INTRODUCTION
The human hair is a whole system with distinct physicochemical features, in which the hair shaft has three major structural components of the cuticle, the cortex, and the medulla [1]. Chemically, hair fiber is composed of several chemical components, including keratin, lipids, water, colors, and trace elements [2]. Keratin is the most significant component of hair, making up to >90% of dry hair weight [3], and consists of 22 amino acids, with cysteine having the largest amount (up to 15.5%) [4]. Keratin peptide chains have numerous cysteine residues internally cross-linked via disulfide bonds, allowing keratin molecules to connect securely and form a thick structure that can withstand the impacts of external physicochemical factors [5]. Nevertheless, extreme environmental conditions and the process of hair styling/dyeing, with heat or chemical treatments, can severely damage the outer cuticle layer. This damage can cause the keratin fibers to disconnect, resulting in split ends and leaving the hair dry, frizzy, brittle, and uneven at the ends [6]. These damaged hairs are permanent; thus, no specific current treatment is available for hair split-ends, besides general hair cosmetics such as conditioners and serums. However, these leave-on products are short actions, thus, they are commonly advised to be used twice or thrice daily, which discomfort the users. Additionally, most hair care cosmetics for treating split-ends contain silicones (i.e., dimethicone, cyclomethicone, and cyclopentasiloxane), quaternium compounds (i.e., quaternium-18 and quaternium-80), and polyquaternium compounds (i.e., polyquaternium-7 and polyquaternium-10). These compounds, although possessing effectiveness, demonstrate various limitations of (1) difficulty in removal, making the hair heavy and greasy, (2) blocking moisture absorption, leading to hair dryness and scalp issue, (3) non-biodegradable, posing environmental risks, and (4) potential allergic/sensitivity reactions due to the positively charged moieties (i.e., (poly) quaternium compounds), resulting in scalp irritation or dermatitis [7,8]. A potential approach for overcoming these issues is the utilization of nature-based nanoparticles. The natural biodegradable materials could reduce the irritation/allergic and environmental issues, whereas the nanoparticles could strongly adhere to the hair surfaces due to their nano-sizes, form a complete coating on the hair due to the interactions (i.e., van der Waals forces) between their compositions and the hair components (i.e., keratin), consequently prolonging the re-binding efficacy and preventing the moisture blockage [9]. Therefore, this work aimed to develop novel leave-on natural nanoparticulate products with long duration of action to treat hair split-ends.
For that reason, biological materials (i.e., fibroin, chitosan, alginate, and pectin) with biocompatibility and biodegradability properties are increasingly being employed in the manufacture of cosmetics, as well as other biomedical fields [7,10–17]. Among numerous biomaterials, fibroin, the primary protein in the core of the silk fibers (accounting for ~75% of the silk mass), is a potential one [18]. Fibroin has been extensively studied in medicine, cosmetic, and pharmaceutical areas [19–21]. Specifically for hair treatments, fibroin’s anti-parallel β-sheet and amphiphilic structure make it flexible, elastic, hard, and resistant to decomposition agents [19,22]. Thus, fibroin could be applied to the hair and protect it from physicochemical factors. Moreover, fibroin is a protein that possesses a similar structure to keratin, namely the motifs arginine-glycine-aspartic acid that facilitates intermolecular interactions [23,24]. Therefore, it is hypothesized that fibroin and keratin could strongly interact with each other, consequently enhancing the re-binding and restoration of the hair fibers. To further improve the binding capacity and the duration of action, fibroin has been formulated in nanoparticle form (FNP), without/with the functionalization of a polymer.
To this end, 4 typical cosmeceutical polymers have been selected to make hybrid fibroin/polymer nanoparticles, including poly(ethylenimine) (PEI), poly(vinyl pyrrolidone) K30 (PVP K30), poly(vinyl alcohol) (PVA), and poly(ethylene glycol) 400 (PEG 400) (Fig. 1). All four polymers are all safe for human uses, with limited environmental impacts, and have been long utilized in numerous pharmaceutical/cosmeceutical products and applications. Specifically, PEI is a positively charged polyamine that has previously been utilized to complex and transport DNA into a variety of cell lines and tissues, both in vitro and in vivo [25], due to its low cytotoxicity, cost-effectiveness, simplicity of manufacture, and adaptability [26]. PVP K30, a water-soluble polymer composed of the monomer N-vinylpyrrolidone, is commonly used in the cosmetics industry to formulate hair conditioners, hairsprays, shampoos, and other hair care products, because of its safety, binding/adhesive, and moisture-prevention properties [27,28]. PVA is a non-toxic, biocompatible, and biodegradable polymer with a wide range of cosmetics applications due to its ability to create a moisture-locking layer, preventing dehydration while also providing moisture to the skin [29,30]. Similarly, PEG 400, with its non-toxic and biodegradable properties, is a viable polymer in a variety of cosmetic products in gel, suspension, or emulsion platforms [31]. Overall, these materials, including fibroin, PEI, PVP K30, PVA, and PEG 400, are highly biodegradable, aligning with eco-friendly practices, thus reducing environmental impacts.
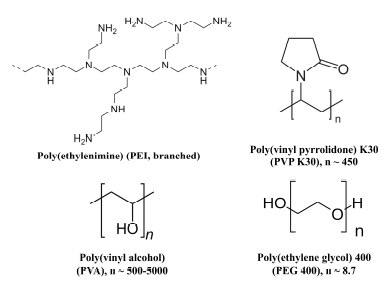 | Figure 1. Chemical structures of the polymers utilized in the study. [Click here to view] |
Ultimately, this work developed the polymer/silk fibroin hybrid nanoparticles as a potential treatment for hair split-ends, employing the compatibility of fibroin and hair keratin, as well as the adhesive properties of the polymers (PEI, PVP K30, PVA, and PEG 400). The particles were formulated using simple mixing and desolvating processes, characterized in terms of size, zeta potential, morphology, chemical interaction, and skin irritation. Then, the particles’ ability to re-bind and restore the hair split-ends was assessed using the damaged hair model. This study aligns with the United Nations Sustainable Development Goals, particularly SDG 12 (Responsible Consumption and Production), by promoting biodegradable and sustainable cosmetic formulations.
MATERIALS AND METHODS
Materials
Raw silk cocoons were acquired from Nam Dinh, Vietnam. Xilong, China, provided chemicals such as sodium carbonate (Na2CO3), calcium chloride (CaCl2), ethanol, and calcium nitrate tetrahydrate. Sigma-Aldrich, Singapore, supplied PEI, PVP K30, PVA, PEG 400, and dialysis membranes. The split-ends damaged hair fibers (length 5–7 cm, observable split-ends) were gathered at Traky Hair Salon, Can Tho, Vietnam. Before experiments, the obtained hair fibers were cleansed with decyl glucoside (5% w/v) non-ionic shampoo thrice, rinsed with distilled water, and dried naturally.
Extraction of fibroin from silk cocoons
Fibroin was extracted from the silk cocoons following the standard protocol [22] (Fig. 2). Briefly, raw cocoons were heated at 100°C with Na2CO3 (0.5% w/v) for 60 minutes to produce sericin-free silk fibers, which were then washed thrice with distilled water. The sericin-free fibers were then dissolved in a salt mixture of CaCl2: H2O: Ca(NO3)2: EtOH (weight ratio of 30:45:5:20) at 90°C for 2 minutes, dialyzed against distilled water for 72 hours to remove the salt ions, centrifuged at 6,000 rpm, 4°C, to remove residues, and the obtained fibroin solution was freeze-dried at −55°C, 10−4 Torr to generate fibroin powder for further experiments.
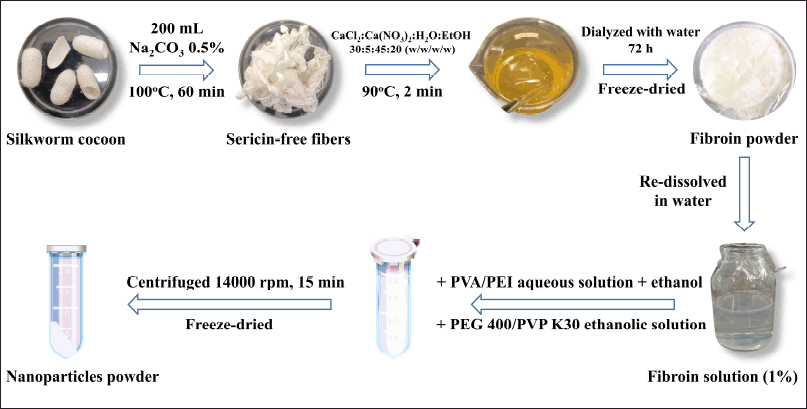 | Figure 2. Fibroin extraction and nanoparticles preparation process. [Click here to view] |
Preparations of nanoparticles
In this study, utilizing the simple desolvation method [32] (Fig. 2), five kinds of fibroin-based nanoparticles were formulated, namely the FNP (without polymer functionalization), FNP/PEI (Fibroin and PEI hybrid nanoparticles), FNP/PVP (Fibroin and PVP K30 hybrid nanoparticles), FNP/PVA (Fibroin and PVA hybrid nanoparticles), and FNP/PEG (Fibroin and PEG 400 hybrid nanoparticles). For this, fibroin powder was dissolved in distilled water to get fibroin solution (1% w/v), PEI was dissolved in distilled water at room temperature with pH adjusted to 7, PVA was dissolved in distilled water at 60°C for 1 hour, PEG 400 and PVP K30 were dissolved absolute ethanol. The nanoparticles were one-pot prepared by homogeneously mixing 1 ml of fibroin solution (without/with PEI/PVA at a final polymer concentration of 0.5%, 1%, or 2%) with 2 ml of ethanol (without/with PVP/PEG 400 at a polymer concentration of 0.5%, 1%, or 2%) for 20 minutes. The spontaneously formed nanoparticles were obtained by centrifugation (14,000 rpm, 15 minutes), rinsed thrice with distilled water, and freeze-dried at −55°C and 10−4 Torr.
Characterizations of nanoparticles
The nanoparticles were physicochemically characterized regarding the particle size, zeta potential, morphology, and chemical interaction.
For the particle size and zeta potential, the dynamic light scattering (DLS) method was employed with the Zetasizer Advance (Malvern, USA). DLS analyzes particle size distribution based on light scattering caused by Brownian motion. When a laser beam is directed at the suspension, the light scatters off the particles, and the light intensity fluctuates over time due to the movement of particles, which were analyzed to calculate the particle size based on the Stokes–Einstein equation. The freeze-dried particles were re-dispersed in distilled water until a count rate of ~500 kcps and the measurements were conducted following the machine instructions. The data were determined in triplicate and presented as mean ± SD.
The particle morphology was assessed using a scanning electron microscope (SEM, JCM 7000, JOEL, Japan). The freeze-dried particles were re-dispersed in water and subjected to the SEM stub, air-dried, and gold-coated. The particles were then observed following the SEM instructions in a nitrogenic environment.
The chemical interactions between fibroin and polymers were assessed by Fourier-transform infrared spectroscopy (FT-IR) and X-ray diffraction (XRD). For FT-IR, the pure polymers, the freeze-dried fibroin powder, and the nanoparticles were mixed with KBr and pressed into thin pellets. The pellets were then analyzed using a Jasco 4600 spectrophotometer (Jasco, Japan), with a wavenumber range of 4,000−500 cm−1, a resolution of 4.0 cm−1, in a desiccated air purge. The empty KBr pellet was used to estimate background signals, which were then eliminated from the sample measurements. For XRD, the samples were subjected onto a quartz stub and analyzed from 15° to 30° (2 theta) using a D2 Phaser machine (Bruker, USA) with Cu Ka radiation.
Irritation test
The skin irritation/compatibility level of the nanoparticles was tested using the standard hen’s egg test on the chorioallantoic membrane (HET-CAM) model [33]. In particular, 8-day-old fertilized hen eggs were kept at 37°C and 65% humidity for 24 hours, followed by chorioallantoic membrane exposure by removing the eggshell. The eggs were separated into seven groups, one group for the negative control (NaCl 0.9% w/v), one group for the positive control (sodium dodecyl sulfate 1% w/v), and five groups for the nanoparticles (FNP, FNP/PEI, FNP/PVP, FNP/PVA, and FNP/PEG re-dispersed in NaCl 0.9%). After chorioallantoic membrane (CAM) exposure, 200 μl of the samples were subjected directly to the CAM surface and monitored for vascular alterations, including coagulation, lysis, and bleeding, at 30-, 120-, and 300-second (i.e., 0.5, 2, and 5 minutes) intervals. The sample irritation scores were determined and the irritability level was assessed.
In vitro test on hair split-ends
To test the ability of the particles to re-bind/restore the split-ends, 63 split-end hair fibers (5–7 cm length, observable split-ends, obtained from a single donor, cleansed with non-ionic shampoo and water, dried at room temperature), were randomly selected. Each hair fiber was then mounted onto a glass slide using sticky tape (enclose the hair fibers at one end between two sticky tapes). Each hair fiber was treated with 20 μl of the sample (nine fibers per sample, nine replicates), including distilled water, FNP, FNP/PEI, FNP/PVP, FNP/PVA, FNP/PEG, and a commercial product (containing cyclomethicone and dimethicone as main active ingredients). The samples (FNP and FNP/polymer hybrids) were dispersed in distilled water and applied to the hair fiber, whereas the commercial product was applied directly to the hair fiber. The 20-μl dose is approximately 3%–5% of the dry hair weight, which is recommended for most hair cosmetics. The sample was then gently smoothed from top to bottom, simulating the hair massage process as the consumers would when applying hair cosmetics in real life. All samples were imaged before, during, and after treatments using a camera-linked inverted microscope (Axio Observer, Zeiss, Germany). The hairs were observed and photographed every 15 min for the first 3 hours, then every 6 hours for the next 3 days [9]. The required time for the hair fibers to be re-split was determined.
Data replication and analysis
All experiments, both the qualitative and quantitative analyses, were conducted at least three times and the results were presented as mean ± SD for the quantitative data. For the qualitative data, the best representative images/figures were demonstrated.
RESULTS AND DISCUSSIONS
Existing leave-on hair care products for split-ends hair treatments, such as silicones and quaternium-based conditioners, are limited by their short duration of action, inadequate efficacy, and potential skin irritation, emphasizing the urgency for alternative formulations. Thus, we developed the FNP and fibroin/polymer hybrid nanoparticles with the hypothesis that the systems could effectively restore split-ends and prolong the action for a couple of days. The study results could address a significant need in the cosmetic industry for safer, more effective, and longer-lasting hair care solutions for hair split-ends. The study’s importance lies in its potential to revolutionize hair care by providing a safer, sustainable, and highly effective product, setting a new standard for non-silicone-based treatments.
Preparations and characterizations of nanoparticles
The FNP and FNP/polymer were produced using the simple one-pot desolvation method, which our group previously developed [22,34,35]. To that end, the fibroin concentration was fixed at 1% (w/v) (the optimal concentration from the previous studies) and the polymer concentrations were varied at 0.5%, 1%, and 2% (w/v) (Table 1).
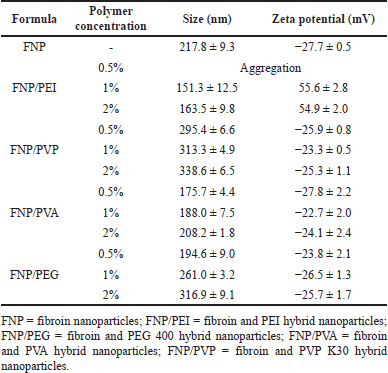 | Table 1. Particle size and zeta potential of FNP formulas at different polymer concentrations (n = 3). [Click here to view] |
First, all formula sizes ranged from 151.3 to 338.6 nm, suggesting good adhesive properties on the hair surface [36]. The particle size of the non-functionalized FNP was 217.8 ± 9.3 nm, in agreement with our previous studies [22,37]. Interestingly, when being functionalized with polymers to form hybrid nanoparticles, the particle size is altered depending on the polymer types. For instance, the incorporation of non-ionic polymers of PVA, PEG 400, and PVP K30 slightly increased the particle size. This could be due to the fact that PVA, PEG 400, and PVP K30 interacted with fibroin molecules via hydrogen bonding and van der Waals forces, thus accumulating the fibroin molecules and increasing the particle size. The PVP K30 formed the biggest particles, which was because PVP K30 possessed stronger fibroin-polymer interactions than other polymers, with the additional π-π interactions of the pyrrolidone rings of PVP K30 and benzene rings of fibroin (present in the aromatic amino acid residues of tyrosine, phenylalanine, and tryptophan), as well as the high molecular weight of ~40,000 Da and large structure of this polymer. It is also worth noticing that at a small concentration of 0.5%, the non-ionic polymers could act as a stabilizer that prevents the aggregations of fibroin molecules due to their steric hindrance effects, resulting in smaller size [22,38,39]. On the other hand, the functionalization of the positively charged PEI decreased the particle size, which was due to the strong ionic interactions between the positively charged PEI and the negatively charged fibroin molecules, which tightened the particles [22]. Another issue to note is that the PEI at 0.5% caused particle aggregation, probably due to an inadequate polymer amount that could not completely cover the whole particle surface. In this case, a phenomenon called bridging flocculation occurs, as the polymer chains adsorb onto the surface of multiple nanoparticles, creating physical connections between them and causing the particles to come close together and form aggregates [40].
Second, regarding the particle zeta potential, most formulas possessed the inherent negative surface charge of fibroin molecules, at approximately −25 mV, with the exception of FNP/PEI (Table 1). Fibroin structure contains numerous carboxyl groups (-COOH) from the residue amino acids (i.e., aspartic acid), making it being negatively charged at pH 7.0. When being combined with non-ionic polymers such as PVA, PEG 400, and PVP K30, the particle surface potential did not significantly alter. On the other hand, the FNP/PEI showed a positive charge, indicating the successful incorporation of the positive PEI protonated amine groups in the hybrid system [22]. The typical human hair isoelectric point is in the range of 2.45–3.17 range, attributed to the carboxyl groups of glutamine/aspartic acid and sulfonic acid groups in the keratin structure [41]. Thus, at a physiological pH of 7.0 and in most leave-on product pH of 5–6, the hair possesses a negative charge. Therefore, the positive charge FNP/PEI could strongly adhere to the hair structure via ionic interactions and could enhance and prolong the re-binding/restoration of split-ends. The zeta potential is influenced by polymer type (i.e., PEI has an inherent positive charge at pH 7.0, while others are neutral at this pH), pH level, and ionic strength, which determine the electrostatic interactions of nanoparticles. Further research could focus on these issues when developing nanoparticles for hair treatment.
Conclusively, the formula with a polymer concentration of 1% was selected as representative for further experiments.
In terms of the chemical interactions, the FT-IR spectrum of fibroin powder exhibited the distinctive amide group peaks at 1,645 cm−1 (amide I, C=O vibrations) and 1,557 cm−1 (amide II, N-H vibrations), which correspond to the intrinsic silk-I structure (i.e., random coils and α-helices) (Fig. 3) [22,37,42]. In the FNP spectrum, these two characterized peaks were shifted to smaller wavenumbers, 1,620 cm−1 for amide I and 1,515 cm−1 for amide II, suggesting the successful transformation of silk-I to silk-II anti-parallel β-sheet structure [22]. Interestingly, when formulated as the fibroin/polymer hybrid nanoparticles, these two peaks shifted back to the silk-I positions. This phenomenon indicated that the polymers could interfere with the formation of FNP. The transformation of fibroin molecules to FNP is an auto-assembly process, where the water-soluble random coils and α-helical structures of fibroin (i.e., silk I) gradually change to water-insoluble anti-parallel β-sheet structures of FNP (i.e., silk II), upon contacting with an organic solvent such as ethanol [19,34]. This alteration is mainly based on the formation of intermolecular bonding between basic amino acids of fibroin (i.e., alanine, glycine, and serine). Thus, the presence of polymers could reduce these bonding amounts by creating fibroin-polymer hydrogen bonding, consequently making the particles have more silk-I content. For instance, PVP K30 could form hydrogen bonding (=O of PVP K30 and -H of fibroin, -O/-N of fibroin and -H of PVP K30), van der Waals forces, and π-π interactions with the fibroin molecules, consequently interfere the fibroin intermolecular bonding [42,43]. Unexpectedly, the FNP/PEI did not change much to the silk-I structure but remained in the silk-II one, which could be due to the strong ionic interactions between the positively charged PEI and the negatively charged fibroin [22]. These interactions overcome the fibroin-PEI hydrogen bonding, thus, maintaining the silk-II structure. The FNP/polymer particles with more silk-I are beneficial for the leave-on hair care products since the fibroin random coils and α-helices are more flexible, water absorbable, and elastic than the rigid water-insoluble silk-II structure [19]. Thus, the particles could be more compatible with the hair structure. It is worth noting that the polymer signals did not appear in the FNP/polymer spectra, which might be due to the low amounts of polymers incorporated in the hybrid systems.
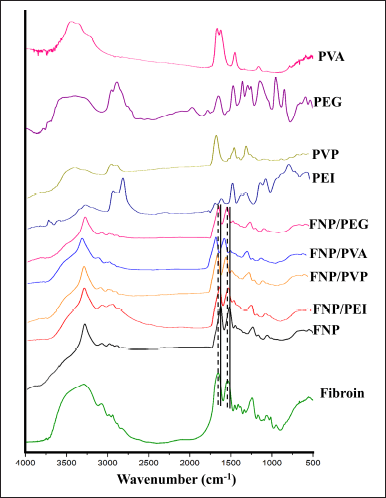 | Figure 3. Fourier-transform infrared spectroscopy spectra of fibroin, fibroin nanoparticles, fibroin and PVA hybrid nanoparticles, fibroin and PEG 400 hybrid nanoparticles, fibroin and PVP K30 hybrid nanoparticles, fibroin and PEG 400 hybrid nanoparticles, and the respective polymer. The dashed line (- - -) and the solid line (––) indicate the fibroin silk-I and silk-II characterized peaks, respectively. [Click here to view] |
The XRD data (Fig. 4) also suggest the same phenomenon as described in the FT-IR spectra. The natural fibroin sample shows a broad peak at 2θ of ~20°, which is typical of the β-sheet crystalline structure in silk fibroin [22]. The broadness and moderate intensity suggest semi-crystalline behavior. Expectedly, when being transformed into FNP, the nanoparticles exhibit a similar broad peak at 20°, but with higher intensity compared to the native fibroin, indicating the silk-II formation. Interestingly, when formulated as the fibroin/polymer hybrid nanoparticles, the peak intensity reduced for FNP/PVA, FNP/PEG, and FNP/PVP, suggesting the reduction in silk-II content, in agreement with the FT-IR data. This phenomenon, again, indicated that the polymers interfere with the formation of FNP by forming additional intermolecular bonding between fibroin amino acids, which making the particles have more silk-I content. Notably, the FNP/PEG demonstrated the lowest crystallinity, nearly similar to that of raw fibroin. This is possibly due to the small molecular weight and structure of PEG 400, which highly interferes with the fibroin structure, consequently decreasing the amount of hydrogen bonding between fibroin molecules and reducing the silk-II content.
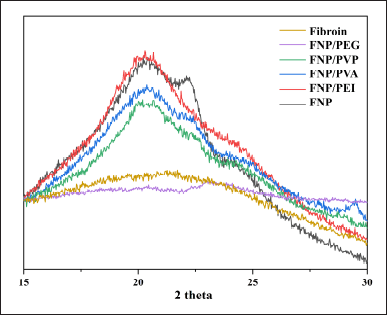 | Figure 4. X-ray diffraction graphs of fibroin, fibroin nanoparticles, fibroin and PVA hybrid nanoparticles, fibroin and PEG 400 hybrid nanoparticles, fibroin and PVP K30 hybrid nanoparticles, and fibroin and PEG 400 hybrid nanoparticles. [Click here to view] |
Regarding the particle morphology, all formulas had spherical shapes with relatively uniform size, smooth surface, and particle aggregations (Fig. 5). The SEM images reveal particle shape and surface morphology, aiding in understanding their interactions with hair fibers. As previously discussed, these polymers, on the one hand, could stabilize the particles and reduce the particle sizes by the steric hindrance effects, on the other hand, could accumulate the particles (i.e., bridging flocculation effect) by forming additional fibroin-polymer interactions [40,42]. In this case, the aggregated particles could be favorable for the hair leave-on product. As the main purpose of hair care leave-on products is to maintain their efficacy for the longest possible duration on the hair, these products usually employ highly viscous platforms such as gels and creams with lots of excipients (i.e., thickener, emulsifier, and stabilizers), thus toxifying and uncomforting the products [7]. Therefore, the nanoparticles/polymers network systems, in their aqueous dispersion form, could help increase the retention time compared to the individual particles, and limiting the uses of cosmeceutical excipients (discussed in the next sections).
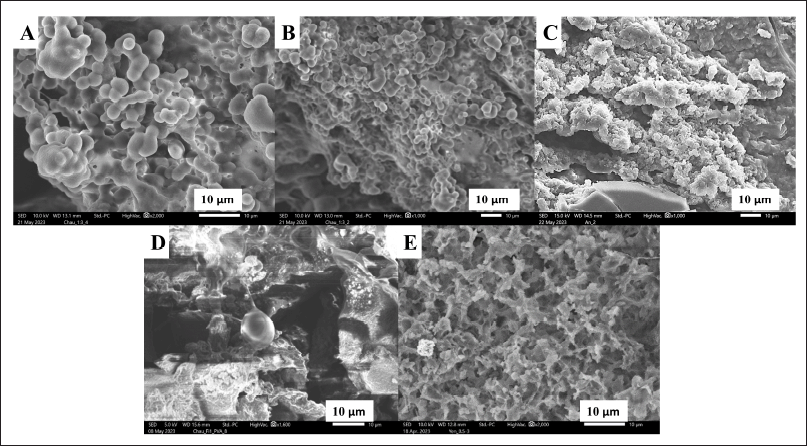 | Figure 5. Scanning electron microscope micrographs of (A) fibroin nanoparticles, (B) fibroin and PVP K30 hybrid nanoparticles, (C) fibroin and PEI hybrid nanoparticles, (D) fibroin and PVA hybrid nanoparticles, and (E) fibroin and PEG 400 hybrid nanoparticles, at polymer initial concentration of 1% (w/v). [Click here to view] |
Irritation test
Skin irritation tests are generally utilized to evaluate the safety of cosmetics formulations. As such, for a leave-on hair product that is applied directly on the user skin, it is crucial to evaluate its irritation and skin compatibility. One of the most common skin irritation tests is the HET-CAM assay, which utilizes a fertilized hen’s egg, where the CAM is highly vascularized and sensitive to irritants. The HET-CAM test is recognized as a useful alternative to in vivo methods by various regulatory bodies and is included in international guidelines for the safety assessment of cosmetics and chemicals [44].
The irritation levels were assessed as (1) none (< 1), (2) slight (< 5), (3) moderate (< 9), and (4) severe (9–21), following the scores described in Table 2. Accordingly, all formulas demonstrated no significant level of irritation on the CAM, with irritation scores of 0 (Fig. 6). The positive control showed significant irritation with blood coagulation at 120 and 300 seconds (score of 15) and the negative control exhibited no irritation (score of 0), indicating the method’s dependability. Interestingly, although the positively charged PEI, a cationic polymer, and the PVP K30, PVA, and PEG 400, non-ionic surfactants, have been proven to cause potential skin irritation [45], the FNP/polymer hybrid systems did not show any irritation. This could be due to the low amount of these polymers presented in the nanoparticles. In summary, all formulas were skin compatible and could be used as hair care leave-on products.
 | Table 2. Irritation scores of the hen’s egg test on chorioallantoic membrane test. [Click here to view] |
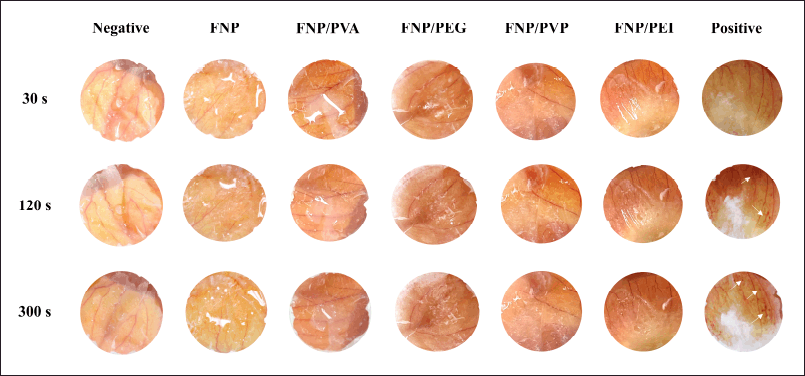 | Figure 6. Photographs of hen’s egg test on chorioallantoic membrane test results of the positive control (irritation score of 15, white arrows indicate blood coagulation), the negative control (irritation score of 0), and fibroin nanoparticles, fibroin and PVA hybrid nanoparticles, fibroin and PEG 400 hybrid nanoparticles, fibroin and PVP K30 hybrid nanoparticles, and fibroin and PEI hybrid nanoparticles (irritation score of 0). [Click here to view] |
In vitro test on hair split-ends
Due to external factors of chemical treatments (i.e., dyeing), mechanical styling, and environmental deterioration, hair could be significantly damaged, making hair fibers more brittle and prone to developing longitudinal split-ends. For the leave-on product for hair treatments, consumer commonly use them for as long as 2 days (48 hours), before washing their hair intentionally or unintentionally (i.e., by bathing). Hence, in this study, the effects of nanoparticles, together with the commercial product, were investigated for a maximum of 72 hours. Moreover, we conducted the split-end restoration test under normal ambient conditions, the condition that most consumers are in when using the products. Ultimately, these experiments closely mimicked the real situation where the users use the products.
Accordingly, to evaluate the efficacy and duration of action of the fibroin/polymer hybrid nanoparticles for the restoration of hair split-ends, the hair samples were subjected to one drop of the particle aqueous dispersion. The distilled water and the commercial product, containing cyclomethicone and dimethicone as the main active ingredients, were used as references (Fig. 7 and Table 3). Obviously, the distilled water could re-bind the split-ends for only 7.0 ± 3.2 minutes, before the hair being split again. For the commercial product, the hair re-split after 1.5 ± 0.3 hours post-treatment, indicating its short binding time. Nevertheless, as a hair leave-on product, this duration is inadequate for maintaining the hair appearance for a suitable time, preferably 24 hours. Thus, novel products are necessary.
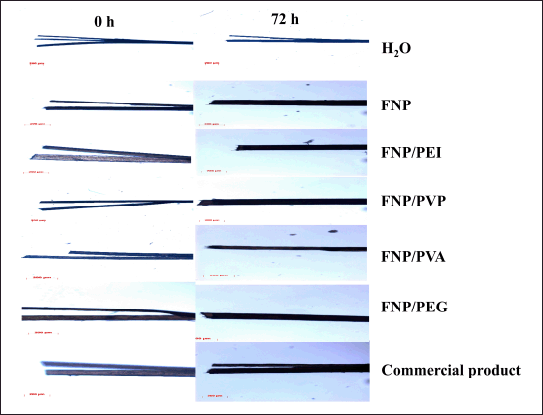 | Figure 7. The hair micrographs (scale bar 200 μm) at 0 hour (the initial time of product application) and at 72 hours post-treatments with water, fibroin nanoparticles, fibroin, and PEI hybrid nanoparticles, fibroin and PVP K30 hybrid nanoparticles, fibroin and PVA hybrid nanoparticles, fibroin and PEG 400 hybrid nanoparticles, and commercial product. [Click here to view] |
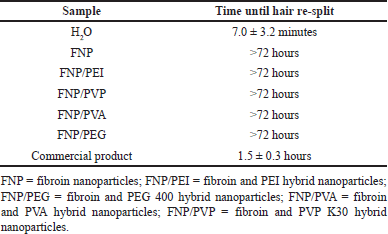 | Table 3. Time until the split-ends hair re-split after the treatments (n = 9). [Click here to view] |
Interestingly, all the formulas, FNP, FNP/PVP, FNP/PVA, FNP/PEI, and FNP/PEG, could re-bind the split-ends for more than 72 hours, with no observable signs of re-split, indicating the superiority of both the negatively charged and positively charged fibroin-based nanoparticles, compared to the commercial product. Moreover, these particles were applied on the hair fibers in the aqueous liquid form (i.e., particles dispersed in water), with no support from other cosmeceutical excipients. The efficacy of a leave-on product for hair split-ends generally depends on its adhesion ability and its capacity to form a complete coating on the hair [9]. The adhesion ability could be increased by decreasing the applied particle sizes and promoting more interactions between their compositions and the hair components (i.e., keratin). The coating-forming ability could be facilitated through van der Waals forces between nanoparticles. As discussed in previous sections, the fibroin-based particles possessed favorable properties for these two processes. First, the particles’ nano-sizes of 151.3–338.6 nm provide a large interfacial surface area, making them mainly stay and adhere on the hair surfaces [36]. Second, the particle adjustable zeta potentials (i.e., positive charged FNP/PEI) could form ionic interactions with the negatively charged hair keratin, thus enhancing the adhesion ability. Third, the particle-dominant silk-I hydrophilic structures could absorb more water and are more compatible with the hair, which yielded better hair adhesion than the lipophilic structures [46]. Fourth, after being applied to the hair and water eventually evaporated, the particles could form a matrix pattern (as observed in the SEM micrographs, Fig. 5), thus enhancing the van der Waals forces between particles, therefore accelerating the coating-forming process and prolonging the product duration of action. Fifth, the non-irritation property makes the particles comfortable for the users. Finally, the unique interactions between fibroin and hair keratin result in the strong hair adhesion, discussed in the next section. Last but not least, the nanoparticles primarily adhere to the hair surface due to their size and interaction properties, rather than penetrating the hair shaft. Thus, these nanoparticles focus on restoring the external structure of the hair without significant alteration to internal structures.
Notably, the data suggest no significant differences, in terms of hair split-end treatments, between FNP and FNP/polymer hybrid systems. Nevertheless, the inclusion of polymers in the hybrid systems could offer several benefits for further investigations, especially when a drug/pharmaceutical compound is incorporated into the systems [22,47–49]. For instance, these particles enhanced drug loading and encapsulation efficiency of hydrophobic drugs, which may be beneficial for drugs with poor aqueous solubility. Additionally, these polymers can provide a diffusion barrier, leading to extended drug release profiles compared to native FNP, which may be advantageous for sustained therapeutic effects. Moreover, cationic PEI can aid in surface modification (i.e., alter the particle charge to positive), potentially enabling functionalization for targeted delivery.
Propose interactions between fibroin and hair keratin
As previously discussed, besides the benefits of nanoparticles on the hair split-ends, the interactions between the fibroin molecule itself and the hair keratin also play an important role in prolonging the product duration of action. These interactions could be explored through various mechanisms, including molecular interactions and structural compatibility (Fig. 8). The main interactions are (1) hydrophobic interactions between 12 hydrophobic domains of fibroin [22] and the hydrophobic cysteine-rich regions of keratin, (2) π-π interactions between the aromatic amino acid residues (i.e., tyrosine, phenylalanine, and tryptophan) of the two proteins, (3) π-cation interactions between the negatively charged aromatic rings and the positively charged amino groups in the two proteins, (4) van der Waals forces, (5) ionic bonding (electrostatic interactions) between the local positively charged moiety at the C-terminal of fibroin and the negatively charged keratin, and (6) hydrogen bonding between the hydrogen bond donors (i.e., OH in hydroxyl groups, O in carbonyl groups, N in amine groups) and acceptors (i.e., H in amide in peptide backbones) of the two proteins. Moreover, the structural similarity and compatibility of fibroin and keratin make it easier for these interactions to occur [50]. For instance, the fibroin β-sheet formation could interact favorably with the β-sheet regions in keratin, leading to potential alignment and stacking interactions. Both proteins have amorphous regions that may intermix, leading to enhanced flexibility and blending at a molecular level.
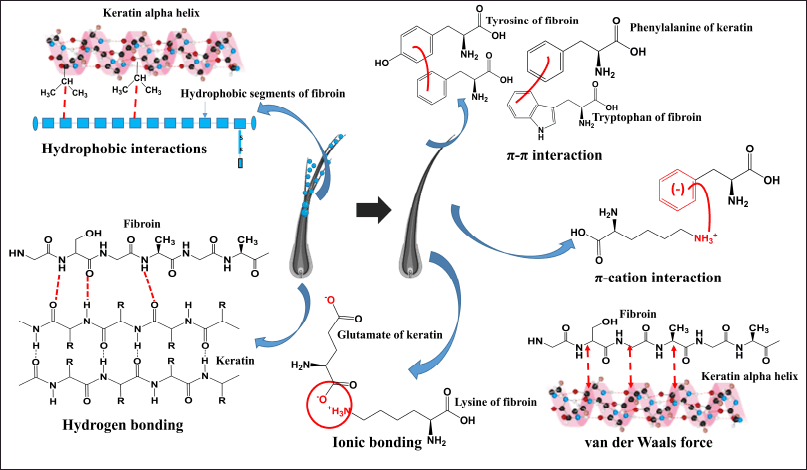 | Figure 8. Possible chemical interactions between fibroin and hair keratin. [Click here to view] |
Study limitations
This study has some limitations that could be focused in future works. First, the particle thermal stability, determined using thermogravimetric analysis and/or differential scanning calorimetry, was not employed, it is recommended for future studies to assess nanoparticle thermal stability. Second, environmental factors such as humidity and temperature might influence the durability of the nanoparticle treatment. Further research might investigate the product effects at different humidity and temperature conditions. Third, considerations on the particles long-term safety, particularly regarding prolonged use of the products, should be employed in future studies. Fourth, the general characteristics of the products should be focused, including moisture retention and reduction, hair softness, and shine.
CONCLUSION
In summary, the silk fibroin and fibroin/polymer hybrid nanoparticles were successfully developed, demonstrating remarkable potential as a next-generation treatment for hair split-ends. These formulations exhibited optimal characteristics for a leave-on hair care product, including nano-sizes ranging from 151.3 to 338.6 nm, tunable zeta potentials, dominant silk-I structures, matrix-patterned particles, and excellent skin compatibility. Notably, unlike conventional commercial products that provide only short-term repair lasting 1.5 hours, these innovative nanoparticles maintained effective hair fiber re-binding for over 72 hours without the need for additional cosmeceutical excipients. This breakthrough highlights the transformative potential of FNP and FNP/polymer systems as natural, long-lasting, and safer alternatives for hair split-end repair, paving the way for further development of advanced hair care products. Future studies could focus on the optimization of polymers and concentrations to improve product stability, functionality, and user experience; as well as the consumer usages and feedbacks to enhance product acceptance and marketability.
LIST OF ABBREVIATIONS
DLS, dynamic light scattering; FNP, dibroin nanoparticles; FNP/PEI: fibroin and PEI hybrid nanoparticles; FNP/PEG, fibroin and PEG 400 hybrid nanoparticles; FNP/PVA, fibroin and PVA hybrid nanoparticles; FNP/PVP, fibroin and PVP K30 hybrid nanoparticles; PEI, poly(ethylenimine); PEG 400, poly(ethylene glycol) 400; PVA, poly(vinyl alcohol); PVP K30, poly(vinyl pyrrolidone) K30; SEM, scanning electron microscopy.
ACKNOWLEDGMENTS
The authors sincerely thank Can Tho University, Can Tho University of Medicine and Pharmacy, and Naresuan University for their supportive helps.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Hashimoto K. The structure of human hair. Clin Dermatol. 1988;6:7–21. doi: CrossRef
2. Lee H, Noh K, Lee SC, Kwon IK, Han DW, Lee IS, et al. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng Regen Med. 2014;11:255–65. doi: CrossRef
3. Cassoni AC, Freixo R, Pintado AIE, Amorim M, Pereira CD, Madureira AR, et al. Novel eco-friendly method to extract keratin from hair. ACS Sustain Chem Eng. 2018;6:12268–74. doi: CrossRef
4. Shavandi A, Jafari H, Zago E, Hobbi P, Nie L, De Laet N. A sustainable solvent based on lactic acid andl-cysteine for the regeneration of keratin from waste wool. Green Chem. 2021;23:1171–4. doi: CrossRef
5. Sinclair RD. Healthy hair: what is it? J Investig Dermatol Symp Proc. 2007;12:2–5. doi: CrossRef
6. Thieulin C, Vargiolu R, Zahouani H. Effects of cosmetic treatments on the morphology, biotribology and sensorial properties of a single human hair fiber. Wear 2019;426–427:186–94. doi: CrossRef
7. Gavazzoni Dias MFR. Hair cosmetics: an overview. Int J Trichology. 2015;7:2–15. doi: CrossRef
8. Bains P, Kaur S. Silicone in dermatology: an update. J Cutan Aesthet Surg. 2023;16:14–20. doi: CrossRef
9. Prasertpol T, Tiyaboonchai W. Development and characterization of cream containing nanostructured lipid carriers for hair split-end repairing. Int J Appl Pharm. 2019;11:354–8. doi: CrossRef
10. Bergfeld WF, Andersen FA. Natural products for hair care and treatment. In: Whitting DA, Blume-Peytavi U, Tosti A, Trüeb RM, editors. Hair growth and disorders. Berlin, Heidelberg: Springer; 2008. pp 515–24. doi: CrossRef
11. Huynh DTM, Le M-NT, Tran VD, Pham DT. Antibacterial hydrogel containing Piper Betel L. extract for acne treatment, an ex vivo investigation. Pharm Sci Asia 2022;49:372–80. doi: CrossRef
12. Murugan E, Akshata CR, Ilangovan R, Mohan M. Evaluation of quaternization effect on chitosan-HAP composite for bone tissue engineering application. Colloids Surf B Biointerfaces 2022;218:112767. doi: CrossRef
13. Luong HVT, Diep MT, Nguyen NY, Pham DT, Cao LNH, Ha TMP. Alginate–functionalized Fe3O4 nanoparticles as a drug delivery system for targeted controlled release. J Drug Deliv Sci Technol. 2024;93:105465. doi: CrossRef
14. Akshata CR, Murugan E, Harichandran G. Alginate templated synthesis, characterization and in vitro osteogenic evaluation of strontium-substituted hydroxyapatite. Int J Biol Macromol. 2023;252:126478. doi: CrossRef
15. Nguyen NY, Luong HVT, Pham DT, Tran TBQ, Dang HG. Chitosan-functionalized Fe3O4@SiO2 nanoparticles as a potential drug delivery system. Chem Pap. 2022;76:4561–70. doi: CrossRef
16. Akshata CR, Harichandran G, Murugan E. Effect of pectin on the crystallization of strontium substituted HA for bone reconstruction application. Colloids Surf B Biointerfaces 2023;226:113312. doi: CrossRef
17. Cao-Luu N-H, Luong H-V-T, Nguyen T-V, Nguyen-Thi B-T, Pham DT, Pham N-C, et al. Curcumin-loaded co-axial electrospun chitosan/polyvinyl alcohol/calcium chloride nanofibrous membranes for wound healing enhancement. ChemistrySelect 2024;9:e202402644. doi: CrossRef
18. Nguyen NY, Nguyen TNP, Huyen NN, Tran VD, Quyen TTB, Luong HVT, et al. Onto the differences in formulating micro-/nanoparticulate drug delivery system from Thai silk and Vietnamese silk: a critical comparison. Heliyon 2023;9:e16966. doi: CrossRef
19. Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv. 2020;27:431–48. doi: CrossRef
20. Pham DT, Tiyaboonchai W. Fibroin-coated poly(ethylenimine)-docusate nanoparticles as a novel drug delivery system. Curr Sci. 2021;121:775–80. doi: CrossRef
21. Pham DT, Huynh QC, Lieu R, Nguyen VB, Tran VD, Thuy BTP. Controlled-release Wedelia trilobata L. flower extract loaded fibroin microparticles as potential anti-aging preparations for cosmetic trade commercialization. Clin Cosmet Investig Dermatol. 2023;16:1109–21. doi: CrossRef
22. Pham DT, Nuttawut S, Tiyaboonchai W. Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: physicochemical properties, crystallinity and structure. J Mater Sci. 2018;53:14087–103.
23. Xu H, Cai S, Xu L, Yang Y. Water-stable three-dimensional ultrafine fibrous scaffolds from keratin for cartilage tissue engineering. Langmuir 2014;30:8461–70. doi: CrossRef
24. Kang Z, Wang Y, Xu J, Song G, Ding M, Zhao H, et al. An RGD-containing peptide derived from wild silkworm silk fibroin promotes cell adhesion and spreading. Polymers (Basel) 2018;10:1193. doi: CrossRef
25. Venkiteswaran S, Thomas T, Thomas TJ. Selectivity of polyethyleneimines on DNA nanoparticle preparation and gene transport. ChemistrySelect 2016;1:1144–50. doi: CrossRef
26. Patnaik S, Gupta KC. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert Opin Drug Deliv. 2013;10:215–28. doi: CrossRef
27. Burnett CL, Heldreth B. PVP (polyvinylpyrrolidone). Int J Toxicol. 2017;36:50S–1S. doi: https://doi.org/10.1177/1091581817716649.
28. Koczkur KM, Mourdikoudis S, Polavarapu L. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2016;1:1–24.
29. Nair B. Final report on the safety assessment of Polyvinyl alcohol. Int J Toxicol. 1989;17:67–92.
30. Demerlis CC, Schoneker DR. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem Toxicol. 2003;41:319–26.
31. Ahn MY, Hwang JS, Yun EY, Kim MJ, Park KK. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol Res. 2015;31:173–80. doi: CrossRef
32. Chomchalao P, Nimtrakul P, Pham DT, Tiyaboonchai W. Development of amphotericin B-loaded fibroin nanoparticles: a novel approach for topical ocular application. J Mater Sci. 2020;55:5268–79. doi: CrossRef
33. Batista-Duharte A, Jorge Murillo G, Pérez UM, Tur EN, Portuondo DF, Martínez BT, et al. The hen’s egg test on chorioallantoic membrane: an alternative assay for the assessment of the irritating effect of vaccine adjuvants. Int J Toxicol. 2016;35:627–33. doi: CrossRef
34. Pham DT, Nguyen TL, Nguyen TTL, Nguyen TTP, Ho TK, Nguyen NY. Polyethylenimine-functionalized fibroin nanoparticles as a potential oral delivery system for BCS class-IV drugs, a case study of furosemide. J Mater Sci. 2023;58:9660–74. doi: CrossRef
35. Pham DT, Saelim N, Tiyaboonchai W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf B Biointerfaces 2019;181:705–13. doi: CrossRef
36. Gu Y, Bian Q, Zhou Y, Huang Q, Gao J. Hair follicle-targeting drug delivery strategies for the management of hair follicle-associated disorders. Asian J Pharm Sci. 2022;17:333–52. doi: CrossRef
37. Pham DT, Ha TKQ, Nguyen MQ, Tran VD, Nguyen VB, Quyen TTB. Silk fibroin nanoparticles as a versatile oral delivery system for drugs of different biopharmaceutics classification system (BCS) classes: a comprehensive comparison. J Mater Res.2022:4169–81.
38. Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, et al. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021;13:10748–64. doi: CrossRef
39. Sonntag H, Ehmke B, Miller R, Knapschinski L. Steric stabilization of polyvinyl alcohol adsorbed on silica/water and water/oil interfaces. Adv Colloid Interface Sci. 1982;16:381–90. doi: CrossRef
40. Hogg R. Bridging flocculation by polymers. KONA Powder Part J. 2013;30:3–14. doi: CrossRef
41. Parreira HC. On the isoelectric point of human hair. J Colloid Interface Sci. 1980;75:212–7. doi: CrossRef
42. Shi P, Goh JCH. Release and cellular acceptance of multiple drugs loaded silk fibroin particles. Int J Pharm. 2011;420:282–9. doi: CrossRef
43. Franco P, De Marco I. The use of poly(N-vinyl pyrrolidone) in the delivery of drugs: a review. Polymers (Basel) 2020;12:1114. doi: CrossRef
44. Gilleron L, Coecke S, Sysmans M, Hansen E, Van Oproy S, Marzin D, et al. Evaluation of a modified HET-CAM assay as a screening test for eye irritancy. Toxicol In Vitro. 1996;10:431–46. doi: CrossRef
45. Lémery E, Briançon S, Chevalier Y, Bordes C, Oddos T, Gohier A, et al. Skin toxicity of surfactants: structure/toxicity relationships. Colloids Surf A: Physicochem Eng Asp. 2015;469:166–79. doi: CrossRef
46. Cao Z, Chen X, Yao J, Huang L, Shao Z. The preparation of regenerated silk fibroin microspheres. Soft Matter 2007;3:910–5. doi: CrossRef
47. Di KN, Ha PTM, Nguyen NP, Nguyen NY, Truong TC, Nguyen TTV, et al. Enhanced anti-inflammatory effects of diclofenac delivered orally via polyvinylpyrrolidone K30/silk fibroin nanoparticles in a murine model of carrageenan-induced paw edema. ChemMedChem. 2024:e202400760. doi: CrossRef
48. Thao NTP, Nguyen NY, Co VB, Thanh LHV, Nguyen MQ, Pan-On S, et al. Formulations of poly(vinyl alcohol) functionalized silk fibroin nanoparticles for the oral delivery of zwitterionic ciprofloxacin. PLoS One. 2024;19:e0306140. doi: CrossRef
49. Pham DT, Nguyen DXT, Nguyen NY, Nguyen TTL, Nguyen TQC, Tu AVT, et al. Development of pH-responsive Eudragit S100-functionalized silk fibroin nanoparticles as a prospective drug delivery system. PLoS One. 2024;19:e0303177. doi: CrossRef
50. Bragulla HH, Homberger DG. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J Anat. 2009;214:516–59. doi: CrossRef