INTRODUCTION
Even though historical cosmetic toner was unpopular because of the irritation caused by skin drying, toner has become favorable these days because of the advancement in the formulating technologies that enhance the oil removal capability of the toner, retain the skin moisture, and refine the care of the skin simultaneously. Botanical products are one of the preferred ingredients used in the toner. Apart from the fact that consumers perceive botanical ingredients as safe and nature friendly, natural ingredients are usually a mixture of chemicals that allow various biological activities. Antioxidants derived from natural sources have become extensively studied and used in the cosmetic industry, especially for skincare products.
Free radicals are unstable molecules produced in the biological processes by cells, for example, superoxide, peroxyl, alkoxylate, and hydroxyl. The radicals create the oxidative stress that involves in the development of several diseases (Phaniendra et al., 2015) and skin aging (Poljšak and Dahmane, 2012). Plant-derived compounds such as phenolic compounds are oxidant scavengers that are ubiquitously found in nature (de Lima Cherubim et al., 2020). Carissa carandas Linn. is a plant that originated in Asia and is commonly found in Thailand, and its fruits contain a high amount of antioxidant substances such as anthocyanin (Le et al., 2019; Sueprasarn et al., 2017) and vitamin C. Phenolics in ripe fruit, for example, isoamyl alcohol, benzyl acetate, lupeol, oxalic acid, tartaric acid, citric acid, malic acid, malonic acid, and glycolic acids, also exhibited antioxidant activity (Khunchalee, 2019). Antioxidant screening of C. carandas fruit was measured from ethanol (Khunchalee, 2019), methanol (Ondee, 2019), and hexane (Normaizatul Afizah et al., 2016) extracts. There is limited data on the water extract of this fruit (Le et al., 2019; Sueprasarn et al., 2017). Therefore, in this study, C. carandas fruit aqueous extract was screened for antioxidant and antityrosinase capacity (Khunchalee, 2019) and fabricated as skincare toner. In addition, the toner preparation was evaluated for its appearance and stability.
MATERIALS AND METHODS
Sample preparation and extraction
C. carandas ripe fruits were collected from Tambon Banlao, Muang District, Chaiyaphum Province. The fruits (625 g) were washed with water, air-dried under the shade at room temperature, then homogenized with 600 ml of distilled water, and filtered through Whatman No. 1 filter paper (GE Healthcare Life Sciences, Marlborough, MA) to remove plant residues. The water extract was put into a –80°C freezer. The frozen sample was lyophilized by freeze dryer (Labconco™ FreeZone™ –105°C 4.5 l Benchtop Freeze Dry Systems, Kansas City, MO) for 3 days at a processing temperature of –103°C and constant pressure in the drying chamber at 47.0 Pa. The lyophilized extract was stored at 4°C for future studies.
Chemicals and reagents used
Ethanol, sodium hydroxide, and hydrochloric acid, all of analytical grade, were obtained from RCI Labscan (Bangkok, Thailand). 3,4-Dihydroxyphenylalanine (L-DOPA), tyrosinase mushroom, sodium phosphate monobasic, sodium phosphate dibasic, kojic acid, and 2, 2-diphenylpicrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, and 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) was purchased from Aldrich (Milwaukee, WI). L-ascorbic acid was obtained from Ajax Finechem (Seven Hills, Australia). The cosmetic grade of triethanolamine, glycerin, propylene glycol, and phenoxyethanol was purchased from Dow Chemical (Midland, MI). Polysorbate 20 (Tween® 20) was obtained from Sino-Japan Chemical (Taipei, Taiwan). Sorbitol syrup 70% was purchased from Chemipan (Bangkok, Thailand).
Antioxidant assays
DPPH radical scavenging activity
The antioxidant potential of the fruit extract was determined using the DPPH method as described previously (Jiangseubchatveera et al., 2017). In brief, the extract was serially diluted with ethanol to a concentration of 1–10 mg/ml. A fresh ethanolic solution of DPPH at a concentration of 66 μg/ml was prepared. 180 μl of DPPH solution was added to 20 μl of the fruit extract in a 96-well microtiter plate and stored in the dark for 30 minutes at room temperature. Then, the absorbance (A) was measured at 520 nm by using a microplate reader (Multimode Microplate Reader, FLUOStar Omega 2020, Ortenberg, Germany). Trolox and ascorbic acid were used as positive controls. All measurements were performed in triplicate. The capability to scavenge the DPPH radical was calculated using the following equation:
DPPH scavenging activity (%) = [(Acontrol – Asample)/Acontrol] × 100
Acontrol is the absorbance of the blank (ethanol), while Asample is the absorbance of the sample or the standard. The inhibitory concentration (IC50) values showed the concentration of the sample which was able to scavenge 50% of DPPH free radicals.
ABTS radical scavenging activity
The antioxidant activity by ABTS radical cation was carried out according to Jiangseubchatveera et al. (2017). The ABTS•+ was generated by the reaction between 7 mM ABTS in water and 2.4 mM potassium persulfate solution at a ratio of 2:1. The mixture was then stored in the dark for 16 hours at room temperature. For the assay, the freshly prepared ABTS·+ solution was diluted with distilled water to obtain the absorbance of 0.70 ± 0.02 at 734 nm using a spectrophotometer (Hitachi U2900, Japan). After that, 20 μl of the fruit extract was mixed with 2 ml of diluted ABTS•+ solution and incubated at room temperature for 5 minutes before the measurement of the absorbance at 734 nm. All the measurements were performed in triplicate. Trolox and ascorbic acid were used as positive controls. The percentage inhibition of free radical (I%) was calculated using the following equation:
ABTS·+ scavenging effect (I%) = [(Acontrol – Asample)/Acontrol] × 100
Acontrol is the absorbance of all reagents except the sample or standard and Asample is the absorbance of the sample or standard. The ABTS of the sample was expressed as mg Trolox equivalent antioxidant capacity (TEAC)/g extract and mg ascorbic acid equivalent antioxidant capacity (AEAC)/g extract, respectively.
Antityrosinase activity
Antityrosinase activity of C. carandas extract was determined using the dopachrome method (Saghaie et al., 2013). The samples were prepared in water at the final concentration of 10 and 50 mg/ml. Twenty microliters of each sample was mixed with mushroom tyrosinase (40 μl, final concentration 500 U/ml), followed by the addition of phosphate buffer (100 μl, 20 mM, pH 6.8). After the incubation at 37°C for 10 minutes, L-DOPA (40 μl, 0.85 mM) was added to the reaction mixture and incubated under the same condition for 20 minutes. The formation of the dopachrome was measured for the visible light absorbance at 492 nm using a microplate reader (Multimode Microplate Reader, FLUOStar Omega 2020, Germany). Kojic acid was used as a positive control. All measurements were carried out in triplicate. The percent inhibition of tyrosinase activity was calculated according to the following equation:
Inhibition (%) = [Acontrol – (Asample – Ablank)/Acontrol] × 100
where Acontrol is the absorbance of the control solution without kojic acid (or the extract), Asample is the absorbance of kojic acid (or the extract) solution with tyrosinase, and Ablank is the absorbance of kojic acid (or the extract) solution without tyrosinase.
Development of the fruit extract skincare product
The fruit extract was incorporated into the skincare toner. The formulation of the toner-based consisted of 70% sorbitol solution (4%), propylene glycol (2%), glycerin (1%), Tween® 20 (1%), phenoxyethanol (1%), allantoin (0.25%), and purified water. To fabricate the preliminary formulation of the fruit extract toner, the toner base was mixed with the fruit extract (1 g). The physical appearances and stability properties of the toner were then evaluated.
Evaluation of the fruit extract skincare product
Physical characteristics
The physical appearances, color, and phase separation of the fruit extract toner were observed after the preparation for 24 hours. The pH values were determined by a pH meter (Mettler-Toledo, Columbus, OH).
Stability study
The stability test was evaluated by two types of assays including a centrifugation assay (Charoenjittichai et al., 2016) and a six-cycle acceleration test. The formulations were centrifuged at 3,500 rpm for 30 minutes at room temperature and observed for the phase separation (Labofuge™ 400 Centrifuges, Thermo Fisher Scientific, Waltham, MA). The acceleration test, six cycles of a heating–cooling cycle, was carried out. Each cycle consisted of storage at 4°C for 48 hours and 45°C for 48 hours. At the end of this experiment, physical appearances, pH values, and antioxidant capacity of the toner were determined.
Effects of light and temperature for the storage condition
The effects of light and temperature on the final product were observed for a month. To determine the effect of light, the fruit extract toner was stored at ambient temperature (25°C) in dark and light conditions. Also, the toner was kept at 4°C to evaluate for the effect of temperature. The physical properties and the antioxidant activity were assessed.
RESULTS AND DISCUSSION
Extraction yield
The ripe fruit of C. carandas was lyophilized to provide red extract with a 6.83% (w/w) yield. The yield in this study was higher than that reported in a previous study (Khunchalee, 2019) which was 5.76 and 2.54% yield using 70 and 100% ethanol as solvents, respectively.
Antioxidant activity
DPPH and ABTS assays were commonly used for the evaluation of the antioxidant activity of plant extracts. The extract showed the concentration-dependent inhibition of radical scavenging activity. The IC50 value of the fruit extract from the DPPH assay still showed less activity than that of Trolox and ascorbic acid (Table 1). According to the ABTS assay, the extract also showed free radical scavenging ability (Table 1). These results revealed that the extract of C. carandas fruit had antioxidant activity. The antioxidant potential of C. carandas fruit extract is from its phenolics and anthocyanins (Chodok and Khumkhom, 2020; Dhar et al., 2017; Le et al., 2019; Sueprasarn et al., 2017). In recent studies, the C. carandas fruit extracts from ethanol, methanol, and hexane fractions also presented the antioxidant abilities by DPPH and ABTS assays (Khunchalee, 2019; Le et al., 2019; Madhuri and Neelagund Shivayogeeswar, 2019; Normaizatul Afizah et al., 2016; Sueprasarn et al., 2017). Moreover, Madhuri and Neelagund Shivayogeeswar (2019) reported that the methanolic extract of this fruit exhibited antioxidant power using ferric-reducing antioxidant power assay (FRAP). Additionally, our results are in agreement with those reported by Chodok and Khumkhom (2020) who found a strong correlation between total phenolic content and FRAP assay. Therefore, our findings agree with other studies that strongly supported the antioxidant activities of C. carandas fruit extracts.
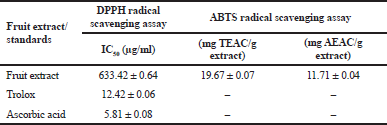 | Table 1. The antioxidant activity using DPPH and ABTS radical scavenging assays of the fruit extract. [Click here to view] |
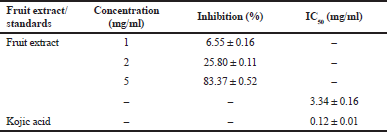 | Table 2. The antityrosinase activity of the fruit extract. [Click here to view] |
Antityrosinase activity
The percentage inhibition of the fruit extract at a concentration of 1, 2, and 5 mg/ml was 6.55 ± 0.16, 25.80 ± 0.11, and 83.37% ± 0.52%, respectively, shown in Table 2. Among these, the tyrosinase inhibitory effect of the extract was in a dose-dependent manner. The IC50 value of the extract and kojic acid was exhibited in Table 2. In this case, the antityrosinase activity of the fruit extract could be attributed mainly to its phenolic compounds (Chodok and Khumkhom, 2020; Dhar et al., 2017). The phenolics of plants have been reported as a source of various pharmacologically active compounds. The hydroxyl groups of phenolics inhibited tyrosinase by copper chelating in the active site (Panzella and Napolitano, 2019).
The evaluation of extracted skincare product
Physicochemical characteristics
The toner from the fruit extract was pink and translucent. The pH value of the freshly prepared extract toner was 3.3, which was improper for the skin application. Therefore, triethanolamine was used to adjust the pH value to be approximately 5.5, mimicking the skin pH. The color of the toner changed to orange-pink but it was still transparent (Fig. 1).
Stability study
The characteristics of cosmetic and pharmaceutical products can be commonly influenced by environmental factors. These factors, such as temperature, pH, light, air, and humidity, contribute to phase separation, color and viscosity changes, loss of activities, and active ingredients degradation (Liu et al., 2019; Restu et al., 2015). Moreover, centrifugation is helpful in the determination of the preliminary shelf life of a product (Khan et al., 2013). The physical appearances of the toner from the fruit extract under centrifugation and accelerated conditions were evaluated. After the centrifugation and acceleration test, there were no phase separation and aggregation. Their color was not significantly changed (orange-pink color), and the pH values remained approximately 5.5. The antioxidant activity of the toner determined using DPPH and ABTS assays was not significantly different in both before and after the heating-cooling tests (Table 3). These outcomes indicated that the toner from the fruit extract was stable; therefore, it could be used as an effective antioxidant skincare product.
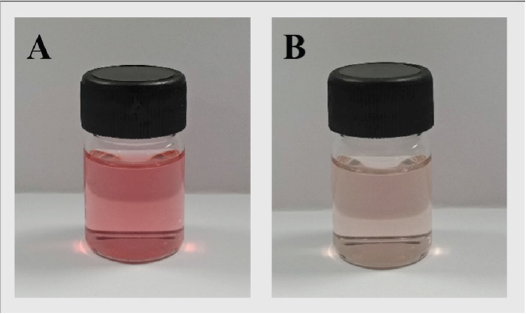 | Figure 1. The appearance of the fruit extract toner before (A) and after adjusting the pH (B). [Click here to view] |
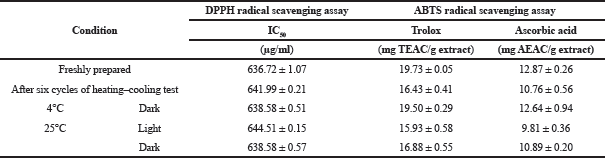 | Table 3. The antioxidant activity using DPPH and ABTS radical scavenging assays on stability test of the fruit extract toner formulation. [Click here to view] |
Effects of light and temperature for storage
After a month of all storage conditions, the physical properties of the toner remained unchanged. The effects of light and temperature on the antioxidant activity of the extract toner were evaluated using DPPH and ABTS radical scavenging assays which were the general method used to determine the antioxidant potential (Ratz-Lyko et al., 2012). The antioxidant properties of the toners are presented in Table 3. For the storage at 4°C and under the light and dark conditions at 25°C, the antioxidant efficiency of the product obtained by both DPPH and ABTS assays was not significantly changed (one-way analysis of variance, p > 0.05). Besides, the increasing temperature slightly decreased the ability of antioxidants as reported by Réblová (2012). However, the cosmetic product was not normally stored in a hot environment. Consequently, the prepared toner does not require light protection and could be stored at ambient temperatures.
CONCLUSION
This study found that C. carandas water extract had antioxidant and antityrosinase activities. The toner formulation containing C. carandas fruit extract was orange-pink, translucent, and stable. Moreover, light and temperature had an insignificant effect on the antioxidant activity of the toner. These results indicated that C. carandas extract can be utilized as an antioxidant from a natural source for further cosmetic purposes, especially in skincare.
AUTHORS’ CONTRIBUTION
The authors confirm contribution to the paper as follows: study conception and design: Nadechanok Jiangseubchatveera and Arpa Petchsomrit; data collection: Nadechanok Jiangseubchatveera; analysis and interpretation of results: Nadechanok Jiangseubchatveera, Charinrat Saechan, and Arpa Petchsomrit; draft manuscript preparation: Nadechanok Jiangseubchatveera, Charinrat Saechan, Nattawut Leelakanok, and Arpa Petchsomrit. All authors reviewed the results and approved the final version of the manuscript.
ACKNOWLEDGMENT
The student research grant (Grant no. 13/2561) and laboratory facilities were supported by the Faculty of Pharmaceutical Sciences, Burapha University.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Charoenjittichai R, Charnvanich D, Panapisal V. Effects of surfactant mixture ratio and concentration on nanoemulsion physical stability. Thai J Pharm Sci, 2016; 40:45–8.
Chodok P, Khumkhom S. Optimization of extraction conditions for improving bioactive compounds and antioxidant activities from Karonda (C. carandas Linn.) fruits using response surface methodology. Burapha Sci J, 2020; 25(2):617–35.
de Lima Cherubim DJ, Martins CV, Fariña LO, Lucca RA. Polyphenols as natural antioxidants in cosmetics applications. J Cosmet Dermatol, 2020; 19(1):33–7. CrossRef
Dhar G, Akther S, Sultana A, May U, Islam MM, Dhali, M, Sikdar D. Effect of extraction solvents on phenolic contents and antioxidant capacities of Artocarpus Chaplasha and C. Carandas fruits from Bangladesh. J Appl Biol Biotechnol, 2017; 5(03):39–44.
Jiangseubchatveera N, Liawruangrath S, Teerawutgulrag A, Santiarworn D, Pyne SG, Liawruangrath B. Phytochemical screening, phenolic and flavonoid contents, antioxidant and cytotoxic activities of Graptophyllum pictum (L.) Griff. Chiang Mai J Sci, 2017; 44(1):193–202.
Khan BA, Akhtar N, Khan H, Braga VA. Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula. Braz J Pharm Sci, 2013; 49(4):763–73. CrossRef
Khunchalee J. Study of free radical scavenging, total phenolic contents and tyrosinase inhibition activity of crude extract from Carissa carandas Linn. SNRUJST, 2019; 11(1):26–34.
Le XT, Huynh MT, Pham TN, Than VT, Toan TQ, Bach LG, Trung NQ. Optimization of total anthocyanin content, stability and antioxidant evaluation of the anthocyanin extract from Vietnamese Carissa Carandas L. Fruits. Processes, 2019, 7(7):468; doi:10.3390/pr7070468 CrossRef
Liu J, Tan Y, Zhou H, Mundo JL, McClements DJ. Protection of anthocyanin-rich extract from pH-induced color changes using water-in-oil-in-water emulsions. J Food Eng, 2019; 254:1–9. CrossRef
Madhuri S, Neelagund Shivayogeeswar E. Anti-oxidant, anti-diabetic activity and DNA damage inhibition activity of Carissa Carandas fruit. Int J Adv Res Dev, 2019; 4(1):75–82.
Normaizatul Afizah I, Ahmad W, Jamshed F, Al-Jasabi S. Quantitative biochemical analysis of antioxidant properties of Carissa carandas fruit ethanolic and n-hexane extracts. Middle East J Sci Res, 2016; 24(8):2418–23.
Ondee S. Antioxidant and antiproliferative activities of Carissa carandas Linn. fruits. Thai Cancer J, 2019; 39(1):6–15.
Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics, 2019; 6(4):57. CrossRef
Phaniendra A, Jestadi D, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem, 2015; 30(1):11–26. CrossRef
Poljšak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract, 2012; 2012:135206. CrossRef
Ratz-Lyko A, Arct J, Pytkowska K. Methods for evaluation of cosmetic antioxidant capacity. Skin Res Technol, 2012; 18(4):421–30. CrossRef
Réblová Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J Food Sci, 2012; 30(2):171–5. CrossRef
Restu WK, Sampora Y, Meliana Y, Haryono A. Effect of accelerated stability test on characteristics of emulsion systems with chitosan as a stabilizer. Procedia Chem, 2015; 16:171–6. CrossRef
Saghaie L, Pourfarzam M, Fassihi A, Sartippour B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res Pharm Sci, 2013; 8(4):233–42.
Sueprasarn J, Reabroy S, Pirak T. Antioxidant properties of Karanda (Carissa carandas Linn.) extracts and its application in Thai traditional fermented pork sausage (Nham). Int Food Res J, 2017; 24(4):1667–75.