INTRODUCTION
According to the latest report of the World Health Organization (WHO) “World Medicines Situation Report 2011”, approximately one-third of the world population does not have access to essential medicines leading to the implementation of a generic drug policy in several countries (Hogerzeil and Mirza, 2011). It is known that the generic medicine market constitutes 38% of the total market in Peru and it is projected to reach up to 882 million in 2021 with an annual growth rate of 6.2% (Pharmaceuticals Export Promotion Council, 2017).
Worldwide, one of the therapeutic classes with more generic presentations are antibiotics but these are among the majority of low-quality and/or counterfeit medicines found in developing countries (Kelesidis and Falagas, 2015) and there are studies that show that exposure to antibiotics of poor quality can promote the development of resistant bacterial strains (Andersson and Hughes, 2014).
In the beginning, the WHO strategy against antimicrobial resistance focused on fighting against the lack of adherence but now, this agency promotes also the quality assurance of generic antibiotics from manufacture to storage. In this way, in countries such as Peru where susceptibility tests are expensive, treatment failure would not be mistakenly attributed to a resistant strain (Nwokike et al., 2018).
Unfortunately, in Peru, from 2000, alerts related to falsification and quality problems affecting the content of the active substance and physical characteristics of one of the most popular antibiotics, amoxicillin powder for oral suspension, manufactured by national and international laboratories, have been reported (DIGEMID, 1997; 2004).
Powders for oral suspensions were developed to face physical and chemical instability of suspensions associated with problems such as a possible increase in the solubility of the drug due to pH changes, chemical degradation, incompatibility of excipients, viscosity problems, polymorphic conversion, crystal growth, caking, and the consequent need for longer-lasting medicines (Ofner CM III et al., 1989) but this delivery system is affected by moisture, which can cause powder aggregation, agglomerates formation, particle size and flow properties changes affecting the dosage (Zeng et al., 2007).
Furthermore, amoxicillin oral formulations should be stored in well-sealed bottles because high temperature and moisture in amoxicillin solid-state formulations (tablets, capsules, and powders) can lead to the hydrolytic breakdown of the beta-lactam ring (Thambavita et al., 2017) and the formation of amoxicilloic acid and amoxicillin diketopiperazine- 2′,5′, to which allergic reactions are attributed (Binson et al., 2019). Although hydrolysis is an important route of degradation of amoxicillin, the main route of degradation is dimerization producing highly antigenic polymeric substances (Florence and Attwood, 2016).
For this reason, in order to guarantee the safety and efficacy of medicines, Good Manufacturing Practices and Good Storage Practices are crucial elements. In this respect, the aim of this research is to have evidence of the impact of real storage conditions in order to promote Good Storage Practices in drug stores.
MATERIALS AND METHODS
Materials
Three Peruvian laboratories with Good Manufacturing Practices certification were selected and 200 units of three different batches of amoxicillin powder for oral suspension (250 mg/5 ml) were purchased from each of them. The sample size was based on the sampling manual for quality control of the National Institute of Health of Peru. The nine batches used were coded as follows: A, B, and C for laboratory 1; D, E, and F for laboratory 2, and G, H, and I for laboratory 3. Record of the batches, product content, packaging materials, and manufacture and expiry dates were kept (Table 1). An Amoxicillin Trihydrate United States Pharmacopeia (USP) reference standard was purchased and all other reagents used were of analytical grade (Merck, Germany).
Quality test
Description of samples
A pool of five bottles was made in order to analyze the color, taste, and smell of non-reconstituted and reconstituted samples. In both cases, the presence of clusters was evaluated.
Deliverable volume
Ten bottles of each batch were taken and reconstituted with ultrapure water following the manufacturer's label instructions and the deliverable volume was measured according to the official method (USP-41, 2018).
pH determination
A pH meter (Mettler Toledo, China) was calibrated and used to determine in triplicate the pH values of the same pool made for the drug content determination at room temperature (25°C), verifying that they were within the official range established (USP-41, 2018), from 5.0 to 7.5. Similarly, the samples refrigerated for 7 days were removed from cold storage and allowed to reach room temperature before pH determination.
Determination of content of amoxicillin by high-performance liquid chromatography
Amoxicillin content was determined using a pool of six bottles per batch reconstituted with ultrapure water by a validated high performance liquid chromatography (HPLC) method verifying that it was within the official range established from 90% to 120% of the label claim (USP-41, 2018). The analyses were undertaken on an AT (Agilent, Technologies) 1260 HPLC system containing diode array detector (DAD), fluorescence detector (FLD), and refractive index detector (RID) with a Zorbax Eclipse L1 (C18) column (150 mm × 4.6 mm × 5 μm) using the ultraviolet detector at 230 nm, a temperature of 30°C, a flow rate of 1 ml/minute, and an injection volume of 10 μl. The mobile phase was a mixture of acetonitrile and 6.8 g/l of monobasic potassium phosphate pH 5.0 ± 0.1 (1:24). The approximate retention time to detect amoxicillin was 4.2 minutes. Each pool was tested in duplicate with two injections per sample.
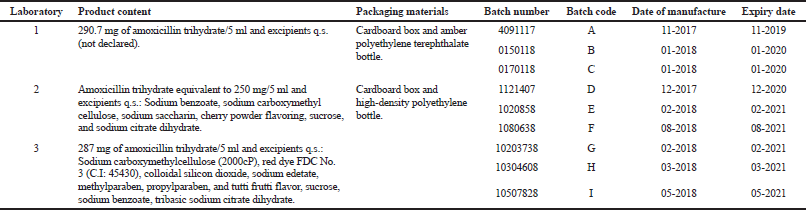 | Table 1. Amoxicillin powders for oral suspension (250 mg/5 ml) used in the study. [Click here to view] |
Stability study
Three drug stores, located at Lima (coast), Huaraz (the Andes), and Tarapoto (jungle), were selected because they had sanitary authorization. After the initial quality analysis, 50 units of each batch were sent to one of the drug stores and were stored properly on shelves, keeping a daily record of temperature and relative humidity of storage. It was verified that ventilation and lighting were appropriate. Twenty-five samples were removed from each batch after being stored for 1 and 3 months and were sent for the determination of parameters such as description, pH, and drug content. In order to test the in-use stability of reconstituted suspensions, each pool (stored in its original container) was kept refrigerated (2°C–8°C) for 7 days after reconstitution and the parameters mentioned above were determined again.
Statistical analysis
A paired sample t-test between means of the same batch was conducted for the stability study using Minitab 18 software to establish the significant effect of the storage, p < 0.05 was considered significant. Also, a one-way analysis of variance test was used to determine whether there were any statistically significant differences between the means of different batches.
RESULTS AND DISCUSSION
The three drug stores presented different storage conditions during the 3 months, the median temperature and relative humidity were 22°C and 62% in Lima, 20.55°C and 41% in Huaraz, and 27.1°C and 64% in Tarapoto.
All the samples were within the expiry period. Initially, the freshly reconstituted and refrigerated samples were orange colored and flavored (laboratory 1), cream-colored and cherry flavored (laboratory 2), and pink colored and tutti frutti flavored (laboratory 3); all of them were slightly bittersweet. There were no variations in the description of the batches after 1 month of storage; however, after 3 months, the taste and smell of the three reconstituted and non-reconstituted batches A, B, and C were a bit unpleasant and cluster formation was observed. This would be explained because the stability of the powders for suspension is affected by storage relative humidity, because alterations of the forces responsible for the flow behavior such as liquid bridge, electrostatic, and van der Waal’s ones depend on moisture level changes (Mukherjee et al., 2019).
Although there were no changes in the deliverable volume, there was a significant drop in the pH after 3 months of storage at Tarapoto (Table 2). In the case of laboratories 1 and 3, all the pH values of the freshly reconstituted samples differed significantly from the initial ones. As indicated in Table 2, regarding the in-use stability study, only some pH values of the refrigerated samples of laboratories 1 and 3 differed significantly from the freshly reconstituted ones but were within specifications (USP-41, 2018). The pH variations found are in agreement with those described in a study in Ethiopia where the pH and water content of generic amoxicillin powders for reconstitution stored at 40°C and 75% RH for 6 months, simulating tropical conditions, changed and were out of specification, although the dosage was acceptable (Mikre and Mekonnen, 2011). This is relevant because, the efficacy and stability of amoxicillin depend on pH because it is an aminopenicillin (Frynkewicz et al., 2013).
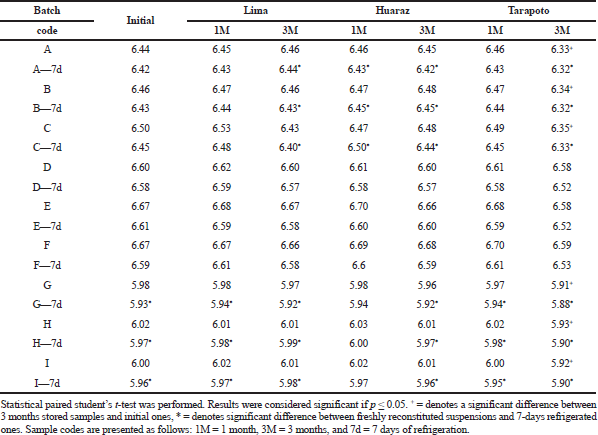 | Table 2. pH results of amoxicillin suspensions 250 mg/5 ml stability samples (n = 3). [Click here to view] |
Chemical stability was studied through the drug content assay. Only after 3 months of storage at Tarapoto, the percentage of the labeled claim of the reconstituted samples decreased significantly across all the different batches (Table 3) but there were no out-of-specification test results, this would be due to overloads made during manufacturing. As shown in Figures 2 and 3, laboratory 3 had the largest variation of drug content and laboratory 2, the lowest one. This can be associated with hydrolytic decomposition and the higher temperature and relativity humidity during the long period of storage (Im-Emsap et al., 2002).
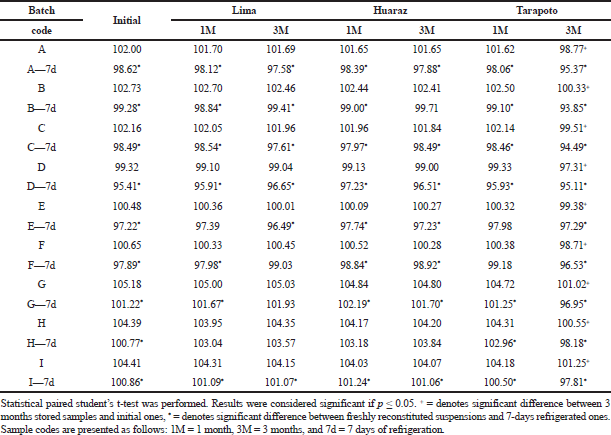 | Table 3. Mean drug content of amoxicillin powder for suspension at zero, one- and three-months storage at Lima, Huaraz and Tarapoto before and after refrigeration. [Click here to view] |
Scientific research has evidenced the effect of storage under tropical conditions on amoxicillin stability. One study carried out in Brazil after having received many claims about the therapeutic inefficiency of generic amoxicillin suspensions regarding reconstitution problems found that the dosage of three out of six brands was out of specification after 7-days storage at room temperature (Pires et al., 2014). Likewise, in Ghana, evaluating the stability of two generic amoxicillin and clavulanic acid suspension powders and the reference one, the dosage of samples stored at room temperature was lower but there were no significant differences in drug content after reconstitution with distilled water, treated tap water, or mineral water (Addotey et al., 2014). On the contrary, a study conducted in Yemen with generic amoxicillin suspensions showed that storage at room temperature and use of tap water for reconstitution have a negative effect due to acidic pH and metal content in water (Al-Tahami, 2014). In Dhaka, a study was conducted to evaluate the stability of reconstituted suspensions stored for 1 week at room temperature, under refrigeration, and at 40°C finding the lowest drug content at the highest storage temperature (Haider et al., 2011).
Taking the medians of temperature and relative humidity of each drug store into account, the drug content results allow us to infer that a higher temperature, relative humidity, and storage time increase the degradation of the active ingredient. In addition, it is well-known that solid-state formulations of amoxicillin are more sensitive to moisture since it promotes the hydrolysis of the beta-lactam ring (Loftsson, 2014).
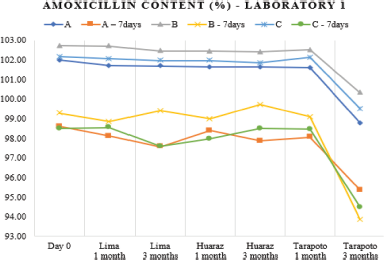 | Figure 1. Amoxicillin content variation (as a percent of label claim) in batches of laboratory 1 at 0-, 1-, and 3-months storage at Lima, Huaraz, and Tarapoto before and after refrigeration. [Click here to view] |
A greater dose reduction would have been expected for laboratory 1 (Fig. 1) because the product should be stored at no more than 25°C, according to the manufacturer’s instructions, instead of laboratory 2, whose indication, same as laboratory 3, is a storage temperature of not more than 30°C. This should not be related to the container material (Table 1) because laboratory 1 bottle material is amber polyethylene terephthalate PET, a better gas insulating material and laboratory 2 one is high-density polyethylene, a better moisture barrier (Chen, 2016); so the results would respond to the differences in the formulation for each laboratory. Differences in the formulation can have a dramatic impact on the dosage, for example, in a study conducted in Yemen with samples from five generic laboratories, a decrease of up to approximately 40% was observed in two laboratories, becoming out of specification on the third day of storage at 25°C; while for samples stored at 40°C, the dosage was reduced by half on the second day (Alburyhi et al., 2013).
Another objective of this work was to evaluate the dosage after a week of refrigeration of the reconstituted suspensions that was optional for all the three laboratories, because the stability of the pharmaceutical product implies not only the evaluation of chemical stability and physical stability but also performance over time (USP-41, 2018); therefore, the analysis of the quality of a powder for oral suspension should be made at the end of the maximum storage time declared by the manufacturer. After 7 days of refrigeration, the variation of the drug content of refrigerated samples with respect to the freshly reconstituted samples was different for each of the batches analyzed (Table 3). This variability, as seen in Figures 1–3, can be attributed to the heterogeneous nature of the suspensions as dispersed systems susceptible to changes related to storage temperature and the existence of caking or experimental error when taking aliquots due to insufficient agitation (Casarolli and Zanin, 2013).
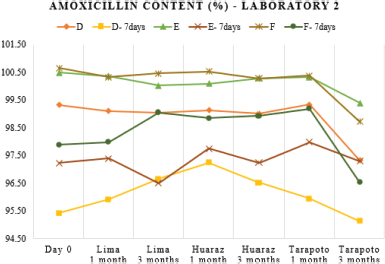 | Figure 2. Amoxicillin content variation (as a percent of label claim) in batches of laboratory 2 at 0-, 1-, and 3-months storage at Lima, Huaraz, and Tarapoto before and after refrigeration. [Click here to view] |
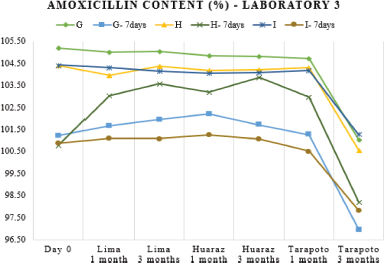 | Figure 3. Amoxicillin content variation (as a percent of label claim) in batches of laboratory 3 at 0-, 1-, and 3-months storage at Lima, Huaraz, and Tarapoto before and after refrigeration. [Click here to view] |
If expiry dates of the reported batches and the dates of the alerts (DIGEMID, 1997; 2004) are considered, it is alarming that some of them were reported the following year of manufacture and not close to their expiration date as expected. This is consistent with the results found in this work, taking into account that the samples of this study were analyzed near their manufacturing date with a sanitary authorization, obtained after long-term stability studies, under controlled storage conditions according to current regulations and not in drug store storage conditions.
CONCLUSION
This research project studied three generic brands of amoxicillin powder for oral suspension at real storage conditions. Even though all the samples met the quality parameters until the end of the study, changes in the description of non-reconstituted and reconstituted powders and a statistically significant drop in pH (laboratories 1 and 3) and in the dosage of amoxicillin (the 3 laboratories) were found only for the samples stored for 3 months in the drug store located in Tarapoto, city with the highest relative humidity and temperature medians, which are factors involved in the decomposition of amoxicillin and in the variation of flow properties of powders. Our results evidence that drug store storage conditions (relative humidity and temperature) and period of storage will cause changes in the quality and stability of the analyzed amoxicillin powders for oral suspension. A strict control over the temperature and humidity of drug stores must be maintained in order to assure the quality and stability of antibiotic powders for reconstitution.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
This research received no external funding.
REFERENCES
Addotey JNA, Yeboah-Awudzi L, Adosraku RK. Stability studies on reconstituted amoxicillin-clavulanic acid oral powder by hplc method development and quantification. Int J Pharm Sci Pract, 2014; 3(1):1–12.
Alburyhi MM, Siaf AA, Noman MA. Stability study of six brands of amoxicillin trihydrate and clavulanic acid oral suspension present in Yemen markets. J Chem Pharm Res, 2013; 5(5):293–6.
Al-Tahami K. Quality and stability of co-amoxiclav products in the Yemeni market. J Chem Pharm Res, 2014; 6(8):565–9.
Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol, 2014; 12(7):465–78. CrossRef
Binson G, Grignon C, Le Moal G, Lazaro P, Lelong J, Roblot F. Overcoming stability challenges during continuous intravenous administration of high-dose amoxicillin using portable elastomeric pumps. PLoS One, 2019; 14(8):1–13. CrossRef
Casarolli R, Zanin D. Estabilidade de suspensões extemporâneas de amoxicilina comercializadas em Cascavel––PR: estudo comparativo. Facultad Assis Gurgacz de Casavel (Online), 2013. Available via https://www.fag.edu.br/upload/graduacao/tcc/53027cab5ea08.pdf (Accessed 18 January 2019).
Chen Y. Packaging selection for solid oral dosage forms. In: Qiu Y, Chen Y, Zhang GGZ, Yu L, Mantri RV, (eds.). Developing solid oral dosage forms: pharmaceutical theory and practice. 2nd edition, Academic Press, New York, NY, pp 564–5, 2016.
DIGEMID. Alertas de la Dirección General de Medicamentos, Insumos y Drogas (Alerts of the General Direction of Medicines, Supplies and Drugs) (Online), 1997. Available via http://www.digemid.minsa.gob.pe/Main.asp?Seccion=371 (Accessed 8 January 2018).
DIGEMID. Observatorio de la calidad de la Dirección General de Medicamentos, Insumos y Drogas (Quality Observatory of the General Direction of Medicines, Supplies and Drugs) (Online), 2004. Available via http://www.digemid.minsa.gob.pe/main.asp?Seccion=443 (Accessed 8 January 2018).
Florence AT, Attwood D. Physicochemical principles of pharmacy. In: Manufacture, formulation and clinical use. 6th edition, Pharmaceutical Press, London, UK, pp 109–50, 2016.
Frynkewicz H, Feezle H, Richardson M. Thermostability determination of broad-spectrum antibiotics at high temperatures by liquid chromatography-mass spectrometry. In: Proceedings of The National Conference on Undergraduate Research (NCUR) 2013. University of Wisconsin La Crosse, La Crosse, WI, pp 333–7, 2013.
Haider SS, Nasrin N, Apu AS, Asaduzzaman M. Accelerated stability and antimicrobial sensitivity studies of amoxicillin dry suspensions marketed in Bangladesh. J Appl Pharm Sci, 2011; 1(9):51–5.
Hogerzeil H, Mirza Z. Access to essential medicines as part of the right to health. In: The World medicines situation 2011. 3rd edition, WHO Press, Geneva, Switzerland, pp 1–12, 2011.
Im-Emsap W, Paeratakul O, Siepmann J. Disperse systems. In: Gilbert SB Christopher TR (eds.). Modern pharmaceutics. 4th edition, Marcel Dekker, New York, NY, pp 243–82, 2002.
Kelesidis T, Falagas ME. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev, 2015; 28(2):443–64. CrossRef
Loftsson T. Drug stability for pharmaceutical scientists. Elsevier Academic Press, Amsterdam, Netherlands, pp 73−4, 2014..
Mikre W, Mekonnen N. Assessment on the quality and influence of tropical storage conditions on amoxicillin formulations marketed in Ethiopia. East Cent Afr J Pharm Sci 2011; 14(1):23–32.
Mukherjee R, Sen K, Fontana L, Mao C, Chaudhuri B. Quantification of moisture-induced cohesion in pharmaceutical mixtures. J Pharm Sci, 2019; 108(1):223–33. CrossRef
Nwokike J, Clark A, Nguyen PP. Medicines quality assurance to fight antimicrobial resistance. Bull World Health Organ, 2018; 96(2):135–7. CrossRef
Ofner CM III, Schnaare RL, Schwartz GB. Reconstitutable oral suspension. In: Lieberman HA, Rieger MM, Banker GS (eds.). Pharmaceutical dosage forms: disperse systems, Marcel Dekker, New York, NY, Vol. 2, pp 243–59, 1989.
Pharmaceuticals Export Promotion Council. Peru pharmaceutical data and market report (Online), 2017. Available via http://www.iphex-india.com/bsm/uploads/PERU.pdf (Accessed 21 January 2019).
Pires P, Ortega B, Wu E, Walter M. Evaluation of the quality and stability of amoxicillin oral suspension. J Appl Pharm Sci, 2014; 4(7):38–40. CrossRef
Thambavita D, Galappatthy P, Mannapperuma U, Jayakody L, Cristofoletti R, Abrahamsson B, Groot DW, Langguth, P, Mehta M, Parr A, Polli JE, Shah VP, Dressman J. Biowaiver monograph for immediate-release solid oral dosage forms: amoxicillin trihydrate. J Pharm Sci, 2017; 106(10):2930–45. CrossRef
USP-41. United States Pharmacopeia and National Formulary USP 41–NF 36. United States Pharmacopeial Convention Inc., Rockville, MD, 2018.
Zeng XM, MacRitchie HB, Marriott C, Martin GP. Humidity-induced changes of the aerodynamic properties of dry powder aerosol formulations containing different carriers. Int J Pharm, 2007; 333(1–2):45–55. CrossRef