Emara, Fatma M. Abdelfattah, Nesrin F. Taha
DOI: 10.7324/JAPS.2017.70822Pages: 156-167

Development and validation of a stability-indicating RP-HPLC method for the detection and quantification of azithromycin in bulk, and self-emulsifying drug delivery system (SEDDs) formulation
Reem Abou Assi, Yusrida Darwis, Ibrahim M. Abdulbaq, Shaik Mohammed Asif
DOI: 10.7324/JAPS.2017.70903Pages: 020-029

Stability-indicating RP- HPLC -DAD method for the simultaneous estimation of Tramadol HCl and Diclofenac sodium
Ramalingam Peraman, D. Subba Rao, Rajesh Reddy Kadiri, Amaranatha Reddy Bommireddy
DOI: 10.7324/JAPS.2017.70912Pages: 085-093

Pharmaceuticals in the tropics: A quantitative study measuring changes in quantity of the active ingredient and microbiological growth
Bharath Raman, Hana Morrissey, Patrick Ball
DOI: 10.7324/JAPS.2017.70922Pages: 160-170

Biochemical Characterization of Recombinant Cu–Zn SOD from Citrus limon Fused to Gliadin Peptides
Ratna Annisa Utami, Sukmadjaja Asyarie, Debbie Soefie Retnoningrum
DOI: 10.7324/JAPS.2018.8117Pages: 115-121

Development and characterization of microemulsions containing Tiliacora triandra Diels as an active ingredient for antioxidant and melanogenesis stimulating activities
Sitthiphong Soradech, Pokchut Kusolkumbot, Sirinan Thubthimthed
DOI: 10.7324/JAPS.2018.8307Pages: 046-054

Formulation and Evaluation of Lactic Acid Bacteria Fermented Brassica juncea (Mustard Greens) Pickle with Cholesterol Lowering Property
Chaiyavat Chaiyasut, Periyanaina Kesika, Sasithorn Sirilun, Sartjin Peerajan, Bhagavathi Sundaram Sivamaruthi
DOI: 10.7324/JAPS.2018.8405Pages: 033-042

A Sensitive, Stability indicating UPLC method for the identification and characterization of forced degradation products for Drometrizole Trisiloxane through MSn studies
M. Ajay Babu, G. V. Krishna Mohan, J. Satish, Pradipbhai D. Kalariya, CH. Krishnam Raju, Sharad D. Mankumare
DOI: 10.7324/JAPS.2018.8609Pages: 065-074

In vitro and In vivo Anti-inflammatory Activities of Coptosapelta flavescens Korth Root’s Methanol Extract
Khemasili Kosala, Moch. Aris Widodo, Sanarto Santoso, Setyawati Karyono
DOI: 10.7324/JAPS.2018.8907Pages: 042-048

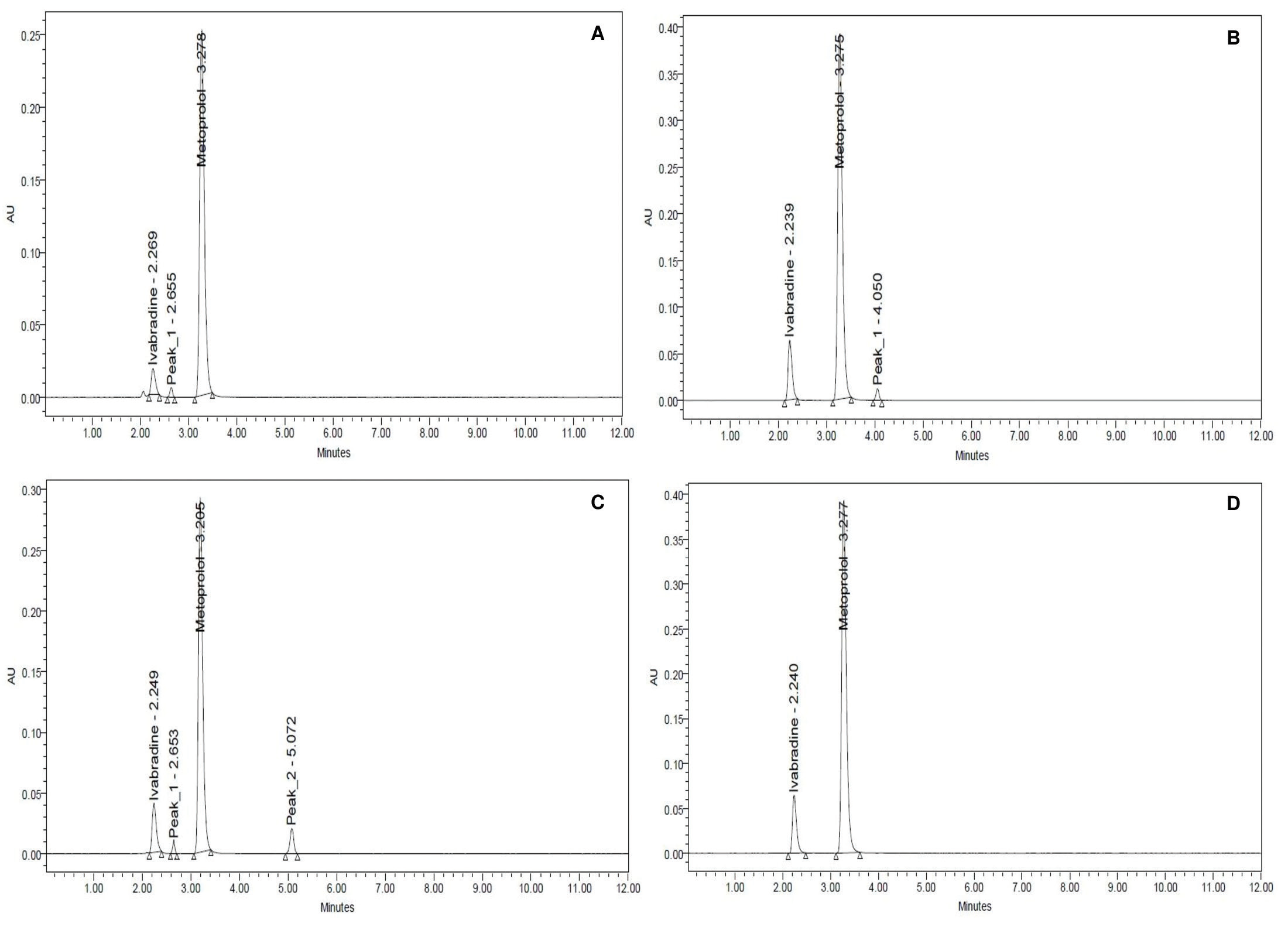
Stability indicating RP-HPLC method for the simultaneous estimation of ivabradine and metoprolol in bulk and tablet formulation
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90418Pages: 137-144
Development of validated stability indicating RP-HPLC method for the estimation of glecaprevir and pibrentasvir in bulk and pharmaceutical dosage form
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90607Pages: 052-060

Inclusion complexes of atorvastatin calcium–sulfobutyl ether β cyclodextrin with enhanced hypolipidemic activity
Anureet Arora, Geeta Aggarwal, Thakur Gurjeet Singh, Manjinder Singh, Gitika Arora, Manju Nagpal
DOI: 10.7324/JAPS.2019.91108Pages: 060-068

Evidencing the impact of drug store storage conditions on the quality and stability of amoxicillin powders for oral suspension marketed in Peru
Consuelo Del Pilar Vega-Zambrano, León F. Villegas Vílchez
DOI: 10.7324/JAPS.2019.91212Pages: 088-093

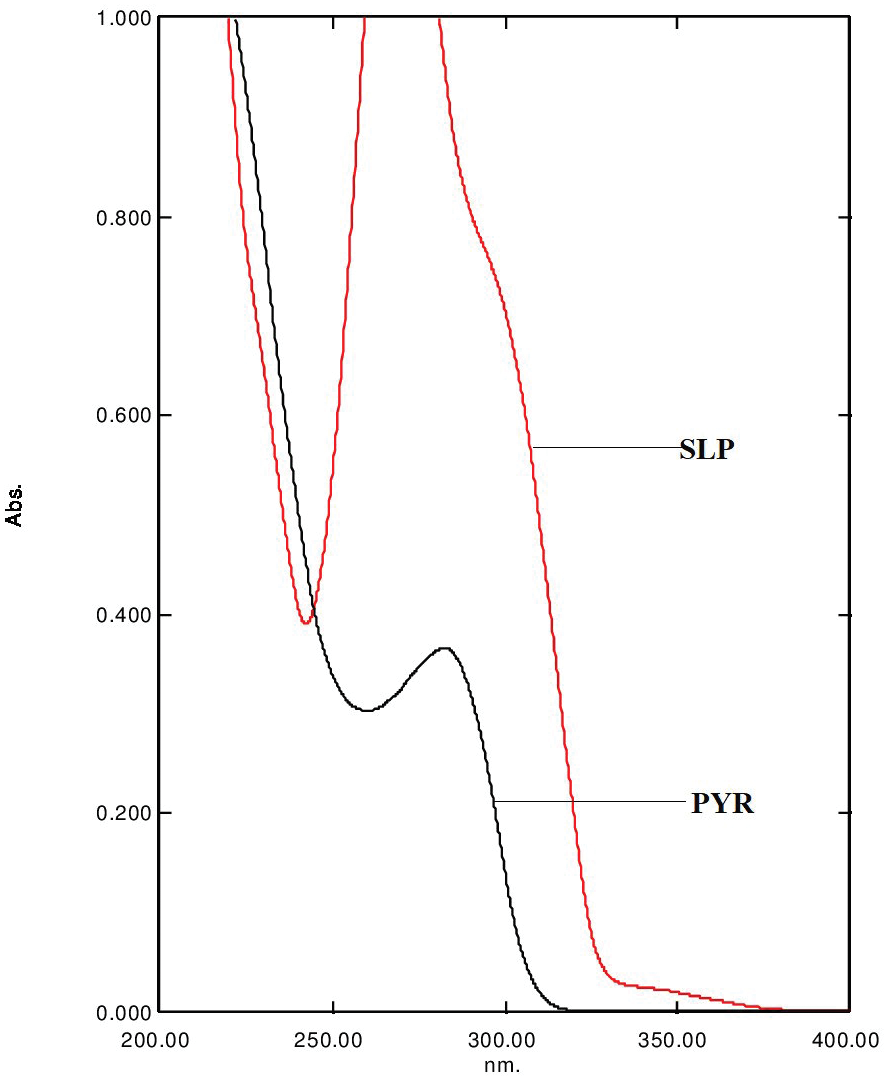
Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107

Formulation and stability studies of metformin hydrochloride in a controlled porosity osmotic pump system
Hanan M. Hashem, Aya R. Abdou, Nesrin F. Taha, Nadia M. Mursi, Laila H. Emara
DOI: 10.7324/JAPS.2020.104013Pages: 100-112

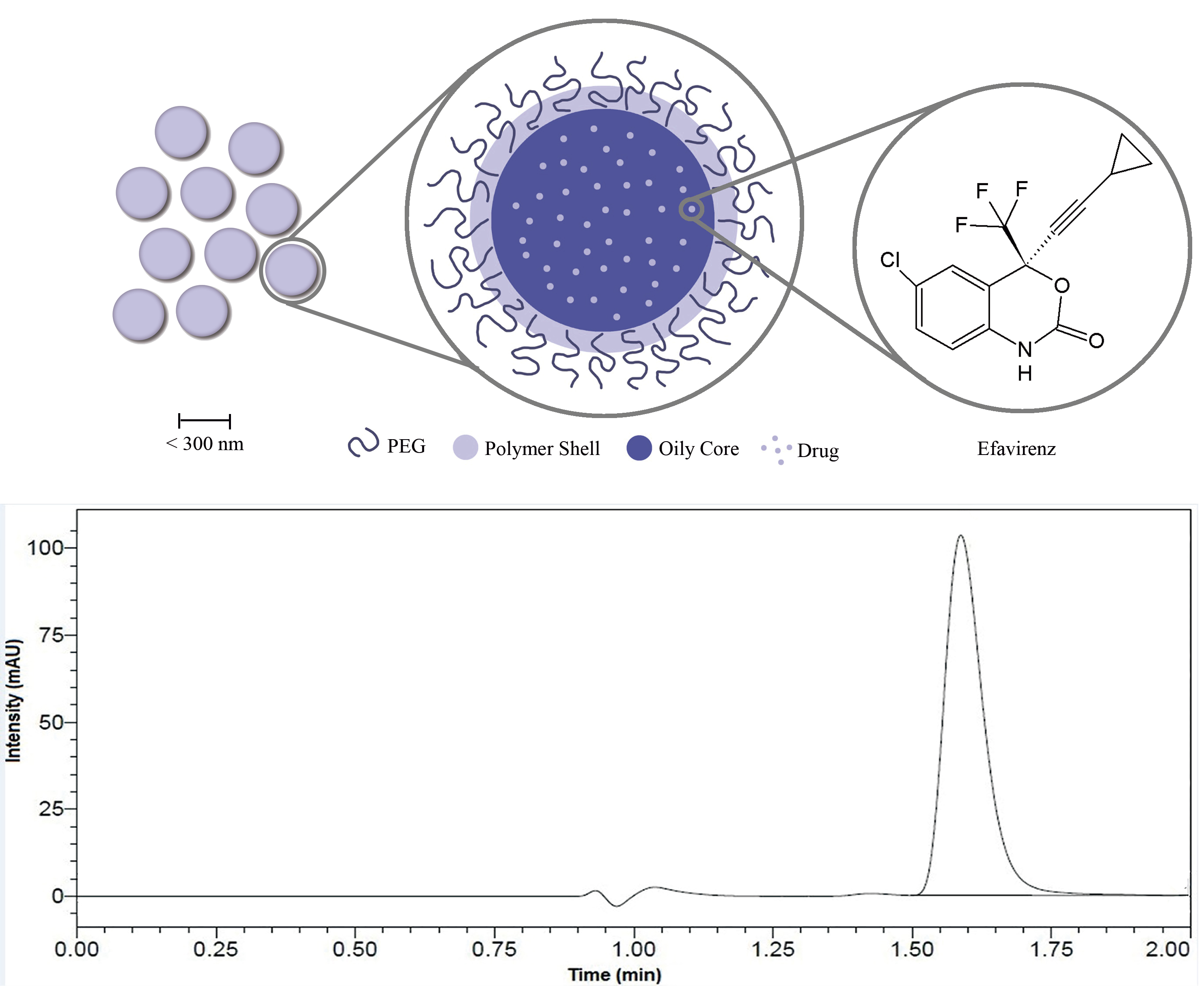
Efavirenz-loaded polymeric nanocapsules: Formulation, development, and validation of an RP-UHPLC-DAD method for drug quantification, determination of encapsulation efficiency, stability study, and dissolution profile
Amanda Martinez Lyra, Juliana Parente Menezes Ribeiro, Jessica Mendes Nadal, Sinvaldo Baglie, Traudi Klein, Andressa Novatski, Paulo Vitor Farago
DOI: 10.7324/JAPS.2021.110212Pages: 093-101
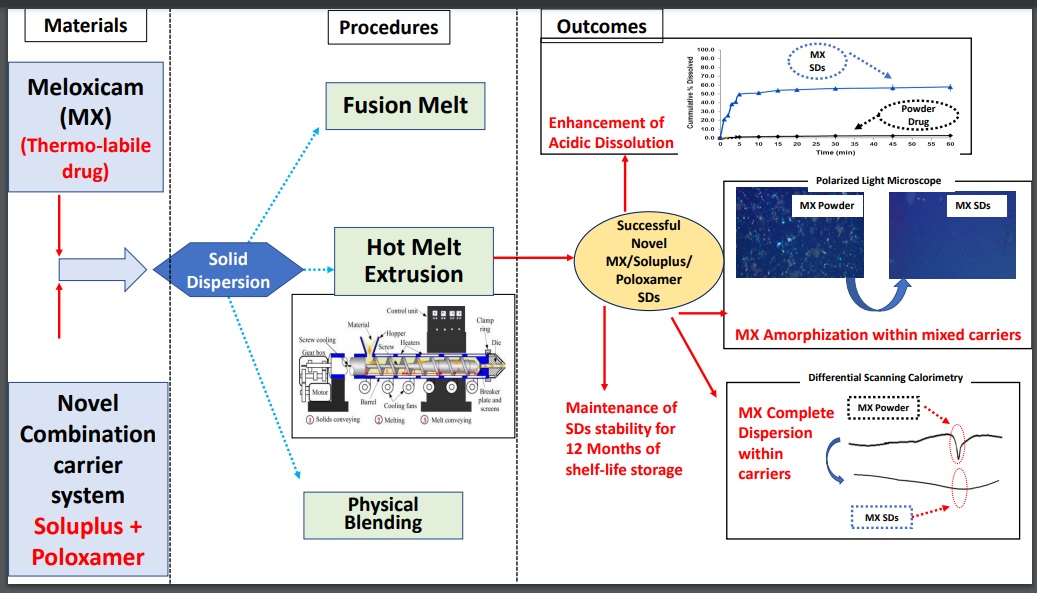
A novel combination of Soluplus® and Poloxamer for Meloxicam solid dispersions via hot melt extrusion for rapid onset of action—part 1: dissolution and stability studies
Maha F. Emam, Nesrin F. Taha, Laila H. Emara
DOI: 10.7324/JAPS.2021.110218Pages: 141-150
Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053

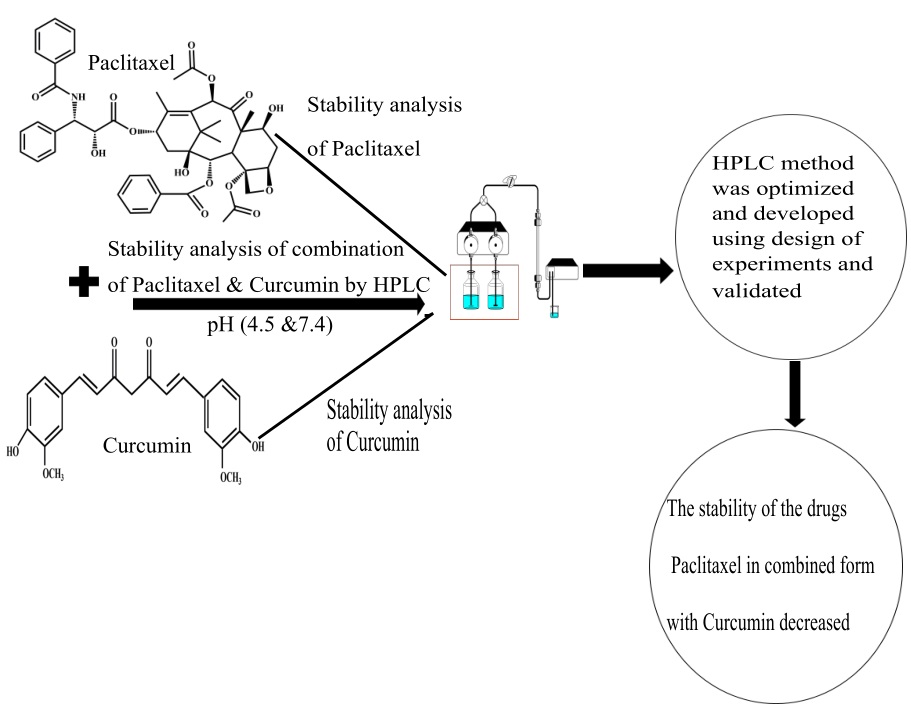
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
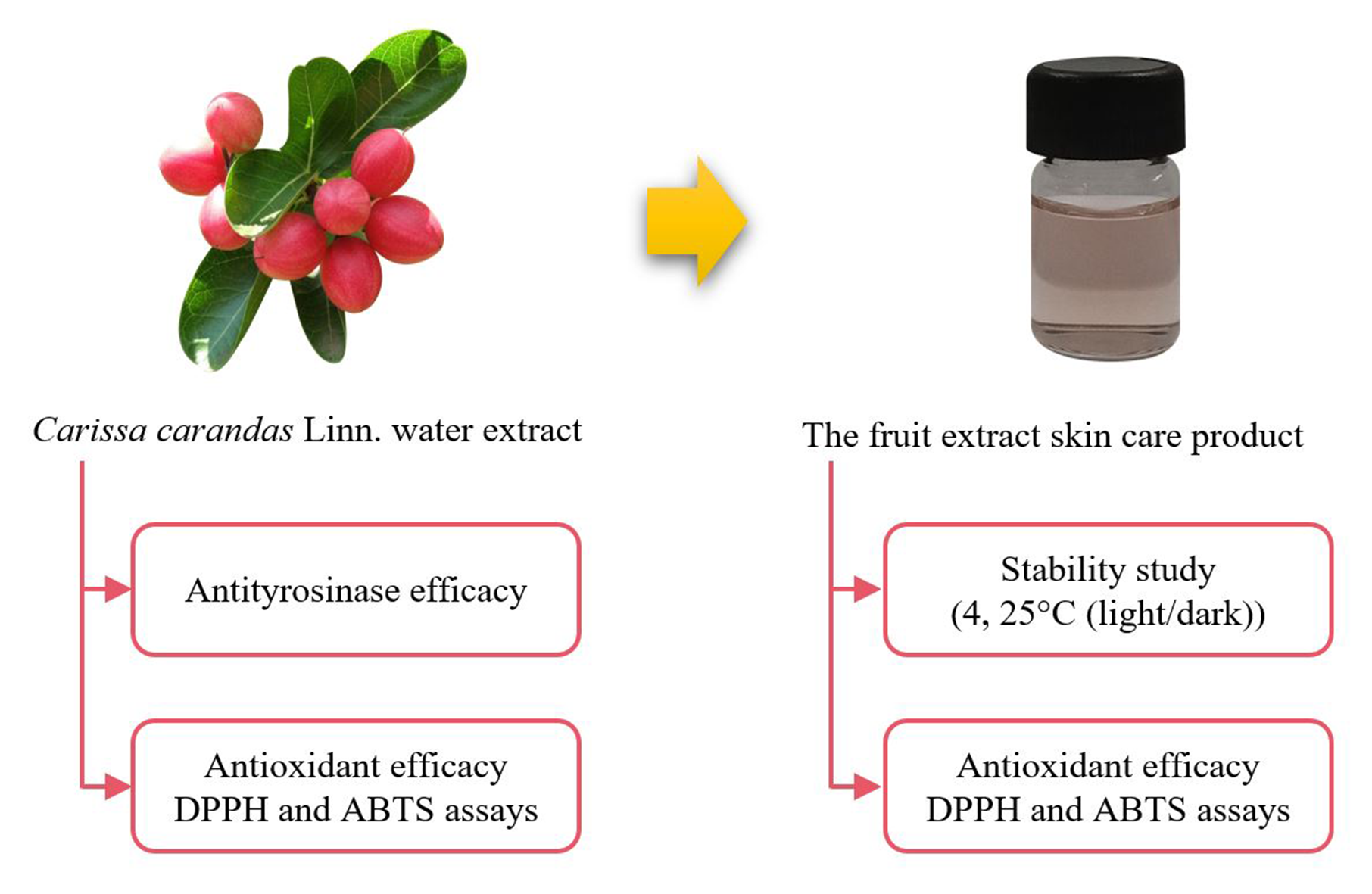
The evaluation of antioxidant and antityrosinase efficacy of Carissa carandas fruit extracts and the development of a preliminary skincare product
Nadechanok Jiangseubchatveera, Charinrat Saechan, Nattawut Leelakanok, Arpa Petchsomrit
DOI: 10.7324/JAPS.2021.110717Pages: 153-157
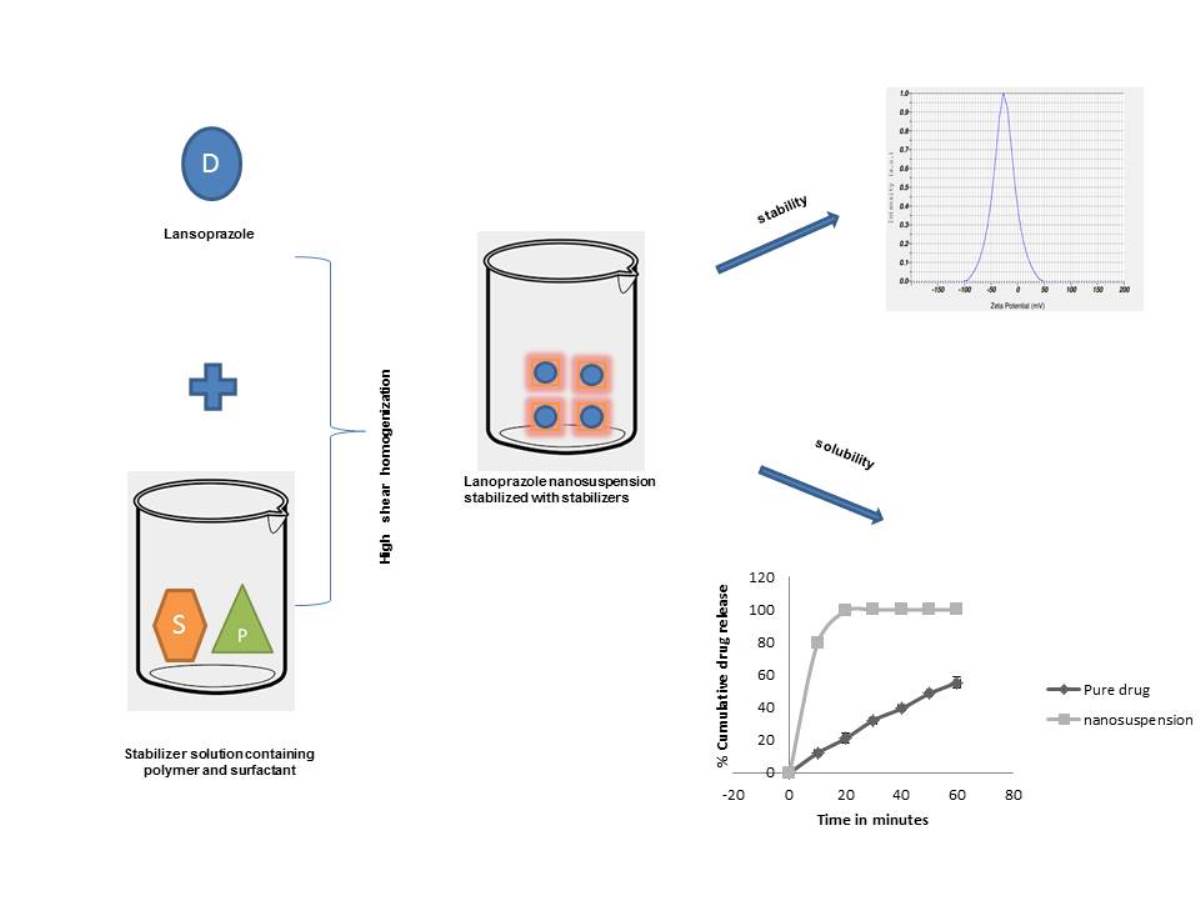
Effect of various stabilizers on the stability of lansoprazole nanosuspension prepared using high shear homogenization: Preliminary investigation
Shobha Ubgade, Aditi Bapat, Vaishali Kilor
DOI: 10.7324/JAPS.2021.110910Pages: 085-092
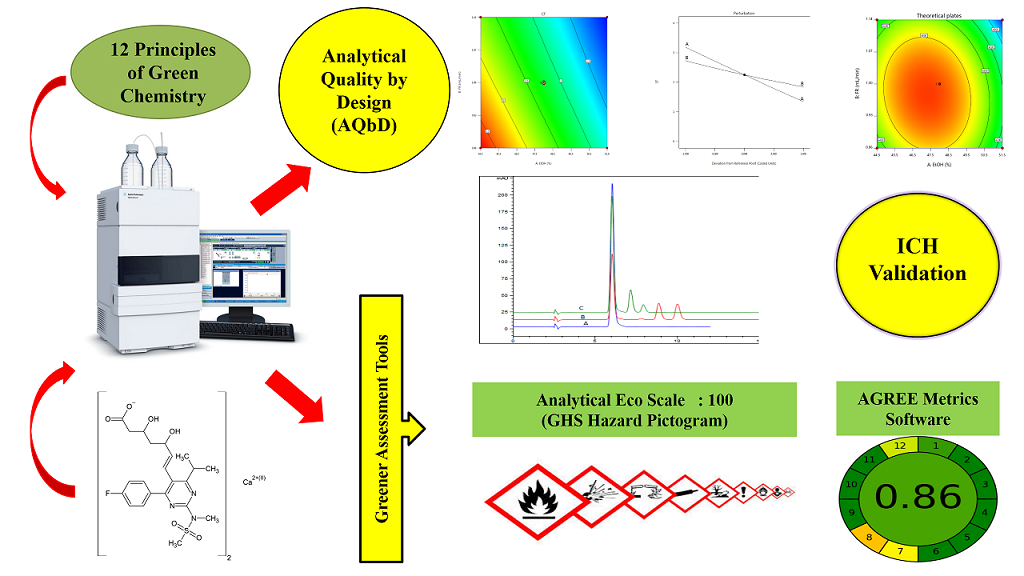
Analytical quality by design approach for estimating rosuvastatin calcium in pharmaceutical formulation by green HPLC method: Ecologically evaluated and stability-indicating
Seetharaman Rathinam, Lakshmi Karunanidhi Santhana
DOI: 10.7324/JAPS.2021.1101119Pages: 150-160
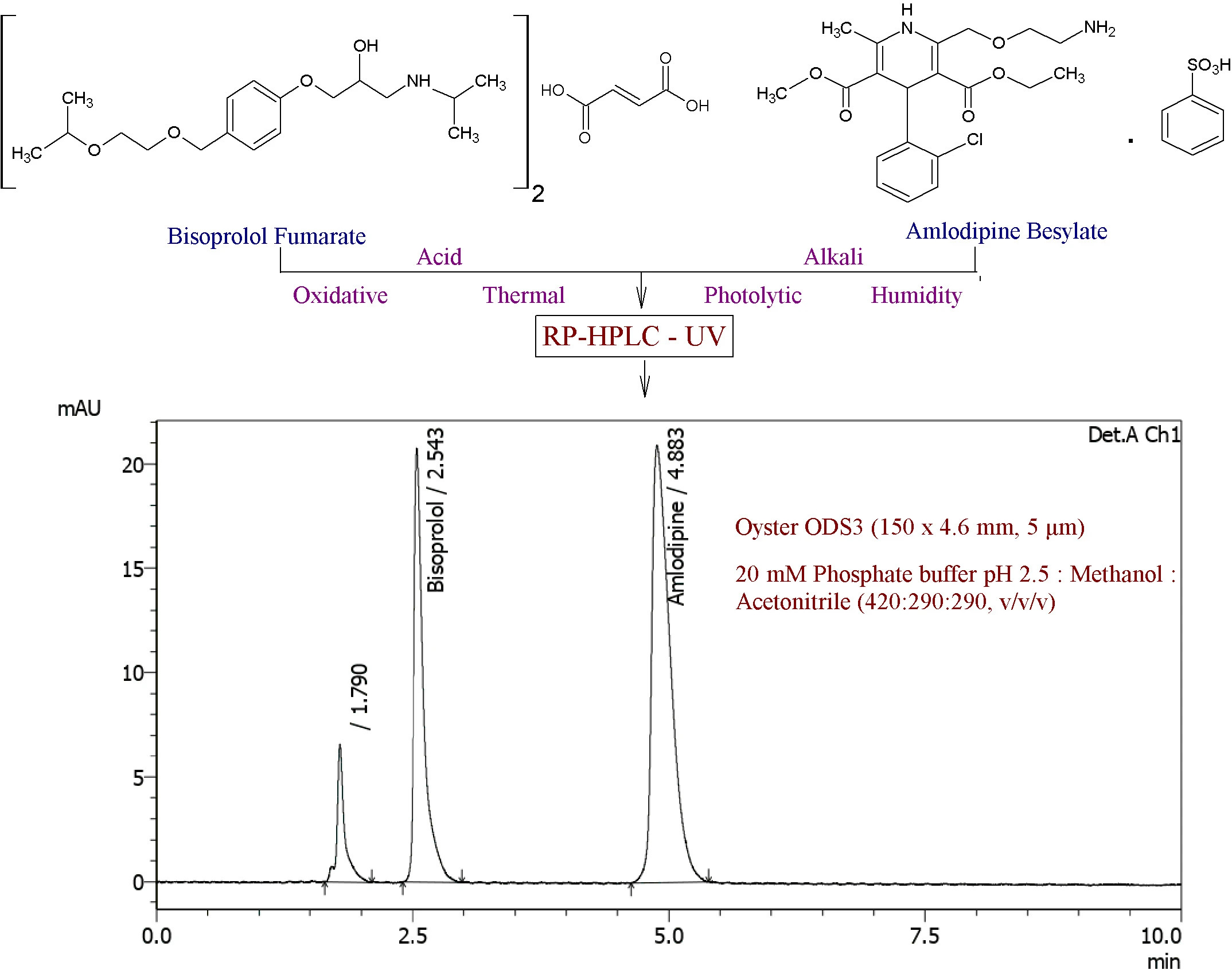
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form
Rameshwar Bhausaheb Gholve, Sanjay Sudhakar Pekamwar, Tukaram Mohanrao Kalyankar
DOI: 10.7324/JAPS.2021.1101211Pages: 121–134
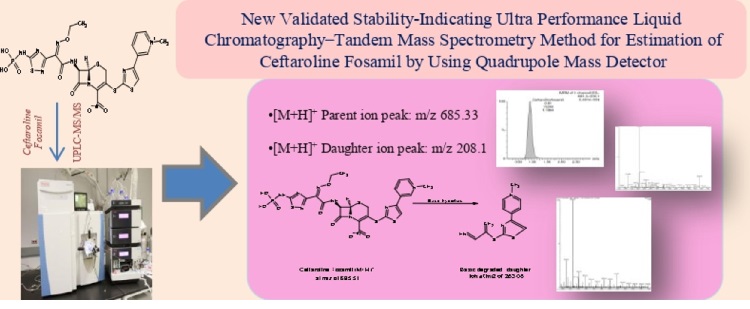
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
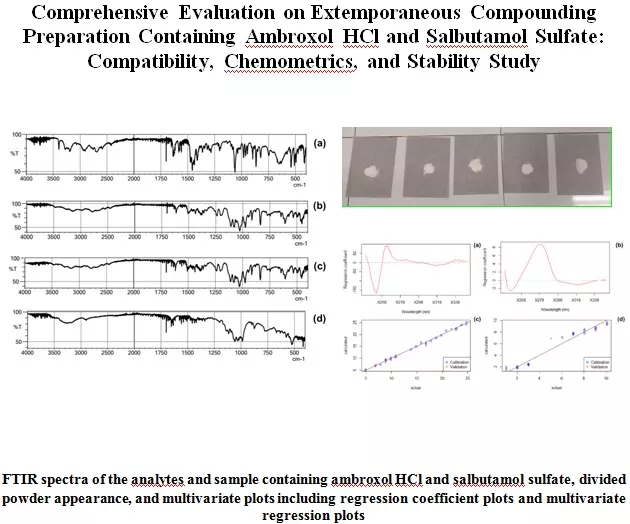
Comprehensive evaluation of extemporaneous preparation containing ambroxol HCl and salbutamol sulfate: Compatibility, chemometrics, and stability study
Michael Raharja Gani, Jeffry Tanriono, Florentinus Dika Octa Riswanto, Dina Christin Ayuning Putri, Dita Maria Virginia, Sri Hartati Yuliani
DOI: 10.7324/JAPS.2022.120912Pages: 105-113
Acid borne oxidative impurities of Naloxone hydrochloride injection: Enrichment, isolation and characterization
Praveen Basappa, Venugopala Rao Dama, M. S. Uma Shankar
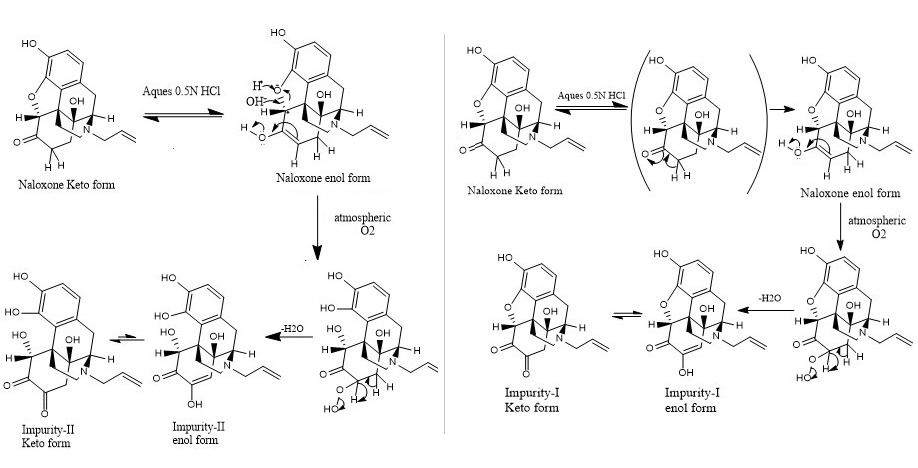
DOI: 10.7324/JAPS.2022.121201Pages: 001-011
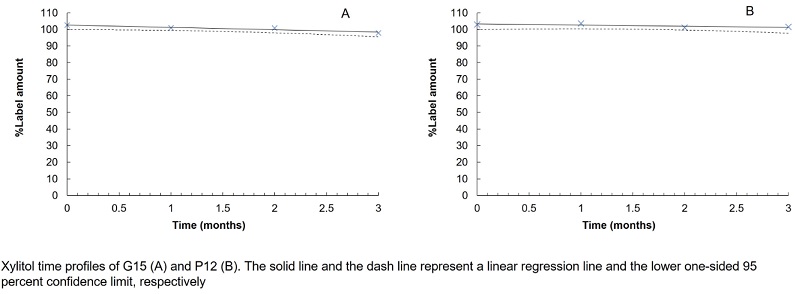
Formulation and evaluation of dental gels and pastilles containing xylitol for dental caries
Walaisiri Muangsiri, Pornpen Werawatganone, Sirilak Sailo, Thanchanit Thaipitakwong
DOI: 10.7324/JAPS.2022.120911Pages: 096-104
Factorial design-based fabrication of biopolymer-functionalized Asiatic acid-embedded liposomes: in-vitro characterization and evaluation
Namdev Dhas, Hosadurga Shantharam Preetha, Akhilesh Dubey, Gundawar Ravi, Induja Govindan, Annamalai Rama, Anup Naha, Srinivas Hebbar
DOI: 10.7324/JAPS.2022.121108Pages: 071-081

Floating tablets incorporating curcumin solid dispersion as a potential pharmaceutical dosage form for stomach cancer treatment
Duyen Thi My Huynh, Viet-Hung Tran, Minh-Ngoc T. Le, Van-Hoa Huynh, Duy Toan Pham
DOI: 10.7324/JAPS.2023.114417Pages: 240-250

A stability-indicating reverse phase-HPLC method development and validation for newly approved drug, Belzutifan in bulk and pharmaceutical dosage forms
Dumpala Sravya, Banoth Ramya Kuber
DOI: 10.7324/JAPS.2023.114351Pages: 233-240

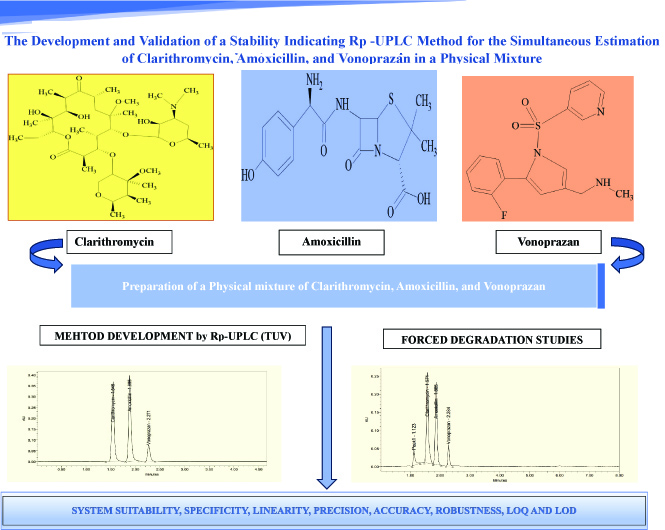
The development and validation of a stability indicating RP-UPLC method for the simultaneous estimation of clarithromycin, amoxicillin, and vonoprazan in a physical mixture
Charumathi Salva, Rajitha Galla
DOI: 10.7324/JAPS.2024.165836Pages: 193-202
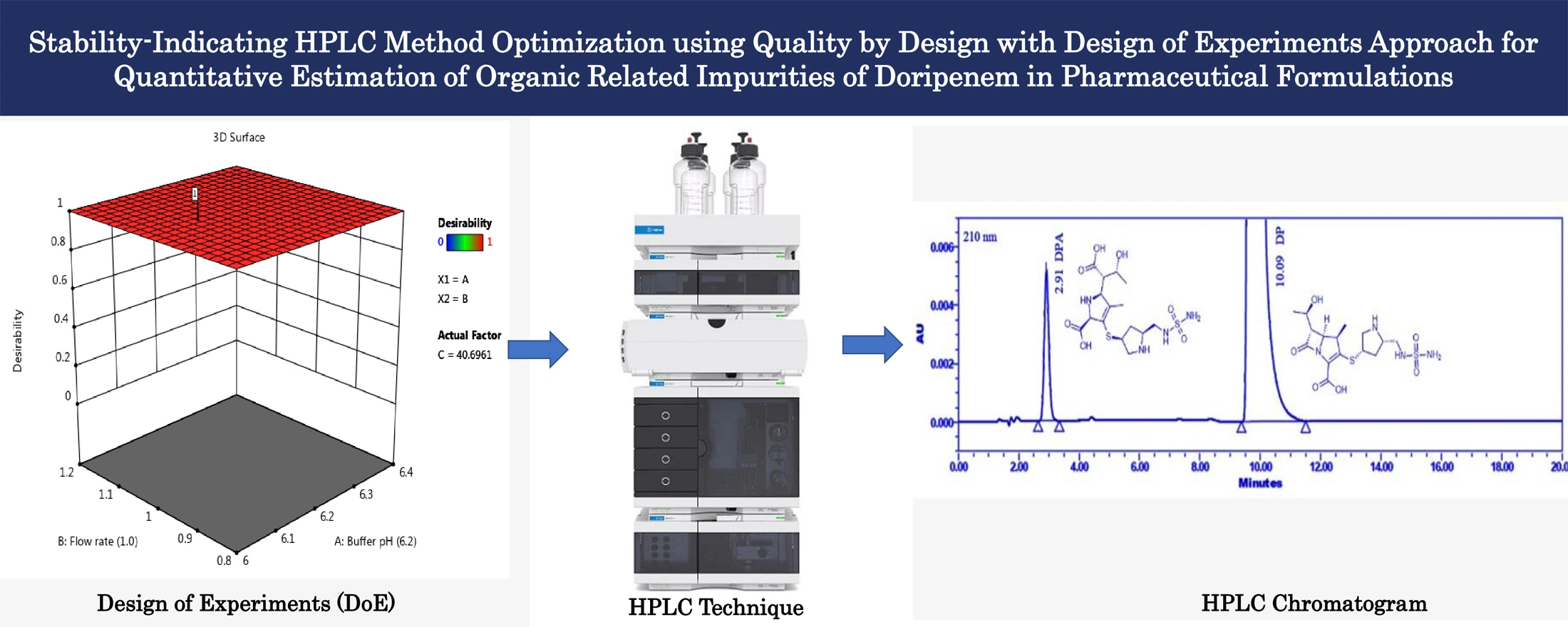
Stability-indicating HPLC method optimization using quality by design with design of experiments approach for quantitative estimation of organic related impurities of Doripenem in pharmaceutical formulations
N. V. V. D. Praveen Boppy, Sharath Babu Haridasyam, Niroja Vadagam, Naveen Sara, Karthik Sara, Eswarlal Tamma
DOI: 10.7324/JAPS.2024.190386Pages: 114-126
Screening of surfactant mixture ratio for preparation of oil-in-water nanoemulsion: A technical note
Shobhit Kumar, Karan Wadhwa, Rakesh Pahwa, Javed Ali, Sanjula Baboota
DOI: 10.7324/JAPS.2024.180648Pages: 121-127
_.jpg)
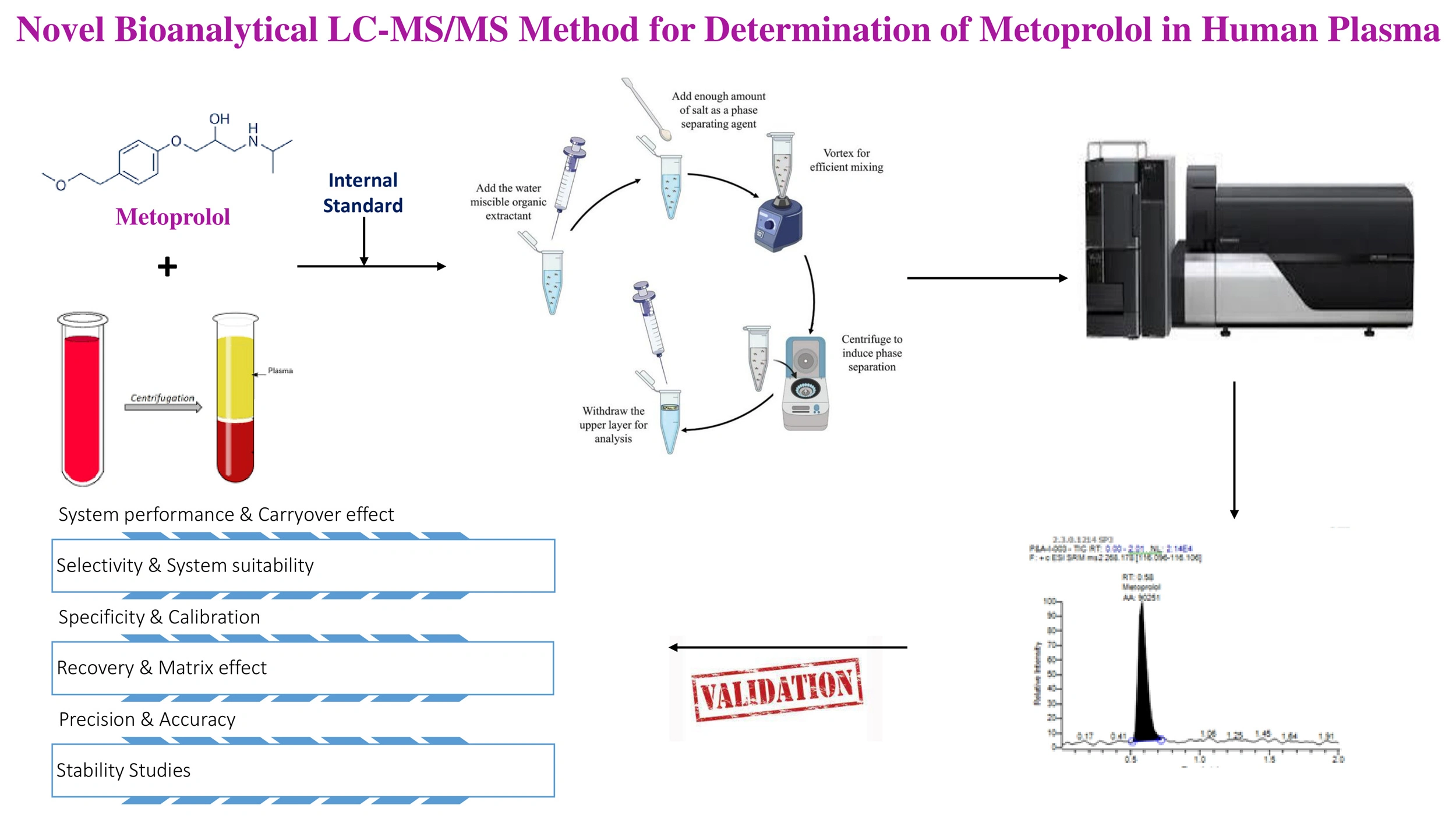
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138
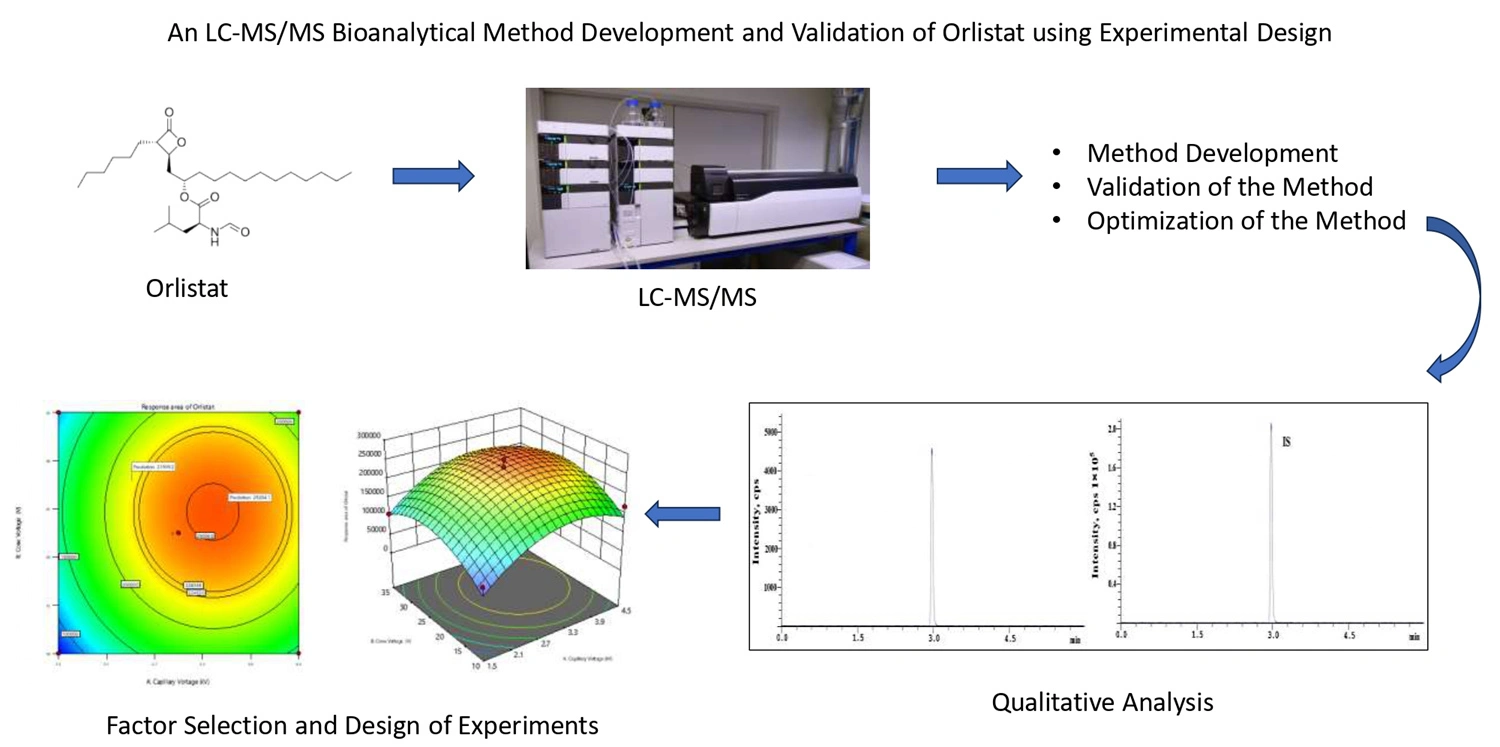
Development and validation of a new LC-MS/MS method for the determination of orlistat in biological matrices using experimental design
Rubina Kauser, Sunil Kumar Chaitanya Padavala, Venkatesan Palanivel
DOI: 10.7324/JAPS.2024.191461Pages: 196-204
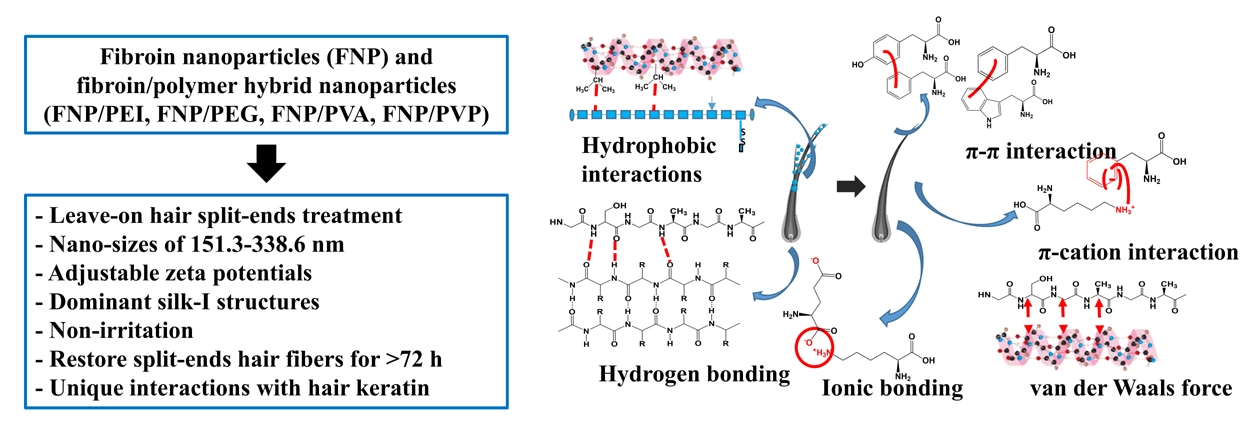
Silk fibroin/polymer hybrid nanoparticles as a potential treatment for hair split-ends
Duy Toan Pham, Bao Chau Nguyen, Hien Triet Nguyen, Ngoc Yen Nguyen, Ngoc Huyen Nguyen, Waree Tiyaboonchai
DOI: 10.7324/JAPS.2025.218439Pages: 166-177
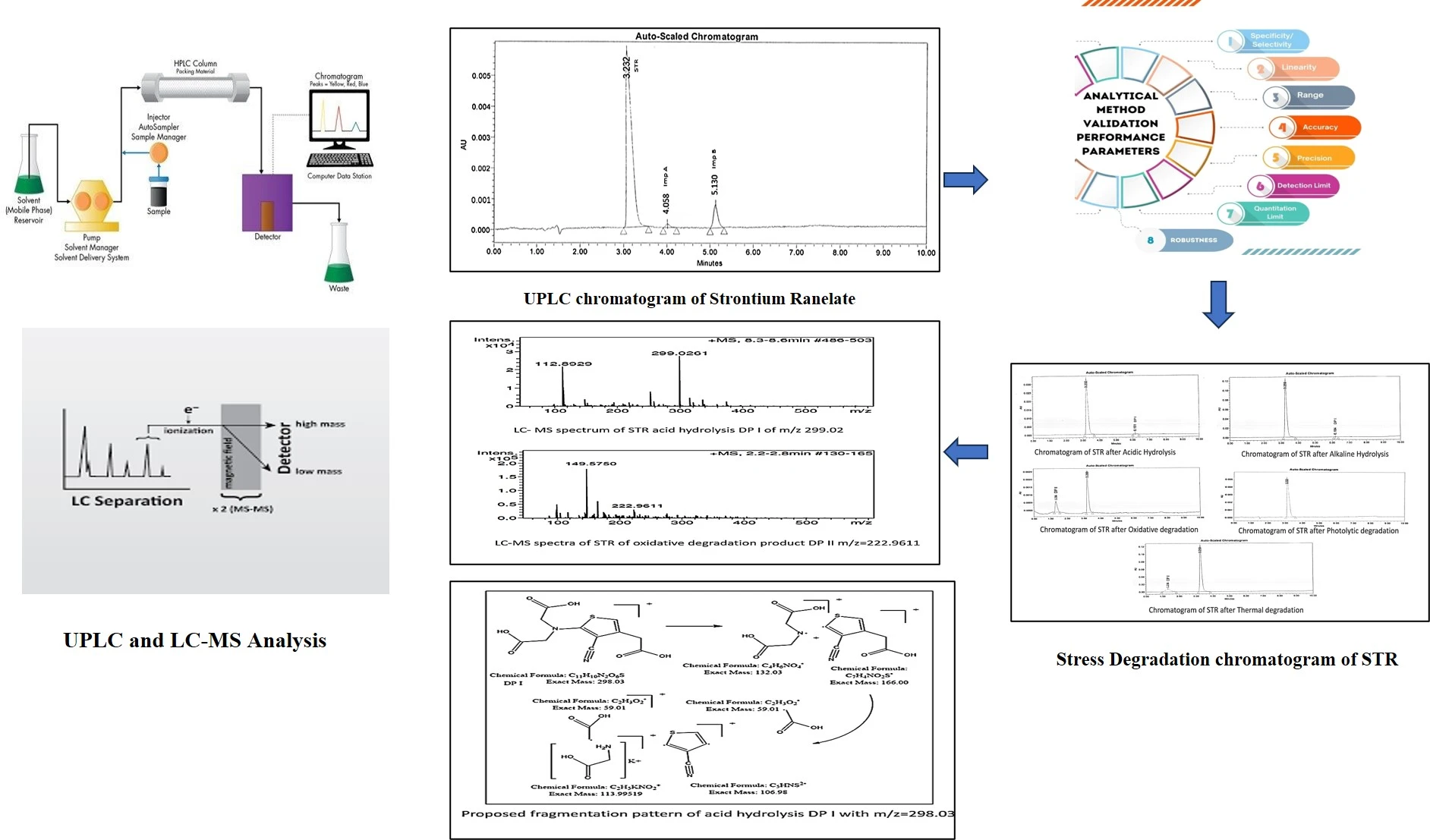
Impurity profiling of the strontium ranelate using stability indicating UPLC method and structural elucidation of degradants by LC-MS-TOF with the greenness assessment
Arti Swami, Aarti Shastri, Vishnu Choudhari
DOI: 10.7324/JAPS.2025.234119Pages: 132-141
Improving rifampicin stability in the presence of modified isoniazid across different pH environment
Sushruta S. Hakkimane, Santosh L. Gaonkar, Vishnu Prasad Shenoy, Bharath Raja Guru
DOI: 10.7324/JAPS.2025.242027Pages: 245-250