INTRODUCTION
A wide range of new active pharmaceutical ingredients (APIs) exhibit poor water solubility and high permeability, hence belong to the Biopharmaceutical Classification System (BCS) class II category and as a result suffer from limited in vivo therapeutic effects (Amidon et al., 1995). Improvement of solubility is still a challenge for researchers in both drug discovery and product development. Various strategies are applied nowadays to solve this problem with the attention being drawn to the application of solid dispersions (SDs) technology (Raimi-Abraham et al., 2015). This technology allows the dispersion of a poorly soluble drug in hydrophilic carriers, with increasing wettability and improving dissolution and bioavailability (Raimi-Abraham et al., 2015). Various techniques are employed to obtain SDs, such as fusion (FUS), spray drying, solvent coprecipitation, comilling, and hot melt extrusion (HME) (Maniruzzaman et al., 2012; Sekikawa et al., 1983; Van den Mooter et al., 2006; Vilhelmsen et al., 2005; Won et al., 2005).
However, a major concern for the production of SDs is the lack of physical stability during storage. The excessive free energy of the amorphous solid led to nucleation and crystal growth which in turn affect the thermodynamic stability upon storage (Lalkshman et al., 2008; Liu et al., 2010). This characteristic thermodynamic instability phenomenon is known as recrystallization. Recrystallization reduces the dissolution and solubility of the API, subsequently decreasing bioavailability. Few drug products prepared via the SDs approach have reached the market, mostly due to physical and/or chemical instability and scaling-up problems (Duncan, 2002; Serajuddin, 1999).
HME provides a continuous, solvent-free, and eco-friendly manufacturing process for preparing a dispersion or glass solution of the drug in the carrier. The mechanical energy exerted together with the short heating time will not cause any significant decomposition for most drugs (Breitenbach, 2002; Kumar et al., 2008; Verreck et al., 2006).
Proper carrier selection is an important aspect in SDs preparation via HME as the glass transition temperature (Tg) or melting temperature (Tm) of carriers should be carefully considered. A major HME drawback involves high melting point drugs (above 200°C), which require high-processing temperatures leading to drug and/or carrier degradation and limit the application of such technique to low melting point drugs (LaFountaine et al., 2016). Up until now, published information on the methods employed to overcome degradation during extrusion of high melting point drugs as well as the availability of carriers in the pharmaceutical market to assess in extrusion process is still limited (Haser et al., 2017).
Meloxicam (MLX), a cyclooxygenase-2 inhibitor, is one of the most promising non-steroidal anti-inflammatory drugs used for the treatment of various inflammatory conditions, including rheumatoid arthritis, postoperative pain, and osteoarthritis (Lugar et al., 1996; Pairet et al., 1998). MLX, a class II BCS drug, is poorly soluble in water, with solubility at pH 1.2 and 4 equals to 0.6 μg/ml, and it has pKa values of 1.1 (hydroxyl group) and 4.2 (thiazole group). The main drawback of MLX is its slow oral absorption, caused by its limited solubility in the acidic solution, triggering treatment failure in severe pain conditions (Lipscomb et al., 1998; Türck et al., 1996). Addressing MLX dissolution problems under acidic conditions is of prime significance for achieving a rapid therapeutic effect.
The enhancement of MLX/SDs dissolution in gastric pH was reported previously utilizing hazardous solvents like dioxane/ethanol and dichloromethane/methanol using solvent evaporation (Shi et al., 2019), freeze drying (Ochi et al., 2016; Suzuki et al., 2018), and spray drying (Shazly et al., 2015) techniques. Data employing the HME technique for the preparation of MLX/SDs are limited up until now. MLX has a melting point of 255°C and tends to degrade at a high processing temperature (Follonier et al., 1994; Hughey et al., 2011), thus preparation of MLX/SDs via HME is still considered a major challenge.
One of the most promising carriers suitable for the HME process, Soluplus® (SOL), is an amphiphilic copolymer of polyethylene glycol (PEG) 6,000, vinylcaprolactam, and vinyl acetate. SOL is considered to be a member of the fourth generation of SDs by acting as a matrix polymer for solid solutions, as well as surface-active solubilizers, allowing micelle formation in water, thus maintaining supersaturation of poorly soluble drugs in the GIT (gastro-intestinal tract) (Hardung et al., 2010). The use of SOL as a carrier to enhance dissolution of the BCS class II drugs has been reported before in the literature (Guo et al., 2014; Linn et al., 2012; Nagy et al., 2012), with an unproductive attempt previously described for the extrusion of MLX using SOL (Hughey et al., 2011).
The addition of plasticizing copolymer with SOL to facilitate the extrusion at a lower temperature is quite favorable (Andrews et al., 2008; Repka et al., 1999; Schilling et al., 2007). Poloxamers 407 (POLOX) (polyoxyethylene-polypropylene-block copolymer), a nonionic surface-active/wetting agent, is employed in the present study due to its low melting point (below 60°C), plasticizing effect, and oral safety (Collett et al., 2009; Ghareeb et al., 2009; Newa et al., 2007, 2008).
Taha et al. (2020) evaluated the rate and extent of MLX absorption from SDs prepared by the HME and FUS techniques using SOL/POLOX as mixed carriers, against the innovator product (Mobic®) in four healthy humans. The HME samples proved to be an ideal alternative for enhancing MLX’s rapid onset of action compared to Mobic® with Tmax value (1.5 hours), almost equal to the reported intramuscular injection (Taha et al., 2020).
The aim of the present study was to employ the challenging approach of HME for the preparation of MLX in SDs and for the improvement of its acidic dissolution using a novel combination of SOL/POLOX as mixed carriers. SDs prepared by HME were compared with the conventional FUS technique and the physical mixture (PM). Solid-state characterizations employing differential scanning calorimetry (DSC) and polarized light microscope (PLM) were carried out to examine the drug-carrier interaction and amorphization, respectively. The present study also explored, for the first time, the effect of the long-term bench storage on MLX/SD stability, in parallel with short-term accelerated storage. Stored formulae were assessed for the MLX content, in vitro dissolution tests, DSC, and PLM.
MATERIALS AND METHODS
Materials
Plain MLX was kindly donated from Delta Pharma (Cairo, Egypt). Polyvinyl caprolactam-polyvinyl acetate-PEG graft copolymer, Soluplus® (SOL) (Mw = 118,000 g/mol, density = 1.08 g/ cm3), Poloxamer (Lutrol® F127) (POLOX) were provided by BASF SE (Ludwigshafen, Germany). All other reagents and solutions were of analytical grade, except for acetonitrile, ethyl acetate, and methanol which were of HPLC (High Performance Liquid Chromatography) grade, purchased from Merck (Germany). Milli-Q purified water (Millipore Corp., Billerica, MA) was used to prepare the dissolution medium.
Methods
Preparation of MLX/SOL/POLOX SDs
SDs were prepared by two different methods: HME and FUS, using different SOL–POLOX ratios. The percentage of MLX was kept constant at 2.50% (w/w) for all formulae. The prepared SDs were compared to the plain MLX powder as well as their respective PMs.
Hot melt extrusion (HME) technique
SDs of MLX/SOL/POLOX were processed using HME at different mixed carriers’ ratios (SOL/POLOX) of 1.0:1.0, 1.5:1.0, and 2.3:1.0 for EXT-0, EXT-I, and EXT-II, respectively (Table 1). A Randcastle Microtruder RC-025 (Randcastle Extrusion Systems, Inc., Cedar Grove, NJ) ¼ inch single screw extruder with a single rod die was employed. The temperatures of the extruder barrel zones and die were controlled as follows: Zone 1 = 120°C, Zone 2 = 120°C, Zone 3 = 115°C, and Die = 105°C. Extrusion pressure was below 1 bar and the screw rotation was set at 30 rpm. PMs were simultaneously melted, homogenized, and extruded in the chamber. Then, the extrudates were collected and cooled at room temperature and ground. Finally, SDs were passed through # 45 mesh sieve (US standard sieves, Fisher Brand, Pittsburgh, PA) to obtain a uniform particle size range of 355–425 um.
Fusion (FUS) technique
Mixed carriers with different ratios (cf. Table 1) were blended with MLX, followed by the melting of each formula in a hot plate on a sand bath maintained at 65°C until a homogenous mixture was obtained. The fused mixture was cooled at room temperature and kept in a vacuum oven overnight to solidify. The solidified mass was ground in a mortar and sieved to obtain a particle size range of 355–425 μm. The FUS mixtures were coded as FUS-I (1.5:1.0) and FUS-II (2.3:1.0) for two different ratios of SOL:POLOX, respectively.
Preparation of PMs
PMs containing MLX/SOL/POLOX were prepared by mixing, using a mortar and pestle, for 5 minutes till a uniform mixture was obtained. The mixtures were then passed through a sieve (# 45 mesh US standard). PM-I and PM-II were prepared in the same weight ratios as the FUS mixtures (Table 1).
Analysis of drug content
The assay of the MLX content in the prepared SDs was evaluated by an HPLC method reported previously (Emara et al., 2016). The HPLC apparatus consisted of Waters’ 600 E multisolvent delivery system controller equipped with a Rheodyne injector P/N 7725i and a UV-visible spectrophotometer (Beckman, DU-650, Golden Valley, MN). A reverse-phase symmetry C18 column (particle size 5 μm, 3.9 cm × 150 mm i.d.) was used. The mobile phase was prepared from a mixture of acetonitrile/water (50:50, v/v, pH 3.0). Samples equivalent to 15 mg of MLX were dissolved in 25 ml of methanol and appropriately diluted and the drug content was determined by HPLC at λ = 360 nm. The flow rate and injection volume were 1 ml/minutes and 50 ul, respectively. The calibration curve was linear (R2 ≥ 0.999) over the tested range, with LOQ (limit of quantification) of 5 ng/ml. Inter- and intraday coefficients of variation for MLX were found to be ≤10%.
Characterization of the prepared SDs
Differential scanning calorimetry (DSC)
Thermograms for pure forms of MLX, SOL, POLOX, and their SDs were obtained. The samples were sealed in aluminum pans and analyzed using a Shimadzu DSC-50 (Kyoto, Japan). Thermal analysis was carried out using a heating ramp in the range of 20°C–400°C at 10°C/minutes under nitrogen purge (20 ml/minutes). The absolute percent crystallinity, x(t), is determined as follows:
x(t) = (ΔHt/ ΔHm)
where ΔHt is the melting enthalpy of MLX at time t, which is calculated as the ratio of melting enthalpy of the sample divided by the composition of MLX, and ΔHm represents the melting enthalpy of 100% crystalline MLX at the same heating rate (Hsu et al., 2015; Yang et al., 2010).
Polarized light microscope (PLM) observation
Representative PLM images of MLX samples were taken using a CX41 microscope (Olympus Co. Ltd., Tokyo, Japan). Samples were examined under various conditions including differential interference contrast and slightly uncrossed polars by using a red wave compensator.
Drug dissolution studies
Dissolution tests
In vitro dissolution studies (n = 3) of MLX powder, PMs, and the prepared SDs (each equivalent to 15 mg MLX) were conducted in filtered, degassed 900 ml of 0.1 N HCl (pH 1.2) at 37°C ± 0.2°C using a USP (United States Pharmacopeia) apparatus II, paddle dissolution tester (AT8, XTEND, Sotax, Switzerland) with paddle rotation of 100 rpm. At designated time points, the samples were drawn and filtered through a 0.45 um filter, replaced by the fresh dissolution medium, and then analyzed using UV spectrophotometry at 351 nm for MLX.
Dissolution data analysis
Mathematical models of release kinetics
The release kinetic models of different SDs were evaluated using the following equations (Costa and Lobo, 2001; Shoaib et al., 2006):
Zero-order kinetic model = Mt/ M∞ = M0 + k0.t
First-order kinetic model = ln (100- Mt) = ln 100 − k1.t
Second-order kinetic model = 1 / (100 – Mt) = k2.t
Higuchi square root of time kinetic model = Mt / M∞ = Mo + kH.t 1/2
Hixson–Crowell’s cube root kinetic model = (M∞) 1/3 – (M∞ − Mt) 1/3 = kHC.t,
where Mo, Mt, and M∞ are the amount of drugs dissolved at the beginning, at time t in the dissolution period, and the total mass of drug dissolved at an infinite time (typically taken to be the mass of drug available in the sample), respectively. The coefficients ko, k1, k2, kH, and kHC represent the zero-order, first-order, second-order, Higuchi, and Hixson–Crowell’s rate constants, respectively.
Dissolution efficiency (DE) determination
Percent dissolution efficiency (% DE) was also evaluated to compare the relative performance of different SDs (Anderson et al., 1998; Emara et al., 2014; Khan and Rhodes, 1972). The magnitude of % DE at 60 minutes (% DE60) for each formula was computed as the % ratio of area under the dissolution curve up to the time t to that of the area of the rectangle described by 100% dissolution at the same time. % DE is expressed as follows:
% DE = [AUC0t / Q 100.t].100.
Mean dissolution time (MDT) determination
For further comparison of dissolution profiles of the prepared SDs, MDT was calculated, as described by (Costa and Lobo, 2001), according to the following equation:
MDT = ∑ n j=1 [t^j.∆Mj] / ∑ n j=1 ∆Mj,
where j is the sample number, n is the number of dissolution sample times, t^j is the time at the midpoint between tj and tj−1, and ΔMj is the additional amount of drug released between tj and tj−1.
Drug stability studies
To investigate the effects of temperature, humidity, and the duration of storage on the final SDs, the stability of the stored samples was assessed. The selected formulae were sealed in a closed glass container and tested for accelerated conditions in a stability chamber (40°C ± 0.5°C with 75% RH (relative humidity)) for 3 and 6 months, as well as room temperature (18°C–33°C) for 12 months. The stored samples were evaluated by DSC, PLM, drug content, and dissolution profile comparison.
The comparative dissolution profiles of stored samples were compared with initial data of freshly prepared ones by calculation of the similarity factor (ƒ2), according to the following equation:
ƒ2 = 50. log {[1+ (1/n Σ t=1 n (Rt – Tt) 2] −0.5. 100},
where n is the number of time points, Rt is the percentage of drug dissolved from the reference product, and Tt is the percentage dissolved from the comparison product at different time points. The ƒ2 value is 50–100 for similarity and ≤50 for dissimilarity in the dissolution profiles (Costa and Lobo, 2001; Food and Drug Administration, 1997; Moore and Flanner, 1996).
RESULTS AND DISCUSSION
Feasibility assessment of SOL:POLOX as mixed carriers for SDs
MLX is a high melting point drug (Tmax of 255°C) and degrades near its melting point, thus presenting significant challenges for thermal processing. Proper selection of a carrier is an important criterion for the formulation of SDs containing high melting point drugs (Hughey et al., 2011). SOL has a low Tg value (approximately 70°C) and possesses the following characteristics: a wide range of extrusion temperature, excellent flowability, and extrudability; thus, it can help to dissolve MLX below its melting point (Zhang et al., 2013).
In the present study, SOL was used as a primary solubilizing carrier for the development of solid solutions. POLOX was incorporated as a plasticizer to improve the wettability and lower the viscosity of the drug-carrier blend and to enable smooth extrusion at a lower temperature and to ensure final product stability. A ratio of 2.50% w/w drug was selected for the preparation of SDs.
Processing HME parameters
The uniqueness of the HME technology lies in its simplicity and economy. HME processing parameters were optimized (i.e., feed rate, the screw speed, and extrusion temperatures) to yield extrudates with acceptable characteristics.
In the present study, the best outcome was achieved with temperatures ranging from 105°C–125°C. The extruder barrels stopped below 90°C due to high torque, and the extrudates became molten above 140°C, leading to the inability to produce uniform continuous mass. The minimum screw speed to maintain a continuous process was found to be 30 rpm.
A previous attempt to extrude 10% MLX with 90% copovidone was carried out and the results stated that a minimum barrel temperature of 140°C and a screw speed of 200 rpm were required for amorphization of the MLX powder (Haser et al., 2017). Another trial was conducted by Hughey et al. (2011), for feasible extrusion of MLX:SOL (1.0:9.0); their results clarified that the minimum extrusion temperature of 175°C was necessary for drug amorphization, and the obtained extrudates were significantly dark indicating drug decomposition.
In the present study, several trials have been carried out to select appropriate copolymer to be mixed with SOL for assisting extrusion at a lower temperature, and hence, coaddition of POLOX with SOL successfully enabled extrusion at 125°C.
Drug content
The percentages of drug content of various MLX/SDs as well as their corresponding PMs were within the range of 98.76 ± 8.08% to 102.94 ± 7.64%, which indicates acceptable uniformity of content for all prepared formulae.
Dissolution of MLX PMs
Figure 1 presented the in vitro dissolution profiles of different MLX samples, and Table 2 showed the MLX dissolution rate in 5, 15, and 60 minutes (Q5, Q15, and Q60, respectively), as well as the % DE at 60 minutes (% DE60) and MDT.
According to Figure 1 and Table 2, plain MLX powder displayed the lowest dissolution rate of 2.8% in 60 minutes, with % DE60 value of 2.3%. This might be attributed to poor solubility and wettability, as well as the agglomeration of the drug during the dissolution test that caused the powder to float on the surface of the dissolution media.
 | Figure 1. Dissolution profiles of MLX/SOL/POLOX from (A) PMs, (B) SDs prepared by FUS, and (C) SDs prepared by hot melt extrusion (EXT) at different ratios of mixed carriers (mean ± SD, n = 3). [Click here to view] |
The PMs showed a 10-fold increase in the MLX dissolution when compared to the plain drug (Fig. 1A). PMs containing SOL:POLOX in both ratios [1.5:1.0 (PM-I) and 2.3:1.0 (PM-II)] dissolved 28.67% and 29.88% of the drug at 60 minutes, respectively. The addition of SOL–POLOX as mixed carriers increased the solubility of plain MLX even by simple physical blending.
The increase in MLX dissolution when physically mixed with hydrophilic SOL in the presence of POLOX as a plasticizer could be ascribed to the increasingly solubilizing effect of mixed carriers in the diffusion layer closely surrounding drug particles. A previous study by Hughey et al. (2011) confirmed that the equilibrium solubility of the MLX powder increased by 15-fold in the presence of SOL as a matrix polymer. However, another study by Shi et al. (2019) showed a limited dissolution of PMs of MLX/SOL in pH 1.2, 4.5, 6.8, and 7.4 compared to plain MLX.
Dissolution of MLX SDs prepared by FUS method
The dissolution of MLX from FUS-II containing a high ratio of SOL to POLOX (2.3:1.0) at the initial dissolution phase was relatively faster than FUS-I (1.5:1.0 SOL to POLOX), i.e., % Q15 equals to 29.32% and 20.17%, respectively (Fig. 1B), while at the end of dissolution test, both formulae showed comparable results (Fig. 1B). SDs prepared by the FUS technique illustrated a marginal increase in MLX dissolution in contrast to PMs (Fig. 1A and B). FUS-I and FUS-II attained Q5 values of 16.37% and 27.03%, compared to 9.1% and 10.91% from PM-I and PM-II, respectively (Table 2). The total % DE60 was equal to 26.49, 30.31, 19.43, and 20.78, with MDT values of 12.93, 7.05, 19.34, and 18.28 for FUS-I, FUS-II, PM-I, and PM-II, respectively (Table 2).
 | Table 1. Composition of various MLX SDs and PMs. Percentage of MLX was kept constant at 2.50% (w/w) for all formulations. [Click here to view] |
 | Table 2. Percent drug dissolved in 5 (% Q5), 15 (% Q15), and 60 (% Q60) minutes, total percent DE in 60 minutes (% DE60), and MDT for MLX PMs and SDs (each value represents a mean ± SD, n = 3). [Click here to view] |
Usually, the thermodynamic state of the API in the resulting SDs has a pronounced impact on its dissolution rate. This was clearly observed during the MLX dissolution from FUS, where its dissolution rate was significantly higher compared to MLX alone, as well as the PMs, which might be due to the beginning of the amorphization step leading to dissolution enhancement of MLX from FUS samples.
Generally, it can be concluded from Figure 1A and B that physical mixing or melting of MLX with SOL–POLOX led to an increase in MLX dissolution, while the increasing ratio of hydrophilic SOL in such systems did not alter its dissolution rates. Also, the difference between dissolution rates of MLX from physical and FUS mixtures was obvious in the early phases of dissolution.
Dissolution of MLX SDs prepared by HME method
The dissolution profile of the MLX from extruded samples was directly proportional to the amount of SOL present in these systems. Figure 1C and Table 2 showed that the percentage of MLX dissolved in both early (% Q15) and late (% Q60) stages of dissolution was in the descending order of EXT-II > EXT-I > EXT-0 samples containing SOL–POLOX at the ratio of 2.3:1.0, 1.5:1.0, and 1.0:1.0, respectively. Total % DE60 values from EXT-0, EXT-I, and EXT-II were 19.23, 34.16, and 53.37, respectively (Table 2). Also, MDT values were in the expected rank order of EXT-II Ë‚ EXT-I Ë‚ EXT-0.
It was quite clear that all HME samples exhibited enhanced dissolution compared to all tested formulae. For SDs containing a high ratio of SOL to POLOX (2.3:1.0), the dissolution of MLX from EXT-II reached 58.05% in 60 minutes, which was about 20.7-, 1.9-, and 1.7-folds higher than that of the plain drug, PM-II, and FUS-II, respectively (Fig. 2). Similarly, for SDs containing a lower ratio of SOL to POLOX (1.5:1.0), the increase in the percentage of MLX dissolved was in the order of EXT-I > FUS-I > PM-I > plain MLX.
These results clearly categorize the superiority of HME over the FUS technique for the enhancement of MLX dissolution rate. HME provides the most intimate dispersion of MLX in the carrier mixture and its transfer to the amorphous form with subsequent enhancement in its dissolution. Moreover, the EXT formulae were successfully extruded below the melting point of MLX (255°C), which ensure the thermostability of the final product. A previous study reported that the preparation of amorphous dispersion of MLX/SOL by HME required processing temperatures of 175°C and yielded only 88% potency (Hughey et al., 2011).
Mechanism of Dissolution
The release kinetic models of different SDs were evaluated, as previously described (Costa and Lobo, 2001; Shoaib et al., 2006). The regression parameters obtained after fitting various release kinetic models to the in vitro dissolution data were presented in Table 3. The best suited kinetic model for most formulae was the Higuchi square root time model, indicating diffusion-restricted release, with R2 values nearer to 1.0. Only EXT-II followed the Hixson–Crowell kinetic model, which describes the release from systems where there is a change in surface area and/or diameter of particles or tablets (Altamimi and Neau, 2017).
Differential scanning calorimetry
The DSC scan of plain MLX, SOL, POLOX, and the prepared SDs was conducted to investigate the solid state of the drug and/or drug-carrier interaction and the results were shown in Figure 3. The DSC thermograms showed a pure crystalline endothermic peak of MLX at 261.92°C with an enthalpy (∆H) of 45.56 J/g. SOL exhibited a glass transition temperature at 74.28°C (Hardung et al., 2010; Nagy et al., 2012). Also, a melting endotherm of POLOX 407 at 59.49ºC was shown in Figure 3. Figure 3 also showed the DSC thermograms of SDs prepared by HME (EXT-II), FUS-II, and PM-II containing SOL to POLOX in the ratio of 2.3:1.0. A slight shift of MLX melting endotherm was detected for all tested samples with a gradual reduction in energy of enthalpy. The enthalpy value of MLX from PM-II was 25.89 J/g, whereas it attained a value of 24.11 J/g for FUS-II, while for EXT-II, the energy of enthalpy further decreased to 18.59 J/g, clearly indicating a higher amorphization degree of MLX by the HME method compared to FUS method. The reduction of the drug melting peaks in all studied SDs was attributable to the gradual conversion of the drug to the amorphous form and/or its dissolution in the melted carriers before reaching its melting temperature; these results correlated with the enhanced in vitro dissolution observed with SDs with respect to the plain drug. The calculated absolute percent crystallinity for PM, FUS, and EXT (i.e., 22.73, 21.17, and 16.32%, resp.) verified the superiority of the HME technique for the gradual conversion of crystalline MLX to its amorphous state which accounts for its higher dissolution.
 | Figure 2. Dissolution profiles of MLX/SOL/POLOX prepared by hot melt extrusion (EXT) and FUS methods against PMs at SOL:POLOX in the ratio of 2.3:1.0 (mean ± SD, n = 3). [Click here to view] |
 | Figure 3. DSC thermograms of MLX, SOL, and POLOX in pure forms, PMs, and fresh SDs. The ratio of SOL to POLOX is 2.3:1.0. [Click here to view] |
 | Table 3. In vitro dissolution kinetic models for different MLX formulae. [Click here to view] |
Inferring the results of the DSC analysis should be carefully interpreted, as the SOL/POLOX mixed carriers melt at considerably lower temperatures than MLX. Hence, residual crystalline MLX might gradually dissolve in the molten carriers during the first scan heating, leading to incorrect DSC readings (Medarevic et al., 2016; Shah et al., 2007). The observed reduction of MLX melting peak from PMs (PM-II) during DSC scan might be due to the above-mentioned postulation. Therefore, the employment of a secondary method of analysis is quite essential for the full elucidation of the physical state of the drug within the SDs.
DeWitt (2015) stated that low drug concentrations within the dosage form might be below the detectable limits of the X-ray diffraction (XRD) instrumentation; hence, employment of XRD for the characterization of the prepared SDs was not recommended in the present study (as drug loading was kept at 2.5% w/w for all formulae). The PLM method was alternatively employed for the interpretation of physical characteristics of the drug within SDs in either fresh and/or stored conditions.
Polarized light microscope
Figure 4 showed the amorphization state of MLX in SDs, observed by PLM analysis. The plain MLX sample clearly showed intense birefringence indicating the presence of the drug in the crystalline state (Fig. 4) (Ochi et al., 2013, 2016). PLM observations displayed evidence of birefringence still visible in the PM-II sample, which confirm the presence of MLX in the crystal state. This highlighted the importance of PLM as a confirmatory, sensitive analytic method for the detection of drug crystallinity compared to DSC. Even though FUS-II and EXT-II samples contained the same ratio of SOL to POLOX; however, weak birefringence was still observed in FUS-II, while EXT-II showed no birefringence (Fig. 4). This confirmed the gradual transfer of crystalline MLX to its amorphous state in EXT-II, which further account for the enhancement of MLX acidic dissolution (Ochi et al., 2013).
 | Figure 4. Morphological observation of MLX samples using PLM. The ratio of SOL to POLOX is 2.3:1.0. [Click here to view] |
Stability results
Stability studies were conducted on FUS-II and EXT-II containing SOL/POLOX in the ratio of 2.3:1.0. Physical observations of all stored SDs samples showed no changes in their color and/or appearance. The analysis of the drug content of different stored samples was investigated and the results showed that the amount of MLX in all stored samples was found to be within 97.5%–105.45% of the initial dose.
Dissolution profiles of stored SDs were compared with initial data of freshly prepared ones by employing the similarity factor (ƒ2), and the results were presented in Table 4 and Figure 5. SD samples prepared by FUS technique (i.e., FUS-II) showed excellent stability under different storage conditions, indicated by ƒ2 ≥ 50 (Table 4 and Fig. 5).
On the other hand, SDs prepared by the HME technique (EXT-II) showed no change in the MLX dissolution rate after bench storage for 12 months (i.e., f2 value = 79, Table 4 and Fig. 5). Yet, a significant decrease in % drug dissolved was detected after 3–6 months of storage at 40°C/75% RH (i.e., ƒ2 Ë‚ 50), which indicates MLX recrystallization from EXT-II sample under such storage conditions.
The changes noted upon storage of MLX/SDs under different conditions were further detected by DSC (Fig. 6a and b) and PLM (Fig. 6c–f) analyses. Figure 6a clearly shows FUS-II samples compared to fresh ones, hence confirming that the amorphous nature of the drug has been reserved; similarly, EXT-II samples exhibited similar stability characteristics after 12 months of bench storage (Fig. 6b). However, the reappearance of MLX crystalline peak was detected by the DSC scan for EXT-II samples stored for 6 months at 40°C/75% RH (Fig. 6b). Figure 6c–f showed the results of PLM analysis of stored SDs, where no clear evidence of birefringence was detected for most stored samples confirming the stability of MLX SDs, where reappearance of birefringence indicate the recrystallization of EXT sample under such storage conditions.
 | Table 4. Similarity factor (f2) values between fresh and stored MLX/SDs under different conditions of temperature and humidity. [Click here to view] |
 | Figure 5. Dissolution profiles of different MLX/SDs stored at different conditions with respect to fresh samples. The ratio of SOL to POLOX is 2.3:1.0 (mean ± SD, n = 3). [Click here to view] |
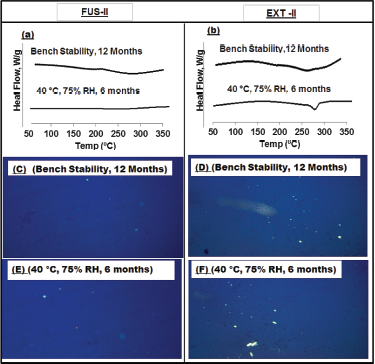 | Figure 6. DSC and PLM analyses of stored MLX/SDs prepared by fusion method (FUS) and hot melt extrusion (EXT). The ratio of SOL to POLOX is 2.3:1.0. (a and b) DSC; (c–f) PLM. [Click here to view] |
This study explored for the first time the difference observed between MLX/SDs long-term bench storage and short-term accelerated storage. Reported data usually focus on conducting the stability testing of MLX SDs at 40°C/75% RH for several months. While some of these formulae remained stable after storage under such conditions (Suhail et al., 2013; Umesh et al., 2012), other SDs changed to semisolid unstable forms, with evidence of drug recrystallization (Ochi et al., 2016; Shi et al., 2019). Differences detected between the two stability protocols frequently employed raise an important query. Emara et al. (2017) stated that attention should be drawn to the differences observed in drug behavior when a certain formula fails to provide the accepted stability criterion under accelerated short-term storage while its stability is confirmed for several years at room temperature. The shelf life extension program was conducted by the US FDA (Food and Drug Administration) in 1986 to extend the expiration dates on qualifying drugs as they retain their potency several years beyond the labeled expiry date. For the approval of extending of shelf life of such drug products, acceptable data should be documented, including full, long-term stability studies on at least three production batches in accordance with FDA guidelines (Kamla, 2016).
CONCLUSION
The present study discussed a successful attempt to formulate SDs for the high melting point MLX via HME and compared it with the conventional FUS melt technique. Reported trials to prepare MLX/SDs by HME were previously unproductive, with most of the published approaches employed for MLX/SDs preparation use mixtures of organic solvents. HME provides a continuous, solvent-free, dust-free, eco-friendly manufacturing technique. The proposed novel combination of SOL/POLOX allowed effective processing of MLX at lower extrusion temperatures. SDs prepared by HME at SOL-POLOX ratio of 2.3:1.0 enhanced acidic dissolution of MLX, as well as maintained its stability for up to 12 months of bench storage.
ACKNOWLEDGMENTS
The authors are thankful to BASF SE (Ludwigshafen, Germany) for providing the gift samples of Soluplus® and Poloxamer 407 to support this study.
ETHICAL CONSIDERATION
This study does not involve the use of animal or human subjects.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data or analysis, and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Altamimi MA, Neau SH. Investigation of the in vitro performance difference of drug-Soluplus and drug-PEG 6000 dispersions when prepared using spray drying or lyophilization. Saudi Pharm J, 2017; 25:419–39 CrossRef
Amidon GL, Lennernas H, Shah VP, Crison J. A theoretical basis for a biopharmaceutic drug classification: the correlation of in-vitro drug product dissolution and in vivo bioavailability. Pharm Res, 1995; 12:413–20. CrossRef
Anderson N, Bauer M, Boussac N, Malek RK, Munden P, Sardaro M. An evaluation of fit factors and dissolution efficiency for the comparison of in-vitro dissolution profiles. J Pharm Biomed Anal, 1998; 17:811–22. CrossRef
Andrews GP, Jones DS, Diak OA, McCoy CP, Watts AB, McGinity JW. The manufacture and characterization of hot-melt extruded enteric tablets. Eur J Pharm Biopharm, 2008; 69:264–73. CrossRef
Breitenbach J. Melt extrusion: from process to drug delivery technology. Eur J Pharm Biopharm, 2002; 54:107–17. CrossRef
Collett JH. Poloxamer. In: Rowe RC, Sheskey PJ, Quinn ME (eds.). Handbook of pharmaceutical excipients. 6th edition, Pharmaceutical Press and American Pharmacists Association, London, UK, pp 506–9, 2009.
Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci, 2001; 13(2),123–33. CrossRef
DeWitt KM. X-ray powder diffraction method development and validation for the identification of counterfeit pharmaceuticals. J Mater Sci, 2015; 1–28.
Duncan QMC. Review: the mechanism of drug release from solid dispersions in water soluble polymers. Int J Pharm, 2002; 231:131–44. CrossRef
Emara LH, El-Ashmawy AA, Taha NF. Stability and bioavailability of diltiazem/polyethylene oxide matrix tablets. Pharm Dev Technol, 2017; 3:1–10. CrossRef
Emara LH, Emam MF, Taha NF, El-Ashmawy AA, Mursi NM. In-vitro dissolution study of meloxicam immediate release products using flow through cell (USP apparatus 4) under different operational conditions. Int J Pharm Pharm Sci, 2014; 6(11):254–60.
Emara LH, Emam MF, Taha NF, Raslan HM, El-Ashmawy AA. A simple and sensitive HPLC/UV method for determination of meloxicam in human plasma for bioavailability and bioequivalence studies. J Appl Pharm, 2016; 6(07):012–9. CrossRef
Follonier N, Doelker E, Cole ET. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained release capsules containing high loadings of freely soluble drugs. Drug Dev Ind Pharm, 1994; 20,1323–39. CrossRef
Food and Drug Administration. Guidance for industry: dissolution testing of immediate-release solid oral dosage forms. Food and Drug Administration, Silver Spring, MD, 1997.
Ghareeb MM, Abdulrasool AA, Hussein AA, Noordin N. Kneading technique for preparation of binary solid dispersion of meloxicam with poloxamer 188. AAPS PharmSciTech, 2009; 10:1206–15. CrossRef
Guo Z, Lu M, Li Y, Pang H, Lin L, Liu X, Wu, C. The utilization of drug-polymer interactions for improving the chemical stability of hot-melt extruded solid dispersions. J Pharm Pharmcol, 2014; 66(2):285–96. CrossRef
Hardung H, Djuric D, Ali S. Combining HME & solubilization: Soluplus® - the solid solution. Drug Deliv Tech, 2010; 10:20–7.
Haser A, Huang S, Listro T, White D, Zhang F. An approach for chemical stability during melt extrusion of a drug substance with a high melting point. Int J Pharm, 2017; 524(1–2):55–64. CrossRef
Hsu HY, Toth S, Simpson GJ, Harris MT. Drop printing of pharmaceuticals: effect of molecular weight on PEG coated-naproxen/PEG3350 solid dispersions. AIChE J, 2015; 61(2):4502–8. CrossRef
Hughey JR, Keen JM, Brough C, Saeger S, McGinity JW. Thermal processing of a poorly water-soluble drug substance exhibiting a high melting point: the utility of KinetiSol® Dispersing. Int J Pharm, 2011; 419(1–2):222–30. CrossRef
Kamla P. Shelf life extension program: revalidating expiry date. J Appl Pharm, 2016; 8(4):69–70.
Khan K, Rhodes CT. Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm Acta Helv, 1972; 47:594–607.
Kumar A, Ganjyal GM, Jones, DD, Hanna MA. Modeling residence time distribution in a twin-screw extruder as a series of ideal steady-state flow reactors. J Food Eng, 2008; 84:441–8. CrossRef
LaFountaine JS, McGinity JW, Williams RO. Challenges and strategies in thermal processing of amorphous solid dispersions: a review. AAPS PharmSciTech, 2016; 17:43–55. CrossRef
Lalkshman JP, Cao Y, Kowalski J, Serajuddin ATM. Application of melt extrusion in the development of a physically and chemically stable high-energy amorphous solid dispersion of a poorly water-soluble drug. Mol Pharm, 2008; 5(6):994–1002. CrossRef
Linn M, Collnot EM, Djuric D, Hempel K, Fabian E, Kolter K, Lejr KM. Soluplus® as an effective absorption enhancer of poorly soluble drugs in-vitro and in vivo. Eur J Pharm Sci, 2012; 45(3):336–43. CrossRef
Lipscomb GR, Wallis N, Armstrong G, Rees WDW. Gastrointestinal tolerability of meloxicam and piroxicam: a doubleblind placebo-controlled study. Br J Clin Pharmacol, 1998; 46(2):133–7. CrossRef
Liu HJ, Wang P, Zhang XY, Shen F, Gogos CG. Effects of extrusion process parameters on the dissolution behavior of indomethacin in Eudragit® E PO solid dispersions. Int J Pharm, 2010; 383(1–2):161–9. CrossRef
Lugar P, Daneck K, Engel W, Trummlitz G, Wagner K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur J Pharm Sci, 1996; 4:175–87. CrossRef
Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN Pharm, 2012; 2012:1–9. CrossRef
Medarevic´ DP, Kachrimanis K, Mitric´ M, Djuriš J, Djurić Z, Ibrić S. Dissolution rate enhancement and physicochemical characterization of carbamazepine-poloxamer solid dispersions. Pharm Dev Technol, 2016;21(3):268-76. CrossRef
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol, 1996; 20:64–74.
Nagy ZK, Baloghm A, Vajnam B, Farkasm A, Patyi G, Kramarics A, György M. Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci, 2012; 101(1):322–32. CrossRef
Newa M, Bhandari KH, Li DX, Kwon TH, Kim JE, Yoo BK, Woo JS, Hoi HG. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int J Pharm, 2007; 343:228–37. CrossRef
Newa M, Bhandari KH, Oh DH, Kim YR, Sung JH, Kim JO, Woo JS, Yong CS. Enhanced dissolution of ibuprofen using solid dispersion with poloxamer 407. Arch Pharm Res, 2008; 31:1497–507. CrossRef
Ochi M, Inoue R, Yamauchi Y, Yamada S, Onoue S. Development of meloxicam salts with Improved dissolution and pharmacokinetic behaviors in rats with impaired gastric motility. Pharm Res, 2013; 30:377–86. CrossRef
Ochi M, Kimura K, Kanda A, Kawachi K, Matsuda A, Yuminoki K, Hashimoto N. Physicochemical and pharmacokinetic characterization of amorphous solid dispersion of meloxicam with enhanced dissolution property and storage stability. AAPS PharmSciTech, 2016; 17(4):932–9. CrossRef
Pairet M, van Ryn J, Schierok H, Mauz A, Trummlitz G, Engelhardt G. Differential inhibition of cyclooxygenases-1 and -2 by meloxicam and its 40-isomer. Inflamm Res, 1998; 47(6):270–6. CrossRef
Raimi-Abraham BT, Mahalingam S, Davies PJ, Edirisinghe M, Craig DQM. Development and characterization of amorphous nanofiber drug dispersions prepared using pressurized gyration. Mol Pharm, 2015; 12:3851–61. CrossRef
Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev Ind Pharm, 1999; 25(5):625–33. CrossRef
Schilling SU, Shah NH, Malick AW, Infeld MH, McGinity JW. Citric acid as a solid-state plasticizer for Eudragit RS PO. J Pharm Pharmacol, 2007; 59(11):1493–500. CrossRef
Sekikawa H, Fukuda N, Takada M, Ohtani K, Arita T, Nakano M. Dissolution behavior and gastrointestinal absorption of dicumarol from solid dispersion systems of dicumarolpolyvinylpyrrolidone and dicumarol-beta-cyclodextrin. Chem Pharm Bull, 1983; 31:1350–6. CrossRef
Serajuddin AT. Solid dispersion of poorly water soluble drugs: early promises problems subsequent and recent breakthroughs. J Pharm Sci, 1999; 88:1058–66. CrossRef
Shah T, Amin AF, Parikh JR, Parikh RH. Process optimization and characterization of poloxamer solid dispersions of a poorly water-soluble drug. AAPS PharmSciTech, 2007; 8:E18–E24. CrossRef
Shazly G, Badran M, Zoheir K, Alomrani A. Utilization of spray drying technique for improvement of dissolution and anti-inflammatory effect of meloxicam. J Pak J Pharm Sci, 2015; 28(1):103–11.
Shi X, Huang W, Xu T, Fan B, Sheng X. Investigation of drug–polymer miscibility and solubilization on meloxicam binary solid dispersion. J Pharm Innov, 2019; 15:125–37. CrossRef
Shoaib MH, Tazeen J, Merchant HA, Yousuf RI. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci, 2006; 19(2),119–24.
Suhail B, Noolkar SB, Jadhav NR, Bhende SA, Killedar SG. Solid-state characterization and dissolution properties of meloxicam–moringa coagulant–PVP ternary solid dispersions. AAPS PharmSciTech, 2013; 14(2):569–77. CrossRef
Suzuki H, Yakushiji K, Matsunaga S, Yamauchi Y, Seto Y, Sato H. Amorphous solid dispersion of meloxicam enhanced oral absorption in rats with impaired gastric motility. J Pharm Sci, 2018; 107(1):446–52. CrossRef
Taha NF, Emam MF, Emara LH. A novel combination of Soluplus®/poloxamer for meloxicam solid dispersions via hot melt extrusion for rapid onset of action. Part 2: comparative bioavailability and IVIVC. Drug Dev Ind Pharm, 2020; 46(8):1362–72; doi: 10.1080/03639045.2020.1791164 CrossRef
Türck D, Roth W, Busch U. A review of the clinical pharmacokinetics of meloxicam. Br J Rheumatol, 1996; 35(Suppl 1):13–6. CrossRef
Umesh MR, Naveen S, Amit C. Physical properties and dissolution behaviour of meloxicam/poloxamer solid dispersions prepared by hot melt method and microwave assisted method. Int J Res Pharm Sci, 2012; 2(2):64–74.
Van den Mooter G, Weuts I, De Ridder T, Blaton N. Evaluation of inutec SP1 as a new carrier in the formulation of solid dispersions for poorly soluble drugs. Int J Pharm, 2006; 316(1):1–6. CrossRef
Verreck G, Decorte A, Heymansa K, Hanna MA. Hot stage extrusion of p-amino salicylic acid with EC using CO2 as a temporary plasticizer. Int J Pharm, 2006; 327:45–50. CrossRef
Vilhelmsen T, Eliasen H, Schæfer T. Effect of a melt agglomeration process on agglomerates containing solid dispersions. Int J Pharm, 2005; 303(1):132–42. CrossRef
Won DH, Kim MS, Lee S, Park JS, Hwang SJ. Improved physicochemical characteristics of felodipine solid dispersion particles by supercritical anti-solvent precipitation process. Int J Pharm, 2005; 301(1):199–208. CrossRef
Yang J, Grey K, Doney J. An improved kinetics approach to describe the physical stability of amorphous solid dispersions. Int J Pharm, 2010; 384(1–2):24–31. CrossRef
Zhang K, Yu H, Luo Q, Yang S, Lin X, Zhang Y, Tian B, Tang X. Increased dissolution and oral absorption of itraconazole/Soluplus extrudate compared with itraconazole nanosuspension. Eur J Pharm Biopharm, 2013; 85,1285–92. CrossRef