INTRODUCTION
Nanoemulsions, stabilized by nanoparticle-sized surfactant molecules, consist of transparent dispersions with oil and water [1,2]. These are kinetically stable systems in which stability is maintained for months [3–6]. Nanoemulsions’ kinetic stability, largely influenced by droplet size, makes them insensitive to gravitational forces and reduces attractive forces between droplets [5–8]. Moreover, the formulation also does not get destabilized by droplet’s flocculation [9].
Surfactants are generally used in the production of nanoemulsions as they lower the interfacial tension between two liquids by getting adsorbed at the interface of oil–water leading to the formation of droplets coated with surfactant; which if further sheared, become interconnected and lead to breaking of droplets into fine ones. As a result of the coating, the movement of oil molecules from the droplet to the bulk aqueous phase is inhibited thereby preventing coalescence or flocculation of the droplets.
Surfactants are commonly small-chain fatty acids or alcohols that are soluble in both water and oil. A molecule’s hydrocarbon portion determines its solubility in oil. In contrast, the polar -COOH and -OH groups have an affinity for water that allows a long chain of nonpolar hydrocarbons to dissolve in it. When these molecules are located at an air/water or oil/water interface, the hydrophilic (water-loving) groups may be trapped in the aqueous phase, while the hydrophobic (water-hating) chains can be released. Surface activity is the adsorption, at an interface, of a monomolecular layer (or monolayer) of oriented surfactant. In other words, surface tension at the interface of air and liquid is the free energy essential for the enlargement of the surface per unit area of the solution. When surfactants are added to a liquid system, the surface tension is reduced owing to the adsorption of these molecules as monolayers. Surface excess concentration (Γ) is a quantity of surfactant adsorption at liquid surfaces. This is the excess total of surfactant per unit area of the surface over the amount that would be present if the surfactant concentration were uniform all the way to the surface. More adsorption of surfactant/co-surfactant mixture (Smix) at the interface leads to a reduction in surface tension.
When surfactant alone fails to sufficiently reduce interfacial tension for nanoemulsion, short-chain co-surfactants are added to achieve near-zero tension. Co-surfactants easily penetrate into the surfactant monolayer and get themselves occupied at empty areas between surfactant molecules resulting in more interfacial fluidity and lowering in bending force of oil–water interface. Therefore, the interfacial film becomes more flexible, and various film curvatures are formed, which are later required for nanoemulsion formation. One more objective of adding co-surfactant in formulation is to reduce the amount of surfactant required. The proper selection of co-surfactants and surfactants and the determination of their minimum concentration in a formulation is important.
The impact of surfactants on reducing interfacial tension is crucial for stabilizing oil/water nanoemulsions. As an example, a hydrophobic surfactant in the oil droplet increases the interfacial tension at the interface, while a hydrophilic one reduces it. In contrast, emulsifiers with more than one molecule at the interface result in a greater reduction of interfacial tension in comparison to surfactants with only one molecule. Throughout the emulsion system, the temperature exaggerated the reduction of interfacial tension, even though the concept of hydrophilic–lipophilic balance (HLB) number focuses on the surfactant molecule itself, not its interactions with water and oil [10].
In one of the previous studies by Mehta et al. [11], water and diesel nanoemulsion have been prepared where a selection of Smix has been done based on surfactant thermodynamic properties such as minimum surface area per molecule (Amin) and surface excess concentration (Γmax). This approach has been used in other published works also where nanoemulsions have been prepared for a pesticide [12], for water-in-diesel fuel nanoemulsion [13].
For nanoemulsions of drugs, the assortment of co-surfactant and surfactant has been based on the miscibility studies, solubility studies, and HLB value and selection of Smix ratio has been done from the pseudoternary phase diagram in almost all the published works [14–17]. Other criteria that can be used for the selection of Smix ratio are based on surfactant thermodynamic properties such as Amin and Γmax [18]. The value of Amin implies the mean area engaged by each adsorbed molecule at the interface [11]. The smallest Amin and the largest Γ value of surfactant indicates that the surfactants are crammed more closely and adsorbed more powerfully at the interface thus increasing the strength of the interfacial film and ensuring that the resultant nanoemulsion formed will exhibit greater physical stability.
The formulation of selegiline nanoemulsion has already been published whereby the criteria for the selection of Smix ratio has been reported on the basis of the construction of a pseudoternary phase diagram [19]. The objective of this paper is to select a ratio of surfactant and co-surfactant for the preparation of nanoemulsion on the basis of Amin value. To the best of our knowledge, the calculation involved in determining the Amin used for selecting Smix ratio has not been reported in detail.
EXPERIMENTAL SECTION
Materials
A gift sample of selegiline was provided by Sun Pharmaceutical Industries Ltd., New Delhi, India, Lauroglycol 90 from Gattefosse, Saint Priest, Cedex, France and Sefsol 218® from Nikko Chemicals, Tokyo, Japan. Tween 80 was procured from Merck, Mumbai, India, and grape seed oil from Falcon, Bengaluru, India. All other solvents and chemicals used during the experiments were of analytical grade.
Screening of Smix on the basis of minimum surface area per molecule (Amin)
In the present study, Tween 80 was used as the surfactant and lauroglycol 90 as the co-surfactant. The chemical structures of Tween 80 and lauroglycol 90 are presented in Figure 1.
Surface tension measurement
Different molar concentrations of Smix were prepared and the surface tensions were calculated at room temperature (20°C ± 2°C) using a stalagmometer that was previously calibrated with distilled water before use. The surfactant solution (Smix) was freshly prepared for measurement. Table 1 explains the preparation of different concentration (mol/l) solutions of tween 80 and lauroglycol 90. For example, to prepare 0.00001 mol/l solution of tween 80 about 0.0123 ml of tween 80 was dissolved in water (q.s. 1l). Table 2 explains the preparation of different concentration (mol/l) solution of Smix. The stalagmometer was filled with the experimental liquid. The numbers of drops were counted as the meniscus moved from the upper mark of the stalagmometer to the lower mark. Similarly, the stalagmometer was filled with reference liquid (i.e., water), and numbers of drops were counted. The surface tension for the different Smix was calculated using equation (1), as explained previously [20]
 | Figure 1. Chemical structure of Tween 80 and Lauroglycol 90. [Click here to view] |
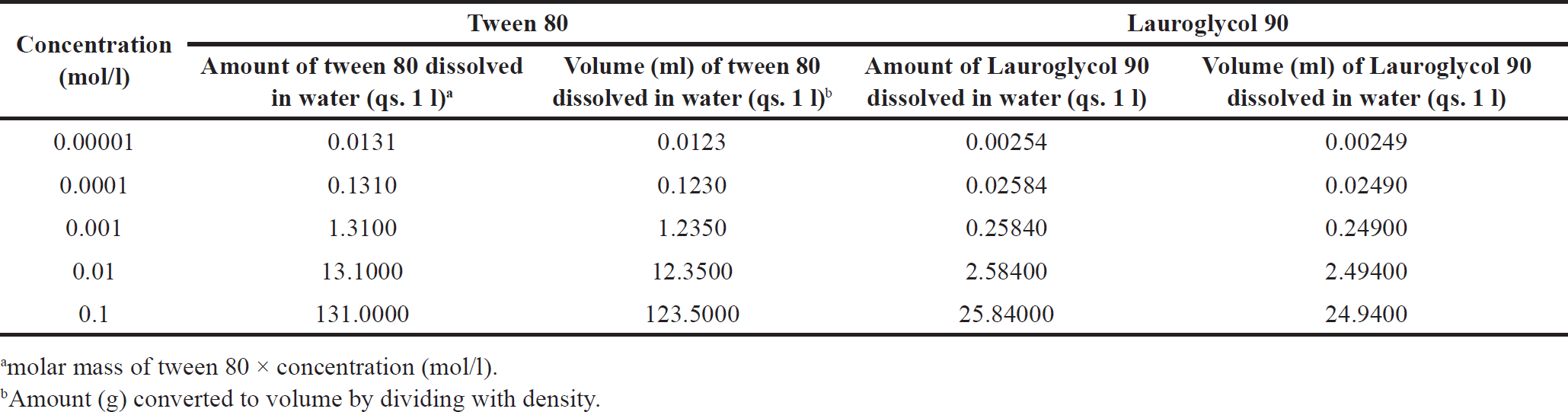 | Table 1. Preparation of different concentration (mol/l) of Tween 80 and Lauroglycol 90 solutions. [Click here to view] |
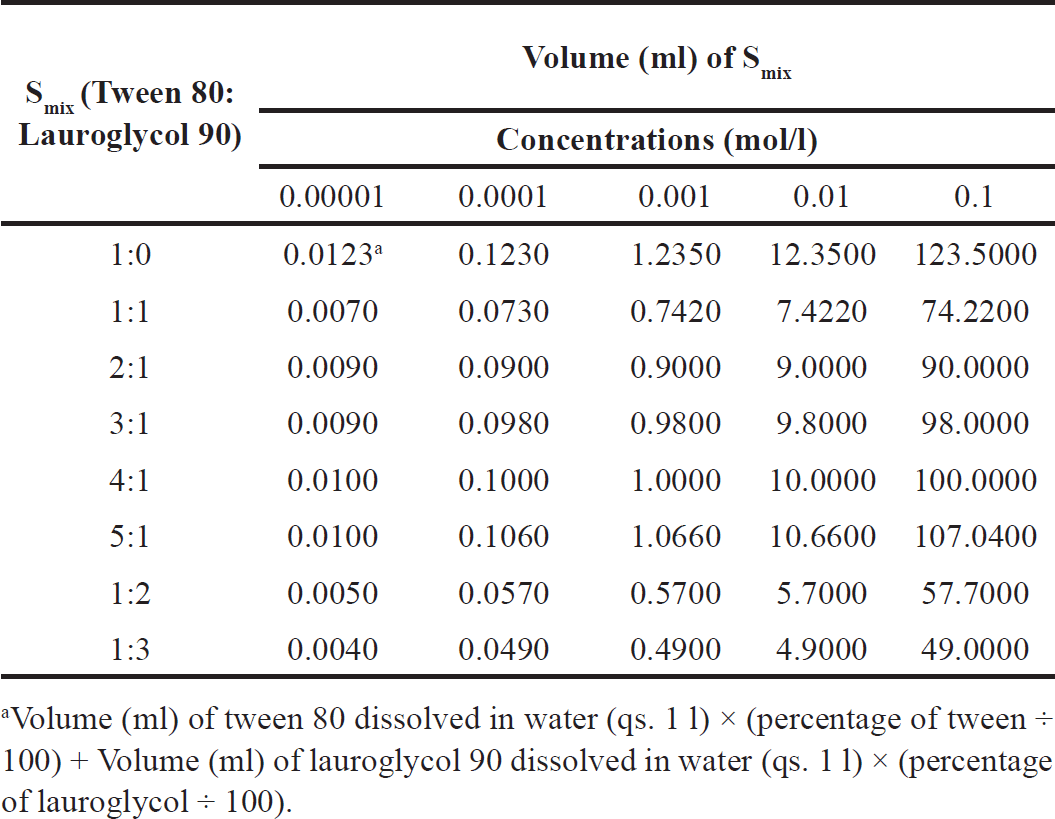 | Table 2. Volume (ml) of Smix dispersed in water (qs. 1 l) to prepare different concentration (mol/l) solutions of Smix. [Click here to view] |
where n1 and n2 are the number of drops produced by the same volume of the two liquids, γ1 and γ2 are the surface tension of test liquid and water (or reference), n1 and n2 are the number of drops of test liquid and water, and d1 and d2 are the density of test liquid and water (i.e., 0.800 g/cm3), respectively. Here, γ2 was 72 dyne/cm at 20°C. The value of d1 was determined using the specific gravity bottle method.
Surface excess concentration (Γmax)
Γmax is a parameter used for determining the maximum adsorption efficiency that can be achieved by surfactants at liquid/liquid or liquid/air interface. In general, higher Γ at the interface means an accretion of larger surfactant molecules may develop binding strength that consequently produces a stable emulsion [21]. Γmax can be determined from equation (2), as explained previously [18,22–24].
where Γmax is the number of molecules adsorbed per unit area of interface (mol/cm2), γ represents the surface tension (units: mNm−1), T is the absolute temperature, R is the
gas constant (8.314 Jmol−1K−1), and is the slope obtained
from the plot between surface tension and log concentration of surfactant which normally shows a linear decrease pattern.
Minimum surface area per molecule (Amin)
Amin is the minimum area per molecule in nm2/molecule at the interface. Lesser the area engaged by surfactant per molecule, the more stable will be the resulting nanoemulsion as the molecules will strongly adsorb themselves at the interface and the direction of the surfactant molecule at the interface will be at right angles to the interface. Alternatively, surfactants occupying a huge surface area per molecule will lead to the adsorption of molecules resulting in parallel packing. The average area occupied by each adsorbed molecule is given by the following equation explained previously [14,17–19]:
where N is Avogadro’s number (6.023 × 1023 molecules/mole).
Nanoemulsion formation
Selegiline (55 mg) was dissolved in the oil phase by using a Vortex Mixer (Nirmal Instrument, India). A specific quantity of Smix was mixed. The resultant solution was mixed with distilled water under constant stirring and then this premix was homogenized using a high-speed homogenizer (Heidolph, Germany) for 15 minutes at 25°C ± 1°C to form a coarse emulsion. Finally, the resulting premix was processed with a high-pressure homogenizer (STANSTED® pressure Cell Homogeniser, Essex CM19 5FN, UK) to form nanoemulsion [19,25,26].
Characterization of nanoemulsion
Nanoemulsion droplet size was evaluated by Zetasizer Ver 6.01 (Malvern Instruments, Ltd, UK). Formulation (1 ml) was taken in a cuvette and mean droplet size and droplet size distribution (PDI) were determined at room temperature (25°C ± 2°C). The percentage transmittance of undiluted formulation was recorded by using a UV–visible double beam spectrophotometer at 630 nm. The refractive index of the formulation was examined at 25°C ± 2°C by using an Abbe’s-type refractometer (Guru Nanak Instruments, New Delhi, India). Zeta potential is a parameter to evaluate the surface charge of dispersed phase droplets; it was measured on the basis of electrophoretic mobility of the dispersed phase droplets by using Zetasizer (Nano-ZS, Malvern Instruments, Worcestershire, UK). Determination of zeta potential was done at a temperature of 25°C ± 2°C.
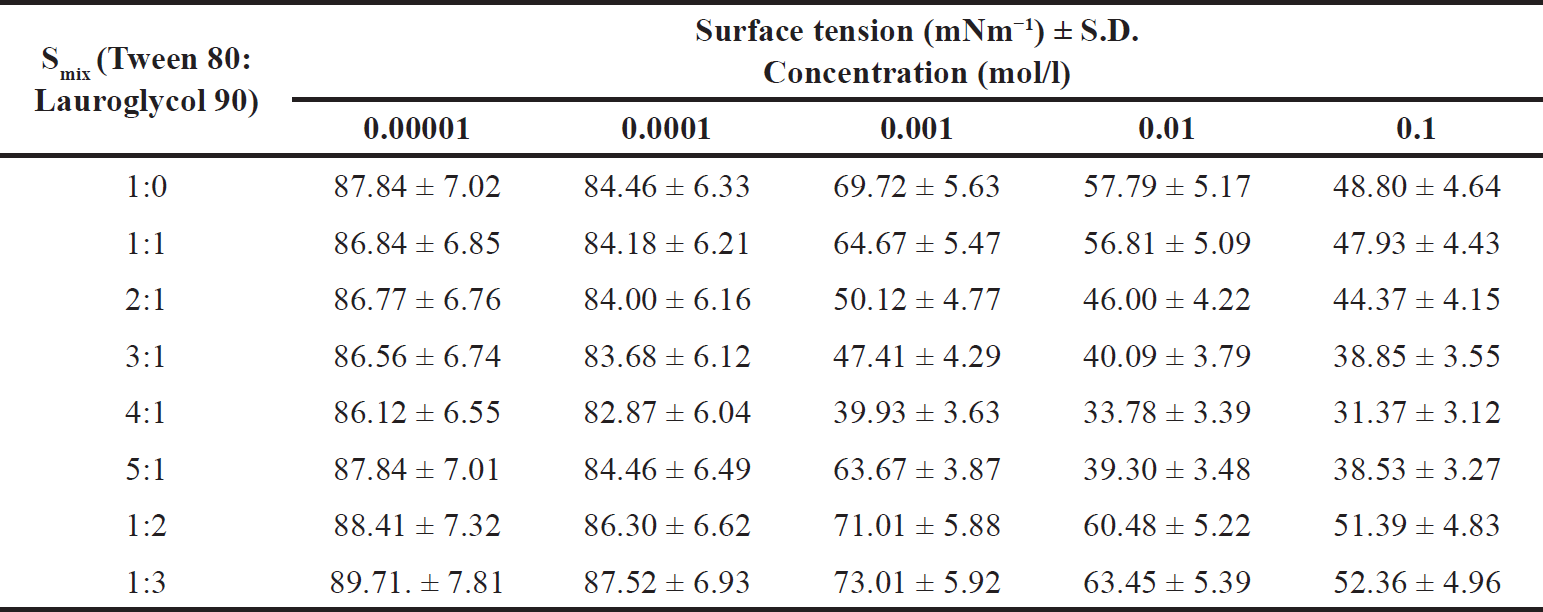 | Table 3. Surface tension values of different Smix. [Click here to view] |
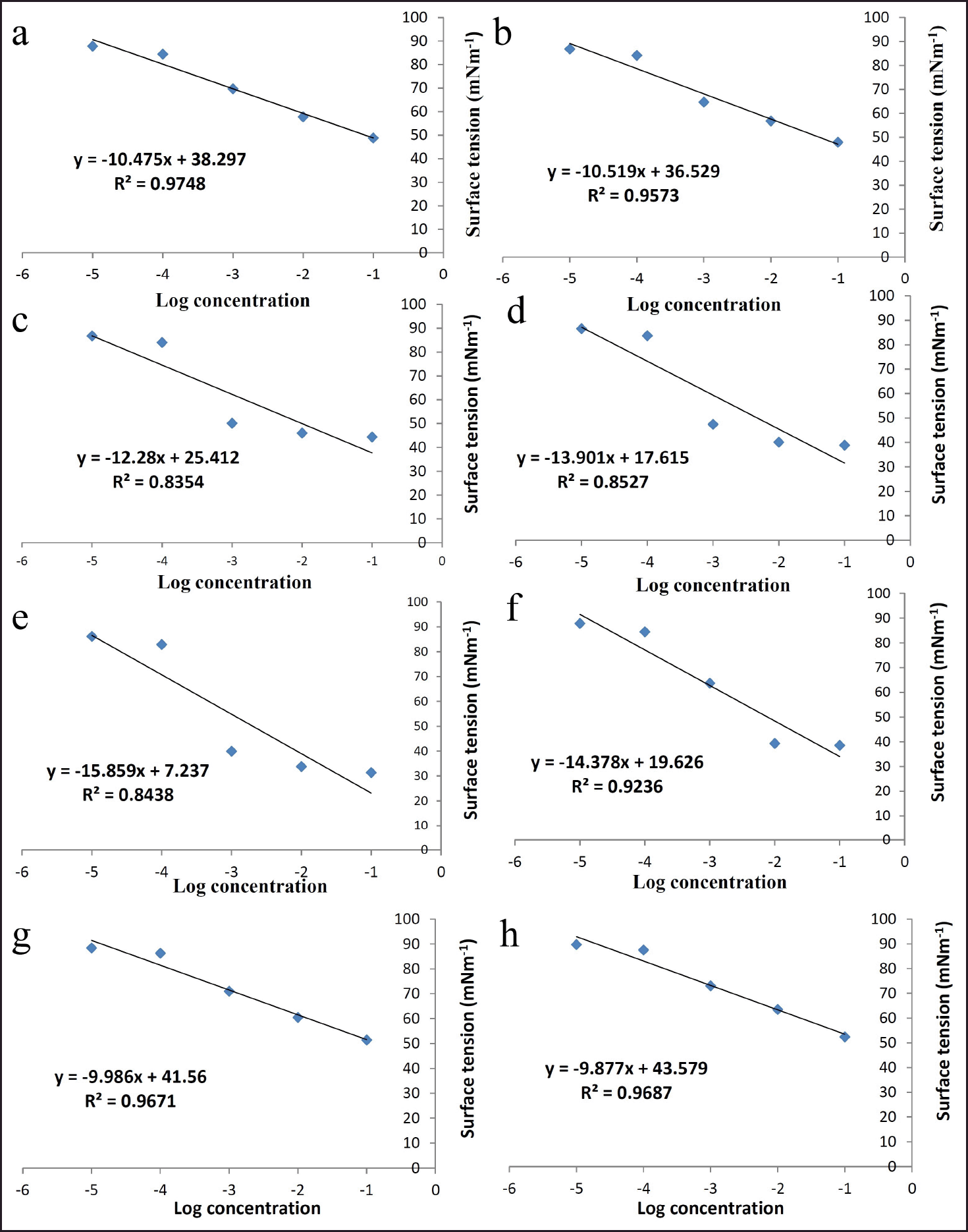 | Figure 2. Plots of surface tension (mNm−1) versus log concentration for different surfactant: co-surfactant ratios (or Smix). (a) 1:0; (b) 1:1; (c) 2:1; (d) 3:1; (e) 4:1; (f) 5:1; (g) 1:2; (h) 1:3. [Click here to view] |
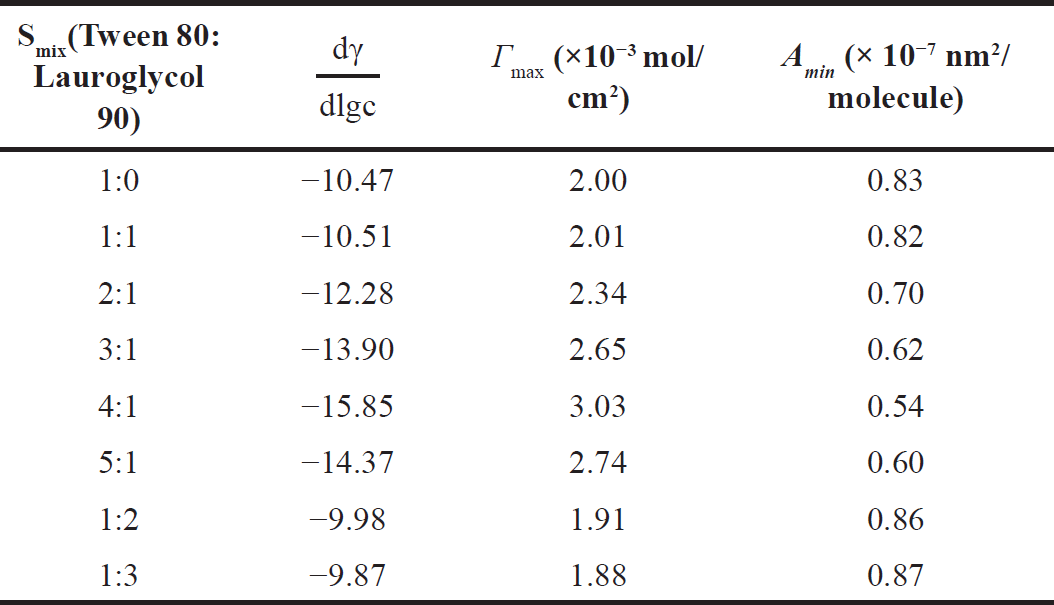 | Table 4. Surface properties of mixed surfactants with different mixing ratios. [Click here to view] |
RESULTS AND DISCUSSION
Several authors have reported that surfactant mixtures result in the formulation of emulsions with the smallest size and greater stability as compared to the emulsions produced using only one surfactant [27,28]. It is possible that this phenomenon is caused due to the fact that the surfactants mixed together can develop a film around dispersed droplets and by strengthening the interfacial film, the droplets are able to maintain their stability [9,29]. It is supposed that the lipophilic and hydrophilic emulsifiers are aligned with each other in a system of this kind providing emulsifier film with an enhanced rigidity and strength by hydrogen bonding [28].
In the present study surface tensions for different Smix were evaluated and are listed in Table 3. A graph of log concentration of Smix versus surface tension (γ) showed that surface tension decreased linearly with surfactant concentration (Fig. 2). The value of Γmax and Amin were determined by using equations (2) and (3), respectively. Table 4 indicates that Smix ratio 4:1 had the highest value of Γ, (3.03 × 10−3 mol/l) and Smix ratio 1:3 had the lowest value (1.88 × 10−3 mol/l). On the other hand, Smix ratio 1:3 had the highest value of Amin (0.87 × 10−7 nm2/molecule) and Smix ratio 4:1 exhibited the least value of Amin (0.54 × 10−7 nm2/molecule), whereas Smix ratios 1:0, 1:1, 2:1, 3:1, 5:1, and 1:2 had middle value between Amin values of Smix ratio 1:3 and 4:1. This may be due to the structure and hydrophobicity of tween 80 and lauroglycol 90. Lauroglycol 90 possesses low molecular weight and a hydrophilic group therefore less number of lauroglycol molecules were required to attain the o/w interface till saturation. Thus, the lowest surface area per surfactant molecule (Amin) was augmented as the concentration of lauroglycol increased. Moreover, a large hydrophobic group was responsible for the fewest moles of lauroglycol being occupied per cm2 to reach surface saturation. There is a large surface area per molecule occupied by these hydrophobic groups; this allows the molecule to be adsorbed and packed parallelly [10,22].
Since both the surfactants, i.e., tween 80 and lauroglycol 90 have different solubility in water, the less water-soluble one (lauroglycol 90) will transfer more hastily between the oil–water interface compared to tween 80, when mixed together, because the surfactant with minor head group adsorbs more at the interface [10]. It can be proposed that the orientation of oil-soluble surfactant at the water-in-oil interface protrudes hydroxyl groups into the aqueous phase, facilitating them to develop hydrogen bonds with the water molecules, thereby decreasing reducing the superfluous link between hydrocarbon chains and water molecules, which consequently, as a result, promote compatibility of both surfactants at the interface [28].
When the ratio of tween 80 and lauroglycol 90 was 4:1, the Smix exhibited a low value of Amin proposing that the oil/water interface was tightly packed thus, at the interface, the surfactant molecules were tilting almost perpendicularly [22]. In this way, the tween 80 and lauroglycol molecules in a 4:1 ratio packed themselves most closely at this ratio. It also suggests that as compared to the other ratios of Smix, they absorbed sturdily at the interface therefore enhancing the potency of the interfacial film. In contrast to 4:1, other mixing ratios had slightly higher values of Amin, and the nanoemulsion stability decreased concomitantly. The nanoemulsion with tween 80: lauroglycol 90 ratio of 4:1 displayed good stability revealing that using a 4:1 mixer ratio, surfactant mixtures produced better synergistic effects and surface activity. The selection of 4:1 ratio among all Smix ratios was justified based on the Amin value required (i.e., the smallest) for the production of a stable o/w nanoemulsion.
It is reported that a low value of Amin corresponds to an interfacial system with low free energy that promotes the forming of microemulsions, as a result, their stability will increase and at the interface, it facilitates molecular exchange. In other words, low Amin means that the Smix molecules removed from the interface to the adjacent bulk phase require lower energy [30].
The droplet size and polydispersity index (PDI) value of selegiline nanoemulsion were found to be 61.43 ± 4.10 nm and 0.203 ± 0.005. This PDI revealed that the formulation had narrow size distribution as well as droplet size uniformity. The confirmation of nanoemulsion stability involves assessing transmittance, a parameter closely linked to droplet size. Therefore, any fluctuation in transmittance indicates alterations in droplet size and distribution. In the case of selegiline nanoemulsion, a transmittance of 98.80% ± 0.04% was observed, indicating its transparency and clarity. Nanoemulsion showed a refractive index of 1.30 ± 0.01, implying the isotropic nature of the formulation. The determined zeta potential for nanoemulsion was found to be −34 mV suggesting the production of a stable formulation.
CONCLUSION
The present work revealed that the optimum Amin value could be used for the screening of Smix. As per the literature search, generally, screening of Smix ratio for preparation of nanoemulsion has been carried out on the basis of a pseudoternary phase diagram which seems to be a time-consuming approach whereas in the present discussed approach the most favorable Smix ratio was identified by calculating thermodynamic properties including Γmax and Amin. This approach is less time-consuming and provides an understanding of the wrapping of surfactant molecules at the oil–water interface so that one can understand the surfactant’s orientation at an interface which in turn provides information regarding the stability of the formulation. The discussed approach is very simple and less time consuming to screen out the surfactant mixture ratio for the production of nanoemulsion.
LIST OF ABBREVIATIONS
Γmax, Surface excess concentration; Amin, Minimum surface area per molecule; Smix, Surfactant mixture.
AUTHOR CONTRIBUTIONS
Concept and design—SK, JA, and SB; Data acquisition—SK, JA, and SB; Data analysis/interpretation—SK, JA, and SB; Drafting manuscript—SK, KW, RP, JA, and SB; Critical revision of manuscript—SK, KW, RP, JA, and SB; Admin, technical or material support—SK, KW, RP, JA, and SB; Supervision—JA, and SB; Final approval—JA, and SB.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bendary MM, Ibrahim D, Mosbah RA, Mosallam F, Hegazy WA, Awad NF, et al. Thymol nanoemulsion: a new therapeutic option for extensively drug resistant foodborne pathogens. Antibiotics [Internet]. 2021 [cited 2024 Feb 14];10:1–13. Available from: https://www.mdpi.com/2079-6382/10/1/25/htm
2. Mosallam FM, Helmy EA, Bendary MM, El-Batal AI. Potency of a novel synthesized Ag-eugenol nanoemulsion for treating some bacterial and fungal pathogens. J Mater Res [Internet]. 2021 [cited 2024 Feb 14];36:1524–37. Available from: https://link.springer.com/article/10.1557/s43578-021-00226-1
3. Anton N, Vandamme TF. The universality of low-energy nano-emulsification. Int J Pharm [Internet]. 2009 [cited 2023 Dec 8];377:142–7. Available from: https://pubmed.ncbi.nlm.nih.gov/19454306/
4. Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res [Internet]. 2011 [cited 2023 Dec 8];28:978–85. Available from: https://pubmed.ncbi.nlm.nih.gov/21057856/
5. Porras M, Solans C, González C, Gutiérrez JM. Properties of water-in-oil (W/O) nano-emulsions prepared by a low-energy emulsification method. Colloids Surf A Physicochem Eng Asp. 2008;324:181–8.
6. Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. Nanoemulsions: formation, structure, and physical properties. J Phys Condens Matter [Internet]. 2006 [cited 2023 Dec 8];18:R635. Available from: https://iopscience.iop.org/article/10.1088/0953-8984/18/41/R01
7. Polychniatou V, Tzia C. Study of formulation and stability of co-surfactant free water-in-olive oil nano- and submicron emulsions with food grade non-ionic surfactants. JAOCS J Am Oil Chem Soc [Internet]. 2014 [cited 2023 Dec 8];91:79–88. Available from: https://onlinelibrary.wiley.com/doi/full/10.1007/s11746-013-2356-3
8. Usón N, Garcia MJ, Solans C. Formation of water-in-oil (W/O) nano-emulsions in a water/mixed non-ionic surfactant/oil systems prepared by a low-energy emulsification method. Colloids Surf A Physicochem Eng Asp. 2004;250:415–21.
9. Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci [Internet]. 2004 [cited 2023 Dec 8];108:303–18. Available from: https://pubmed.ncbi.nlm.nih.gov/15072948/
10. Noor El-Din MR. Study on the stability of water-in-kerosene nano-emulsions and their dynamic surface properties. Colloids Surfaces A Physicochem Eng Asp. 2011;390:189–98.
11. Mehta RN, More U, Malek N, Chakraborty M, Parikh PA. Study of stability and thermodynamic properties of water-in-diesel nanoemulsion fuels with nano-Al additive. Appl Nanosci [Internet]. 2015 [cited 2023 Dec 8];5:891–900. Available from: https://link.springer.com/article/10.1007/s13204-014-0385-3
12. Du Z, Wang C, Tai X, Wang G, Liu X. Optimization and characterization of biocompatible oil-in-water nanoemulsion for pesticide delivery. ACS Sustain Chem Eng. 2016;4:983–91.
13. Noor El-Din MR, El-Hamouly SH, Mohamed HM, Mishrif MR, Ragab AM. Water-in-diesel fuel nanoemulsions: preparation, stability and physical properties. Egypt J Pet. 2013;22:517–30.
14. Mazonde P, Khamanga SMM, Walker RB. Design, optimization, manufacture and characterization of Efavirenz-loaded flaxseed oil nanoemulsions. Pharmaceutics [Internet]. 2020 [cited 2023 Dec 8];12:1–22. Available from: https://www.mdpi.com/1999-4923/12/9/797/htm
15. Gul U, Khan MI, Madni A, Sohail MF, Rehman M, Rasul A, et al. Olive oil and clove oil-based nanoemulsion for topical delivery of terbinafine hydrochloride: in vitro and ex vivo evaluation. Drug Deliv [Internet]. 2022 [cited 2023 Dec 8];29:600–12. Available from: https://pubmed.ncbi.nlm.nih.gov/35174738/
16. Garg H, Mittal S, Ashhar MU, Kumar S, Dang S, Nigam K, et al. Bioavailability enhancement of paroxetine loaded self nanoemulsifying drug delivery system (SNEDDS) to improve behavioural activities for the management of depression. J Clust Sci. 2023;34:223–36.
17. Monika, Sharma S, Shrivastva M, Kumar S, Rabbani SA, Garg A. Novel in-situ nanoemulgel (NEG) of azithromycin with eugenol for the treatment of periodontitis: formulation development and characterization. J Clust Sci. 2022;33:2589–600.
18. Liu Y, Wei F, Wang Y, Zhu G. Studies on the formation of bifenthrin oil-in-water nano-emulsions prepared with mixed surfactants. Colloids Surfaces A Physicochem Eng Asp. 2011;389:90–6.
19. Kumar S, Ali J, Baboota S. Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology [Internet]. 2016 [cited 2023 Dec 8];27:435101. Available from: https://pubmed.ncbi.nlm.nih.gov/27655136/
20. Ohol RM, Vasuki B. Macroscopic mixer for disparate property liquid–liquid mixing in aqueous sanitizer preparation. Chem Pap [Internet]. 2022 [cited 2024 Mar 4];76:701–14. Available from: https://pubmed.ncbi.nlm.nih.gov/34602722/
21. Bangia JK, Singh M, Om H, Behera K, Gulia M. Physicochemical study of nanoemulsions of aqueous cellulose acetate propionate, cellulose acetate butyrate and tween80 with castor, olive and linseed oils from temperature (293.15 to 313.15) K. J Mol Liq [Internet]. 2017 [cited 2023 Dec 8];225:758–66. Available from: https://www.infona.pl//resource/bwmeta1.element.elsevier-72725fa2-9c50-33ae-acf1-176dff0f738b
22. Yang F, Li G, Qi J, Zhang SM, Liu R. Synthesis and surface activity properties of alkylphenol polyoxyethylene nonionic trimeric surfactants. Appl Surf Sci. 2010;257:312–8.
23. Lin LH, Chou YS. Surface activity and emulsification properties of hydrophobically modified dextrins. Colloids Surfaces A Physicochem Eng Asp. 2010;364:55–60.
24. Al-Sabagh AM, Emara MM, Noor El-Din MN, Aly WR. Formation of water-in-diesel oil nano-emulsions using high energy method and studying some of their surface active properties. Egypt J Pet. 2011;20:17–23.
25. Belhaj N, Dupuis F, Arab-Tehrany E, Denis FM, Paris C, Lartaud I, et al. Formulation, characterization and pharmacokinetic studies of coenzyme Q10 PUFA’s nanoemulsions. Eur J Pharm Sci [Internet]. 2012 [cited 2023 Dec 8];47:305–12. Available from: https://pubmed.ncbi.nlm.nih.gov/22732255/
26. Pawar VK, Panchal SB, Singh Y, Meher JG, Sharma K, Singh P, et al. Immunotherapeutic vitamin e nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J Control Release [Internet]. 2014 [cited 2023 Dec 8];196:295–306. Available from: https://pubmed.ncbi.nlm.nih.gov/25459427/
27. Gullapalli RP, Sheth BB. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur J Pharm Biopharm [Internet]. 1999 [cited 2023 Dec 8];48:233–8. Available from: https://pubmed.ncbi.nlm.nih.gov/10612034/
28. Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sci [Internet]. 2009 [cited 2023 Dec 8];330:443–8. Available from: https://pubmed.ncbi.nlm.nih.gov/19038395/
29. Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–10.
30. Pogorzelski S, Watrobska-Swietlikowska D, Sznitowska M. Surface tensometry studies on formulations of surfactants with preservatives as a tool for antimicrobial drug protection characterization. J Biophys Chem [Internet]. 2012 [cited 2023 Dec 8];03:324–33. Available from: http://www.scirp.org/Html/9-7100168_25058.htm