INTRODUCTION
Tuberculosis (TB) has emerged as one of the leading causes of death worldwide over the past century. The World Health Organization and the International Union Against Tuberculosis and Lung Disease recommend controlling TB through the use of multiple drug combinations. The standardized TB treatment regimen consists of two phases and incorporates multiple drugs to enhance patient compliance and reduce the risk of drug resistance. Drug resistance in TB primarily arises from the improper use of prescribed medications; however, good TB control practices were associated with lower or decreasing levels of resistance [1,2]. To mitigate the risk of developing resistance associated with monotherapy, it is advised to administer TB drugs in combination. Therefore, all over the world, multiple drug combinations are used for the treatment of TB. However, there are certain problems that need to be addressed [3,4]. It is well established that the interaction of different drug doses in combination contributes to poor stability and bioavailability [5–7]. A major concern over the last few decades has been the poor bioavailability of rifampicin (RIF) in combination with other anti-TB drug isoniazid (INH) [8,9]. Several reports have indicated that there is a chemical interaction between these two key drugs used in the treatment of TB [10–12]. It is known that RIF degrades in the acidic environment of the stomach [13]. This degradation, along with the chemical interaction with INH, significantly contributes to treatment failures in TB management [14,15]. Literature indicates that RIF and INH can react under specific conditions when administered together, resulting in reduced stability and bioavailability of RIF. Therefore, hydrolysis of RIF in acidic conditions is accelerated in the presence of INH [16–19]. Interestingly, a similar observation was reported in other related studies in the same period; however, that study provided no clue as to how the acceleration of decomposition of RIF was brought about by INH [12,20]. As the currently commercially available method, Ultra performance liquid Chromatography Tandem-Mass Spectrometry (UPLC-MS)/MS enables a comprehensive analysis of RIF stability and metabolism through sample quantification. Moreover, the method’s effectiveness for therapeutic drug monitoring of INH, PZA, and RIF was confirmed during the analysis of clinical samples. Various approaches are in progress to increase the stability of TB drugs in combination. For example, a dissolution medium was formulated for the in vitro evaluation of a fixed-dose combination of the three primary anti-tubercular drugs (RIF, INH, and pyrazinamide) for sustained release. To prevent the atmospheric oxidation of RIF into RIF quinone, ascorbic acid was added to mildly alkaline solutions [21–23]. Additionally, a rapid, accurate, precise, and stability-indicating Reverse-Phase High-performance Liquid Chromatography (RP-HPLC) method was developed for the DOXY-RIF combination. This method is suitable for detecting drugs within nanoparticulate systems. The results suggest that RIF degradation in acidic media, when combined with RIF, can be mitigated by encapsulating both active pharmaceutical ingredients. This strategy ensures their release at different points along the gastrointestinal tract, which could enhance RIF bioavailability [24–28]. This gives reason to suggest that a different approach needs to be developed.
In this study, we demonstrated that RIF is more stable in the presence of IH2, which functions as a prodrug with the same therapeutic efficacy as INH. The instability of RIF in the presence of INH is likely due to the possible reaction between RIF and INH, leading to the formation of a hydrazone with one of the keto groups in RIF. In contrast, the synthesized derivative IH2 does not react with RIF.
In our previous study, we synthesized (N′-benzylideneisonicotinohydrazide) (IH2) by modifying INH, which demonstrated the same therapeutic effect as INH against the Mycobacterium tuberculosis H37Rv strain [29]. Here, we briefly outline the synthesis procedure. IH2 was created by reacting INH with a hydrophobic moiety known as benzaldehyde, a compound commonly used in food supplements. An equimolar mixture of INH and benzaldehyde was refluxed at 70°C in isopropyl alcohol with acetic acid for 2 hours (Fig. 1). Thin-layer chromatography was used to monitor the reaction. Upon adding water with stirring, the product precipitated out, was filtered, and recrystallized from ethanol. The desired product was obtained as a white crystalline powder with an 85% yield. The antimicrobial susceptibility test was conducted using the Becton Dickinson Detection (BACTEC™) Mycobacterium Growth Indicator Tube (MGIT™) 960 instrument against the H37Rv strain, following a technique similar to that used for patient samples in diagnostic laboratories. IH2, at a concentration of 0.16 μg/ml [equivalent to the minimal inhibitory concentration (MIC) of INH at 0.1 μg/ml], effectively restricted the growth of M. tuberculosis H37Rv, demonstrating potency comparable to INH in inhibiting growth [29]. In continuation, this study examines the stability of RIF in the presence of IH2 at various pH environments, comparing it to its stability with INH.
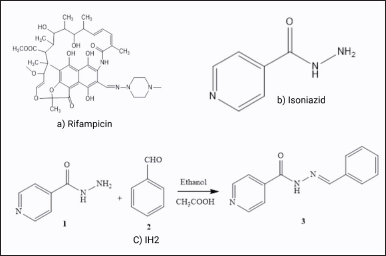 | Figure 1. Antitubercular drugs structure: (a) RIF, (b) INH, and (C) synthesis of IH2. [Click here to view] |
MATERIAL AND METHODS
Instrumentation and reagents
RP HPLC (SHIMADZU, Model No LC-20AD), with an auto sampler, UV detector, and PC installed to integrate chromatograms with LC solution software, electronic weighing balance, stirrer, ultra centrifuge, and Millipore water purification system to collect HPLC grade water. RIF (Duchefa-Genetix Biotech), INH, benzaldehyde, methanol, acetonitrile (MERK Millipore), trifluoroacetic acid, ethyl alcohol (Loba Chemie), isopropyl alcohol, anhydrous monobasic sodium phosphate, anhydrous dibasic sodium phosphate, anhydrous sodium acetate, acetic acid, and all the required chemicals and reagents used were of HPLC grade.
Simultaneous estimation of RIF and IH2
The simultaneous analysis of RIF and IH2 was performed using an optimized method developed for this drug combination. The mobile phases used were water and methanol. The solvents were filtered separately through a 0.45-μm membrane filter and degassed via sonication. A gradient flow was employed, with the organic mobile phase increasing from 50:50 to 10:90, followed by equilibration to the initial concentration over a run time of 20 minutes.
Simultaneous estimation of RIF and INH
Our established RP-HPLC method was used to analyze the drugs RIF and INH [29,30]. Briefly, a mixture of RIF and INH was injected into the HPLC. Gradient elution method by using water and methanol at an initial ratio of 90:10 and gradually moved to 30: 70 and brought back to the initial condition of the equilibrium system in 20 minutes.
Stability studies of the different combinations of drugs in different pH environments
Buffers of pH 2.5, 4.5, and 7.4 were prepared. A known amount (3 mg) of each drug RIF, INH, and IH2 was weighed, and appropriate dilutions were made in the respective buffers. Samples were placed in HPLC vials individually as well as in combinations of RIF and INH or RIF and IH2 as labeled appropriately. The analysis was done in triplicate, in order to avoid bias. Thereafter, samples were incubated at 37°C on a rocker shaker, readings were taken at different time intervals, and results were analyzed. The graph was plotted to analyze the stability of the drug and drug combinations.
Comparative analysis
The stability of pure RIF was assessed using the developed RP-HPLC method across various pH environments. Furthermore, the stability of RIF under different conditions in the presence of INH and IH2 was summarized in a comparative study and presented in graphical form.
RESULTS AND DISCUSSIONS
The synthesized hydrophobic IH2 was eluted separately from the hydrophilic INH using a simultaneous estimation by the RP HPLC method [29]. Briefly, a mixture of INH and IH2 was separated using a gradient elution method with a Phenomenex® Luna C18, 5 μm, 100 Å column, utilizing methanol and water over a 20-minute runtime. The optimized method for estimating INH and IH2 displayed well-resolved peaks with distinct retention times, with the highly hydrophilic INH eluting at 4.9 minutes and the less hydrophilic IH2 eluting at 17.43 minutes [29].
Simultaneous estimation of RIF and IH2
When simultaneous analysis of IH2 and RIF was done, they eluted at 6.3 and 10.4 minutes, respectively. The chromatogram shows two separate peaks with different retention times with many repetitions as shown in Figure 2.
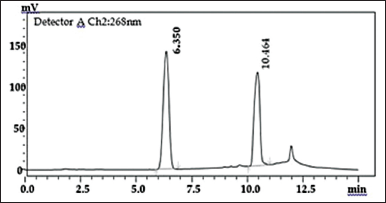 | Figure 2. Chromatogram of IH2 at 6.3 RT and RIF at 10.4 RT. [Click here to view] |
Simultaneous estimation of RIF and INH
INH is a hydrophilic drug eluted initially, and RIF eluted at 15.5 minutes as shown in (Fig. 3). Extra peaks shown in the chromatogram signify the immediate degradation of RIF in the presence of INH. But degradation is significantly reduced when IH2 is estimated simultaneously with RIF [30].
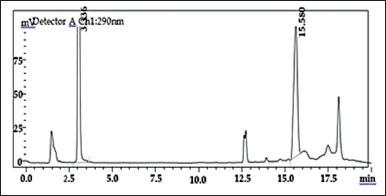 | Figure 3. Chromatogram of INH at 3.3 RT and RIF at 15.5 RT. [Click here to view] |
These methods were further employed to evaluate the stability of drug combinations under various pH conditions. The developed method for simultaneous estimation displayed well-resolved peaks and is both simple and reproducible with a short retention time. Larger sample sizes can be processed in a shorter timeframe, and low concentrations can also be detected. These methods were effective in assessing the degradation levels relative to the actual drug concentrations.
Stability studies in different pH environments
Stability analysis was conducted in triplicate under various pH conditions to mimic physiological conditions. The overall results indicated that RIF exhibited better stability in the presence of IH2 compared to INH. Additionally, IH2 demonstrated greater stability across all pH conditions than INH (Figs. 4–10). The HPLC chromatograms provide precise concentrations of the drugs at different time points. Wavelength used to detect RIF was at 290 nm, and INH and IH2 were detected at 268 nm. In this study, no chemical quenching or neutralization was applied at each time point to stop the reaction, as the objective was to monitor the natural degradation of the drug(s) under specified conditions. The initial sample (time zero) was considered 100% concentration, and samples were taken from incubation (37°C) at predefined time intervals. Each sample was immediately analyzed in triplicate by HPLC to accurately quantify the remaining drug content. This approach ensured that degradation was assessed as it occurred over time, without introducing additional chemical interference.
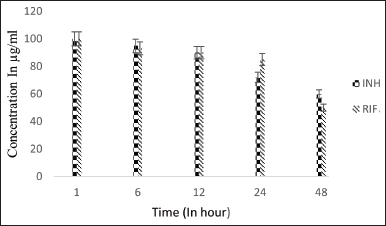 | Figure 4. Stability graph of RIF and INH at pH 7.4. [Click here to view] |
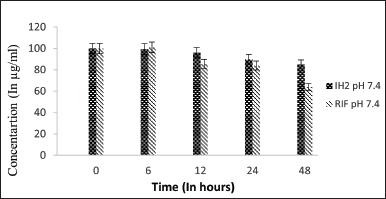 | Figure 5. Stability graph of RIF and IH2 at pH 7.4. [Click here to view] |
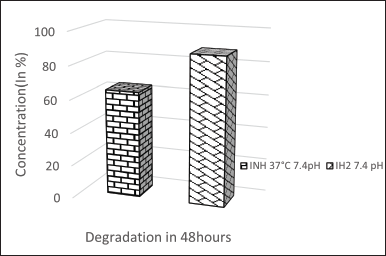 | Figure 6. Stability graph of INH and IH2 at pH 7.4. [Click here to view] |
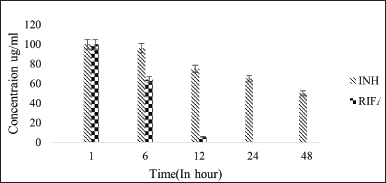 | Figure 7. Stability graph of RIF and INH at pH 4.5. [Click here to view] |
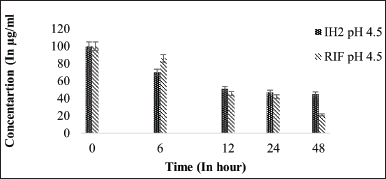 | Figure 8. Stability graph of RIF and IH2 at pH 4.5. [Click here to view] |
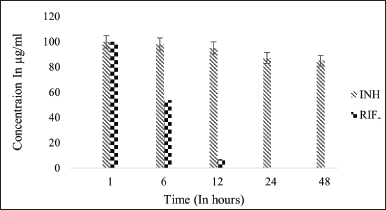 | Figure 9. Stability graph of RIF and INH at pH 2.5. [Click here to view] |
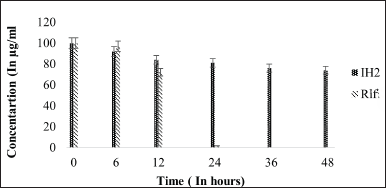 | Figure 10. Stability graph of RIF and IH2 at pH 2.5. [Click here to view] |
The stability of RIF was assessed at various pH conditions over 48 hours, indicating that RIF is more stable at 7.4 pH than in acidic conditions. After 48 hours, pure RIF degraded by approximately 44% at pH 7.4, 80% at pH 4.5, and nearly 95% at pH 2.5. The stability of RIF was compared in the presence of INH and IH2. At pH 7.4, RIF remained stable up to 56%, with only a slight decrease in concentration observed when INH was present, indicating minimal interactive effects. Since INH is unstable at pH 7.4, its interaction with RIF is likely minimal. In contrast, IH2 exhibits greater stability in the basic environment of pH 7.4.
The stability of RIF in the presence of IH2 under basic pH conditions was better than in the presence of INH. This is likely due to minimal interaction between the drugs as a result of the modification of the active group of INH. The stability was similar to the control (equivalent to the stability of RIF under similar conditions). When the hydrophilic INH was modified to the hydrophobic IH2, the stability of IH2 increased substantially compared to INH in basic pH conditions [29].
Under acidic pH conditions, RIF degradation occurs at a faster rate [21]. As it is observed that 80% reduction of RIF concentration was noted in 48-hour time period at 37°C under 4.5 pH condition but in presence of INH, RIF degradation was drastically increased and concentration reduced to zero at pH 4.5 and 2.5 within 48 hours (50% reduction was seen in initial 6 hours followed by 80% reduction and then degraded completely in 12 hours). However, in the presence of IH2, RIF remained stable and presented similar results of individual RIF under controlled conditions. This result shows that the interaction between RIF and IH2 does not exist at all. The stability of RIF has increased substantially in the presence of IH2 compared to INH in acidic pH levels of 4.5 and 2.5.
The stability of RIF under various conditions, in the presence of INH and IH2, is summarized, and the results are analyzed in Figure 11. RIF stability was good in basic pH compared to acidic pH conditions. It was also noticed that RIF with INH undergoes degradation. The probable reason would be that RIF is known to undergo acid-catalyzed degradation in the presence of INH, primarily due to the free hydrazide (-CONHNH2) functional group of INH. Under acidic conditions, this hydrazide moiety reacts with the aldehyde group of RIF, forming a Schiff base or hydrazone intermediate, which leads to decomposition of RIF into inactive products, thus compromising the therapeutic efficacy of both drugs in fixed-dose combinations. However, INH hydrazone is the synthesized condensation product of INH and aldehyde, in which the hydrazide group is already consumed via the formation of a C=N–NH– linkage. This hydrazone bond is more stable and no longer nucleophilic, thereby eliminating the reactive site that would otherwise attack the RIF molecule.
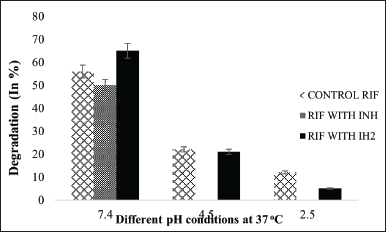 | Figure 11. Stability of RIF at different pH conditions and in combinations for 48 hours. [Click here to view] |
This interaction and degradation of RIF can be reduced, and stability can be enhanced by using IH2, which has a similar therapeutic effect as INH against the M. tuberculosis H37Rv strain. Briefly, the final concentration of IH2 was maintained at 0.16 µg/ml, equivalent to the MIC of INH (0.1 µg/ml), by following the standard procedure. Both free IH2- and IH2-loaded nanoparticles inhibited bacterial growth at the MIC equivalent to the MIC of INH. The control groups (without drug and drug-free NPs) continued to grow in AST. Results obtained following ICH Guidance on Analytical Method Validation can assist national tuberculosis programs in supporting effective case management, aligned with international TB care standards, while accounting for factors that influence drug absorption and therapeutic concentration levels [31].
CONCLUSION
RIF stability in the presence of INH and IH2 was studied using RP HPLC methods. Simple and reproducible methods were developed, and the HPLC chromatogram showed well-resolved peaks in the simultaneous estimation of drugs. A combination study demonstrates that RIF interacts with INH, and the interaction between the two drugs leads to an increase in the degradation of RIF. IH2 does not interact with RIF, therefore, degradation of RIF does not occur due to the presence of IH2. Interaction and degradation of RIF can be reduced, and its stability can be enhanced if IH2 is used instead of INH. The therapeutic efficacy of IH2 is like INH when it is tested on the M. tuberculosis H37Rv strain. Future work will focus on combination formulations and in vivo studies to explore its therapeutic potential.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
Vision Group of Science and Technology (VGST), Karnataka, India K-FIST LEVEL 1 GRD 267, Awarded to Dr. Bharath Raja Guru.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The datasets generated and/or analyzed during this study can be provided by the authors upon reasonable request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Aziz MAbdel. Anti-tuberculosis drug resistance in the world: third global report: the WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance, 1999-2002. Geneva, Switzerland: World Health Organization; 2004.
2. Wilkinson D. Anti-tuberculosis drug resistance in the world. The WHO/IUATLD global project on anti-tuberculosis drug resistance surveillance. Geneva: World Health Organization; 1997. 228 pp.
3. Bhutani H, Mariappan TT, Singh S. The physical and chemical stability of anti-tuberculosis fixed-dose combination products under accelerated climatic conditions. Int J Tuberc Lung Dis. 2004;8:1073–80.
4. Bhutani H, Mariappan TT, Singh S. An explanation for the physical instability of a marketed fixed dose combination (FDC) formulation containing isoniazid and ethambutol and proposed solutions. Drug Dev Ind Pharm. 2004;30:667–72. CrossRef
5. Acocella G. Human bioavailability studies. Bull Int Union Tuberc Lung Dis. 1989;64:38–40; 40–2 discussion.
6. Agrawal S, Singh I, Kaur KJ, Bhade SR, Kaul CL, Panchagnula R. Comparative bioavailability of rifampicin, isoniazid and pyrazinamide from a four drug fixed dose combination with separate formulations at the same dose levels. Int J Pharm. 2004;276:41–9. CrossRef
7. Freire FD, Aragão CFS, de Lima e Moura TFA, Raffin FN. Thermal studies of isoniazid and mixtures with rifampicin. J Therm Anal Calorim. 2009;97:333–6. CrossRef
8. Singh S, Mariappan TT, Shankar R, Sarda N, Singh B. A critical review of the probable reasons for the poor variable bioavailability of rifampicin from anti-tubercular fixed-dose combination (FDC) products, and the likely solutions to the problem. Int J Pharm. 2001;228(1–2):5–17. CrossRef
9. Xu J, Jin H, Zhu H, Zheng M, Wang B, Liu C, et al. Oral bioavailability of rifampicin, isoniazid, ethambutol, and pyrazinamide in a 4-drug fixed-dose combination compared with the separate formulations in healthy Chinese male volunteers. Clin Ther. 2013;35(2):161–8. CrossRef
10. Mariappan T, Singh S. Gastrointestinal permeability studies using combinations of rifampicin and nucleoside analogue reverse transcriptase inhibitors in rats. Indian J Pharmacol. 2007;39:284. CrossRef
11. Immanuel C, Gurumurthy P, Ramachandran G, Venkatesan P, Chandrasekaran V, Prabhakar R. Bioavailability of rifampicin following concomitant administration of ethambutol or isoniazid or pyrazinamide or a combination of the three drugs. Indian J Med Res. 2003;118:109–14.
12. Shishoo CJ, Shah SA, Rathod IS, Savale SS, Kotecha JS, Shah PB. Stability of rifampicin in dissolution medium in presence of isoniazid. Int J Pharm. 1999;190:109–23. CrossRef
13. Freire FD, Câmara MB, Dantas MG, Aragão CFS, de Lima e Moura TFA, Raffin FN. Gastric-resistant isoniazid pellets reduced degradation of rifampicin in acidic medium. Braz J Pharm Sci. 2014;50:749–56. CrossRef
14. Panchagnula R, Kaur KJ, Singh I, Kaul CL. The WHO simplified study protocol in practice: investigation of combined formulations supplied by the WHO. Int J Tuberc Lung Dis. 1999;3:S336–42.
15. Singh S, Mohan B. A pilot stability study on four-drug fixed-dose combination anti- tuberculosis products. Int J Tuberc Lung Dis. 2003;7:298–303.
16. Shishoo CJ, Shah SA, Rathod IS, Savale SS, Vora MJ. Impaired bioavailability of rifampicin in presence of isoniazid from fixed dose combination (FDC) formulation. Int J Pharm. 2001;228:53–67. CrossRef
17. Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Blends of enteric and GIT- insoluble polymers used for film coating: physicochemical characterization and drug release patterns. J Control Release. 2003;89:457–71. CrossRef
18. Singh S, Mariappan TT, Sharda N, Kumar S, Chakraborti AK. The reason for an increase in decomposition of rifampicin in the presence of isoniazid under acid conditions. Pharm Pharmacol Commun. 2000;6:405–10. CrossRef
19. Ohtsuki T, Huang Y, Kamiya A, Nakayama Y, Matsushita M, Morikawa S, et al. Development of an HPLC method using relative molar sensitivity for the measurement of blood concentrations of nine pharmaceutical compounds. J Pharm Health Care Sci. 2024;10(1):35. CrossRef
20. Singh S, Bhutani H, Mariappan TT. Quality problems of anti-tuberculosis fixed-dose combinations (FDCS): a way forward. Indian J Tuberc. 2006;53:201–5.
21. Singh S, Mariappan TT, Sharda N, Singh B. Degradation of rifampicin, isoniazid and pyrazinamide from prepared mixtures and marketed single and combination products under acid conditions. Pharm Pharmacol Commun. 2000;6:491–4. CrossRef
22. Prasanthi B, Ratna JV, Phani RSC. Development and validation of RP-HPLC method for simultaneous estimation of rifampicin, isoniazid and pyrazinamide in human plasma. J Anal Chem. 2015;70:1015–22. CrossRef
23. Siddhartha TS, Prasanthi B, Santosh TA, Ratna JV. Development and validation of high performance liquid chromatographic method for the determination of rifampicin in human plasma. Int J Pharm Pharm Sci. 2012;4(5):362–7.
24. Mwila C, Walker RB. Improved stability of rifampicin in the presence of gastric-resistant isoniazid microspheres in acidic media. Pharmaceutics. 2020;12(3):234. CrossRef
25. Dawre S. Development and validation of stability indicating RP-HPLC method for simultaneous determination of doxycycline and rifampicin in polymeric nanoparticles. Curr Chromatogr. 2022;9(1):54–62. CrossRef
26. Singh H, Bhandari R, Kaur IP. Encapsulation of rifampicin in a solid lipid nanoparticulate system to limit its degradation and interaction with isoniazid at acidic pH. Int J Pharm. 2013;446:106–11. CrossRef
27. Han X, Alu A, Liu H, Shi Y, Wei X, Cai L, Wei Y. Biomaterial-assisted biotherapy: a brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact Mater. 2022;17:29–48. CrossRef
28. Sadaphal P, Chakraborty K, Almossawi HM, Pillay Y, Roscigno G, Kaul A, et al. Rifampicin bioavailability in fixed-dose combinations for tuberculosis treatment: evidence and policy actions. J Lung Health Dis. 2019;3(3):9–15. CrossRef
29. Hakkimane S, Shenoy VP, Gaonkar S, Bairy I, Guru BR. Antimycobacterial susceptibility evaluation of rifampicin and isoniazid benz-hydrazone in biodegradable polymeric nanoparticles against Mycobacterium tuberculosis H37Rv strain. Int J Nanomed. 2018;13:4303–18. CrossRef
30. Hakkimane SS, Guru BR. Nano formulation analysis: analytical method development of isoniazid and simultaneous estimation of antitubercular drugs isoniazid and rifampicin by reverse phase high pressure liquid chromatography. Asian J Pharm Clin Res. 2017;10:330–5. CrossRef
31. Guideline ICH. Validation of analytical procedures: text and methodology. Q2 (R1). 2005;20:05.