INTRODUCTION
Parkinson’s disease (PD), the second most common neurodegenerative disorder, is predominantly found in people aged 60 and above [1]. PD is characterized by the selective loss of dopaminergic neurons and the presence of Lewy bodies in the substantia nigra. These neurons, in normal individuals, secrete dopamine that acts as a messenger between the substantia nigra and corpus striatum, which are the brain areas that are involved in movement coordination [2]. With the loss of dopaminergic neurons, dopamine level is reduced, leading to the four cardinal signs of PD, namely, bradykinesia, tremor, rigidity, and postural instability [1–3].
The pathogenesis of neurodegeneration remains unclear, but autophagy is known to be the main devastating event that leads to neurodegeneration. In normal physiology, autophagy is essential for cell survival. However, in diseased conditions such as PD, the process is dysregulated, where there is an excess of autophagy, leading to neuronal cell death [4,5]. To initiate autophagy, three conditions are believed to be the trigger, namely, oxidative stress, protein aggregation, and mitochondrial dysfunction [4].
Up until now, there is no cure for PD. However, treatments to relieve the motor symptoms are available (termed as symptomatic treatments). Examples of available symptomatic treatments are levodopa, carbidopa, and monoamine oxidase-B (MAO-B) inhibitors. Levodopa is the most effective prescription to date [1]. However, the limitation of levodopa is that long-term use induces dyskinesia. Therefore, many efforts are being carried out to discover novel treatments to address PD.
Recently, a newly approved drug named safinamide, with multiple modes of action, demonstrated higher efficacy and decreased the severity of PD symptoms in clinical trials, where it is given as an add-on therapy to levodopa [6–10]. Interestingly, few studies have been carried out and they suggested that besides being an option for symptomatic treatment for PD, this drug also confers significant neuroprotection [9,11–14]. However, the mechanism of action underlying the neuroprotective effect remains unclear. Studies show that MAO-B activity leads to oxidative stress and mitochondrial dysfunction [12,15]. As safinamide is a MAO-B inhibitor, it is suggested that safinamide works as a neuroprotective agent by inhibiting the enzyme MAO-B, subsequently reducing oxidative stress, mitochondrial dysfunction, and the autophagy process in neuronal cells [15]. Studies on the mechanism of action of safinamide are limited. With no existing in vitro model for safinamide against autophagy, this study aimed to investigate the neuroprotective effect of safinamide against autophagy in a 6-hydroxydopamine (6OHDA)-induced SH-SY5Y cell model of PD.
MATERIALS AND METHODS
Cell culture
SH-SY5Y cells (American Type Culture Collection, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, USA), with a supplement of 10% fetal bovine serum (FBS) (Gibco ™, South America) and maintained at 37°C with 5% carbon dioxide (CO2) at a humidified atmosphere.
Optimization of 6OHDA concentration in SH-SY5Y cells
To determine the percentage of cell viability of SH-SY5Y cells treated with 6OHDA (Sigma-Aldrich, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed. A seeding density of 4 × 105 cells/ml was seeded on a 96-well plate (Sigma-Aldrich, USA). The plate was then incubated at 37°C with 5% CO2 supply to reach a confluency of at least 70% before treatment with 100 µl of 0–200 µM 6OHDA in each well, which was prepared using a two-fold serial dilution from 200 µM to a concentration of 6.25 µM. A control group was treated with DMEM + 1% FBS in which 1% FBS was used in treatment to prevent the growth of cells. After treatment, the plate was incubated at 37°C with a 5% CO2 supply for 24 hours. Subsequently, 20 µl of MTT (Calbiochem, USA) was added into each well and incubated for 4 hours at 37°C with 5% CO2 supply. All solutions were removed before adding 80 µl of dimethyl sulfate (Sigma-Aldrich, USA) to dissolve the precipitates, and the plate was read at 570 nm and 630 nm using a microplate reader. A graph on the percentage of cell viability was plotted with values obtained.
Intracellular acidic vesicles of 6OHDA-treated SH-SY5Y cells were detected using acridine orange. A seeding density of 4 × 105 cells/ml was seeded on a 12-well plate (SPL Life Sciences, Korea). The plate was incubated at 37°C with a 5% CO2 supply to reach a confluency of at least 70% before treatment with 0–200 µM 6OHDA (prepared as above). The plate was then incubated for 24 hours at 37°C with 5% CO2 supply. All solutions in each well were then removed, and the cells were washed with 400 µl phosphate-buffered saline (PBS) (Bio Basic Inc., Canada). Cells were stained with 2 µg/ml acridine orange (Sigma-Aldrich, USA) and incubated for 10 minutes at 37°C. Subsequently, cells were washed with PBS and analyzed under the inverted fluorescent microscope. Five fields were captured per well. Autophagolysosomes appeared as red fluorescent cytoplasmic vesicles, while the nuclei were stained green. A graph showing the ratio of autophagic cells to the total number of cells was plotted.
Evaluation of cytotoxicity of safinamide in SH-SY5Y cells
The percentage of cytotoxicity of safinamide in SH-SY5Y cells was determined using an MTT assay. Cells were treated with seven different concentrations of safinamide mesylate salt (Sigma-Aldrich, USA), which was obtained from a two-fold serial dilution from 50 µM safinamide to a concentration of 0.78 µM. A control group was treated with DMEM + 1% FBS. MTT assay was performed as above, and a graph of the percentage of cytotoxicity was plotted.
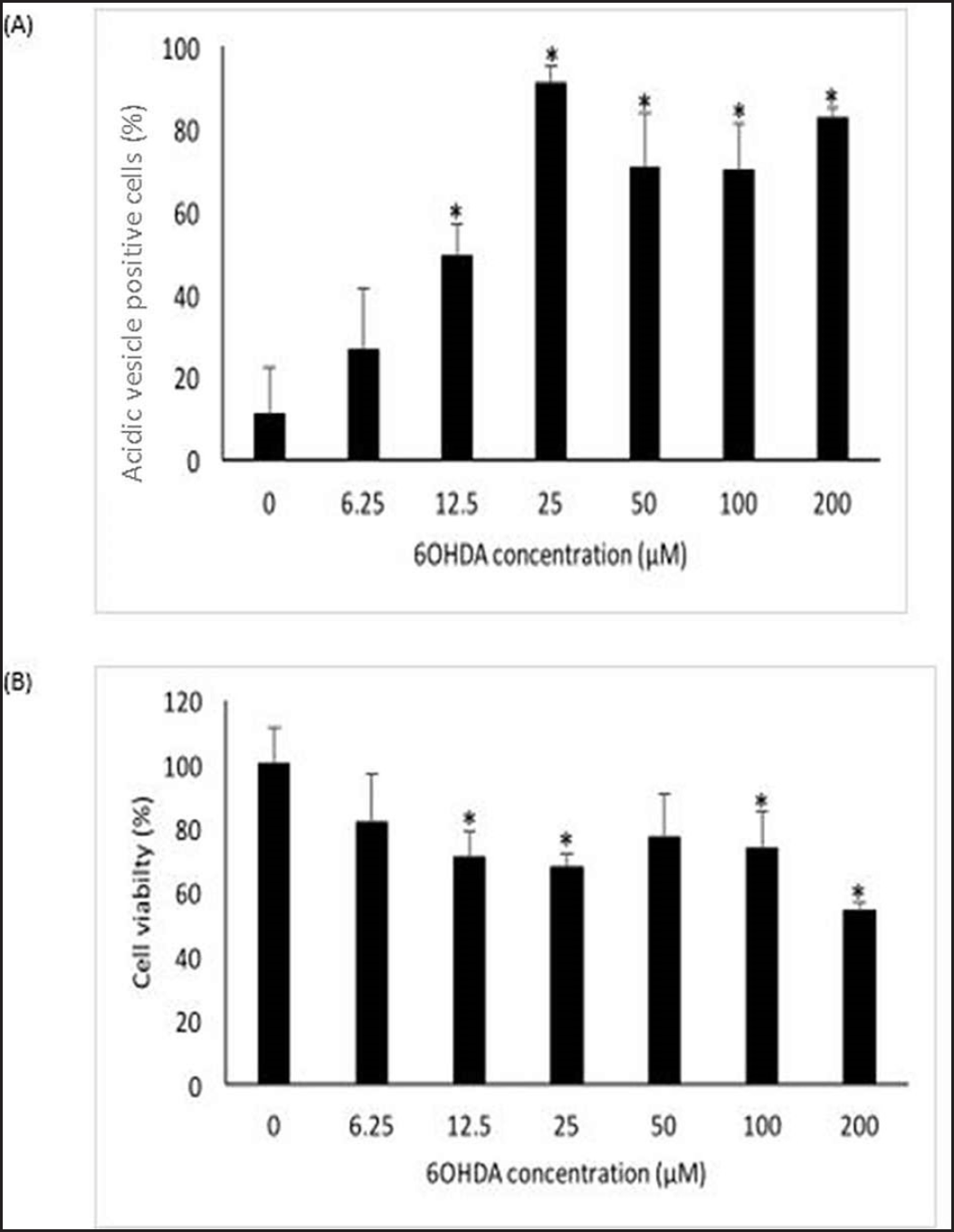 | Figure 1. Effects of 6OHDA on autophagy and cell viability of SH-SY5Y cells. (A) Effects of 6OHDA on autophagy in SH-SY5Y cells. (B) Effects of 6OHDA on cell viability in SH-SY5Y cells. Mean ± standard deviation of data from at least three independent experiments is shown. *p < 0.05. [Click here to view] |
Determination of neuroprotective effects of safinamide in 6OHDA-induced SH-SY5Y cells
To investigate the neuroprotective effects of safinamide in 6OHDA-induced SH-SY5Y cells, cells were treated with 6OHDA concurrently with safinamide for 24 hours, with a total of seven treatment groups: control, 25 µM 6OHDA, 25 µM 6OHDA + 3.125 µM safinamide, 25 µM 6OHDA + 6.25 µM safinamide, 25 µM 6OHDA + 12.5 µM safinamide, 25 µM 6OHDA + 25 µM safinamide, 25 µM 6OHDA + 50 µM safinamide. The percentage of cell viability and intracellular acidic vesicles of SH-SY5Y cells were determined with MTT assay and acridine orange, respectively, as described above.
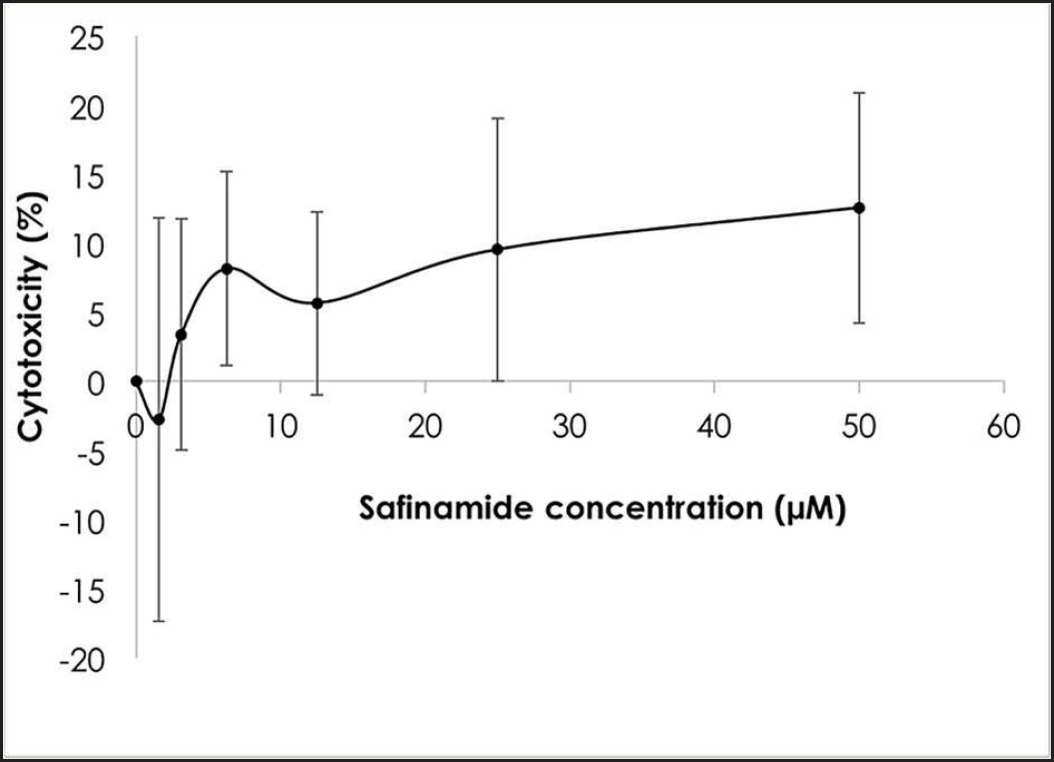 | Figure 2. Cytotoxic effects of safinamide at varying concentrations. Mean ± standard deviation of data from at least three independent experiments is shown. [Click here to view] |
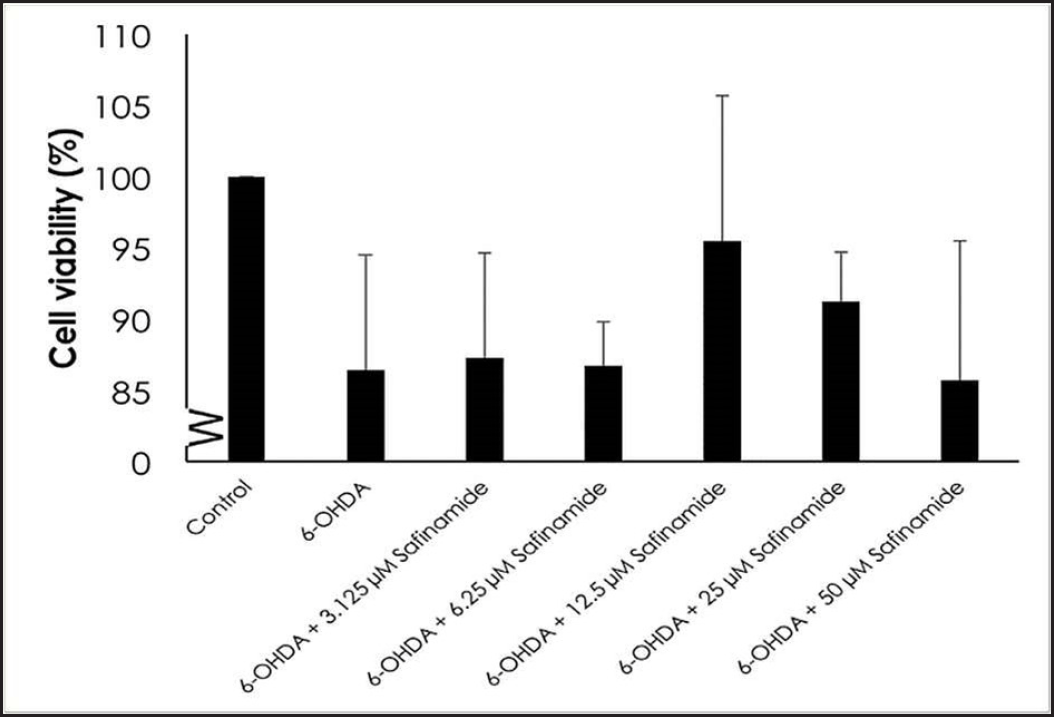 | Figure 3. Effect of safinamide on cell viability in 6OHDA-induced SH-SY5Y cells. Mean ± standard deviation of data from at least three independent experiments is shown. [Click here to view] |
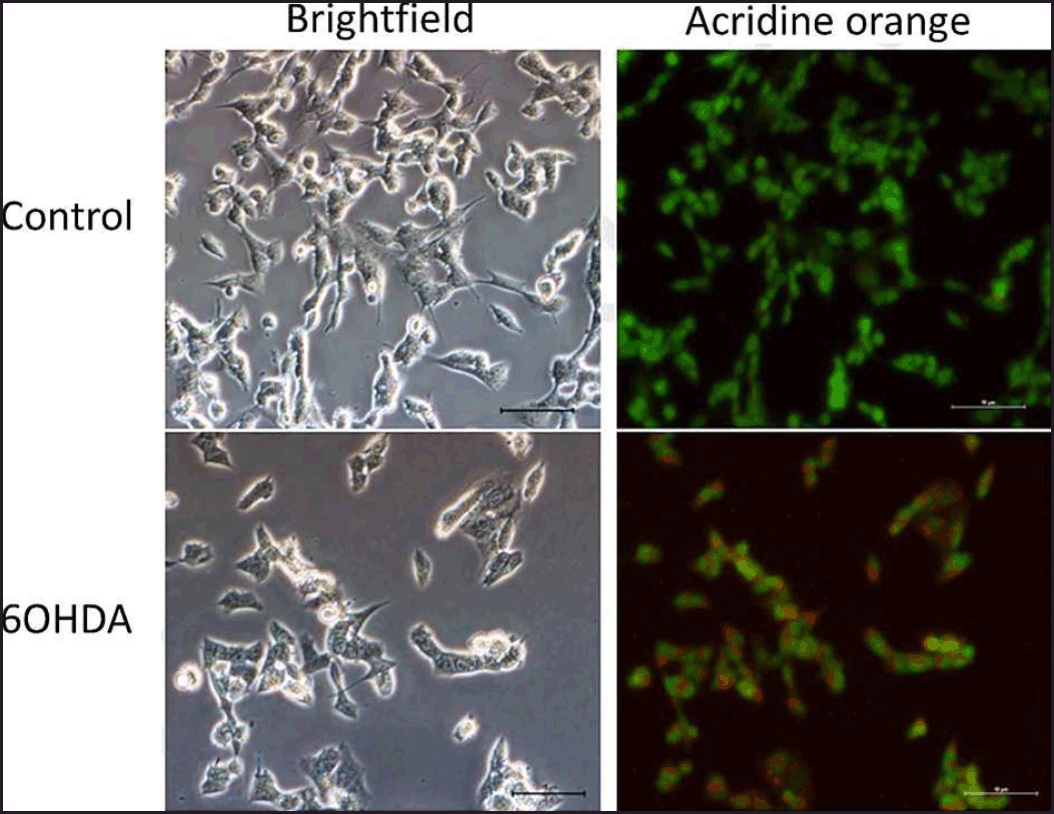 | Figure 4. Effects of 6OHDA on autophagy in SH-SY5Y cells. Micrographs of bright field and acridine orange stained-SH-SY5Y cells at magnification 200× for each treatment group were shown. Nucleus and acidic vesicles are stained green and red, respectively. [Click here to view] |
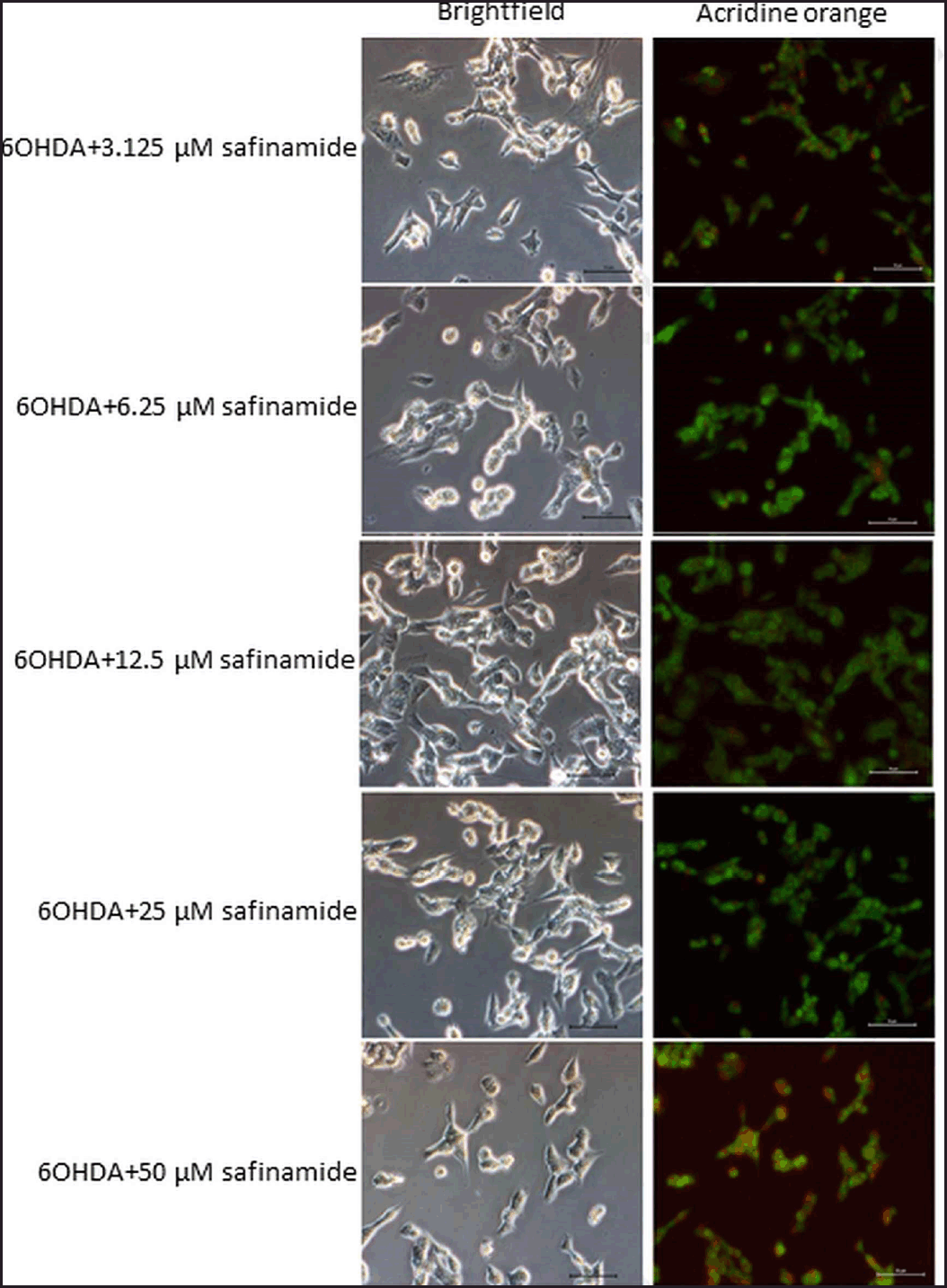 | Figure 5. Effects of safinamide on 6OHDA-induced SH-SY5Y cell autophagy. Micrographs of bright field and acridine orange stained-SH-SY5Y cells at magnification 200× for each treatment group were shown. Nucleus and acidic vesicles are stained green and red, respectively. [Click here to view] |
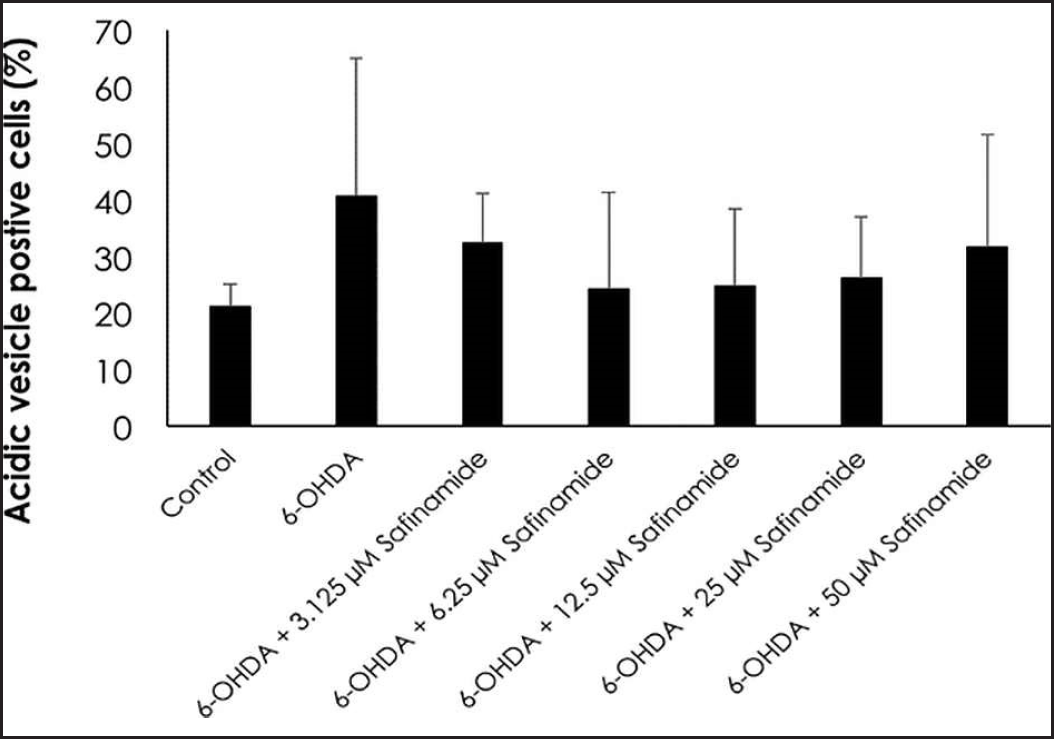 | Figure 6. Percentage of ratio of autophagic cells to total number of cells in each treatment group. Mean ± standard deviation of data from at least three independent experiments is shown. [Click here to view] |
Proteins involved in the autophagy signaling pathway were analyzed using enzyme-linked immunosorbent assay (ELISA). The proteins analyzed were Atg3, Atg5, Atg7, Atg12, Atg16L1, and Beclin-1, LC3A/B (Cell Signaling, USA). SH-SY5Y cells were seeded on a 96-well plate with a seeding density of 4 × 105 cells/ml and incubated at 37°C with 5% CO2 supply to reach a confluency of at least 70% before treatment for 24 hours. Wells for secondary antibodies were treated as in the control group. All solutions in each well were then removed, and 100 µl of −20°C methanol was added into each well to fix the cells for 20 minutes, before washing with Tris buffer saline (Bio Rad, USA) with Tween 20 detergent (TBST) (Calbiochem, USA) for three times, 5 minutes each. Then, each well was incubated with 100 µl 0.6% hydrogen peroxide (Calbiochem, USA) for 30 minutes in the dark. 100 µl 3% bovine serum albumin (BSA) (Cell Signaling, USA) was added into each well and incubated for 1 hour at room temperature after washing with TBST as mentioned previously. BSA was removed from all wells except those for secondary antibodies. 100 µl of primary antibodies were then added to each well and incubated overnight at 4°C. Following this, cells were washed with TBST before the incubation with secondary antibodies (Cell Signaling, USA) for 1 hour at room temperature. 100 µl of 3,3’,5,5’-Tetramethylbenzidine (Nacalai Tesque, Japan) was added to each well. As solutions turned blue, 100 µl of 500 mM sulfuric acid (H2SO4) (Fisher Scientific, USA) was added into each well to stop the reaction. The plate was read at 490 nm with a microplate reader. Subsequently, the solution in each well was discarded before washing with distilled water three times, 5 minutes each, and the plate was dried with an air dryer. 100 µl of 0.04% crystal violet (Merck, USA) in 4% ethanol (Bumi-Pharma Sdn. Bhd, Malaysia) was added to each well, and incubated for 30 minutes at room temperature. The plate was rinsed under running tap water and dried with an air dryer. 100 µl of 1% sodium dodecyl sulfate (OmniPur, USA) was added to each well and tapped gently to fully dissolve the precipitate before reading the plate at 595 nm with a microplate reader.
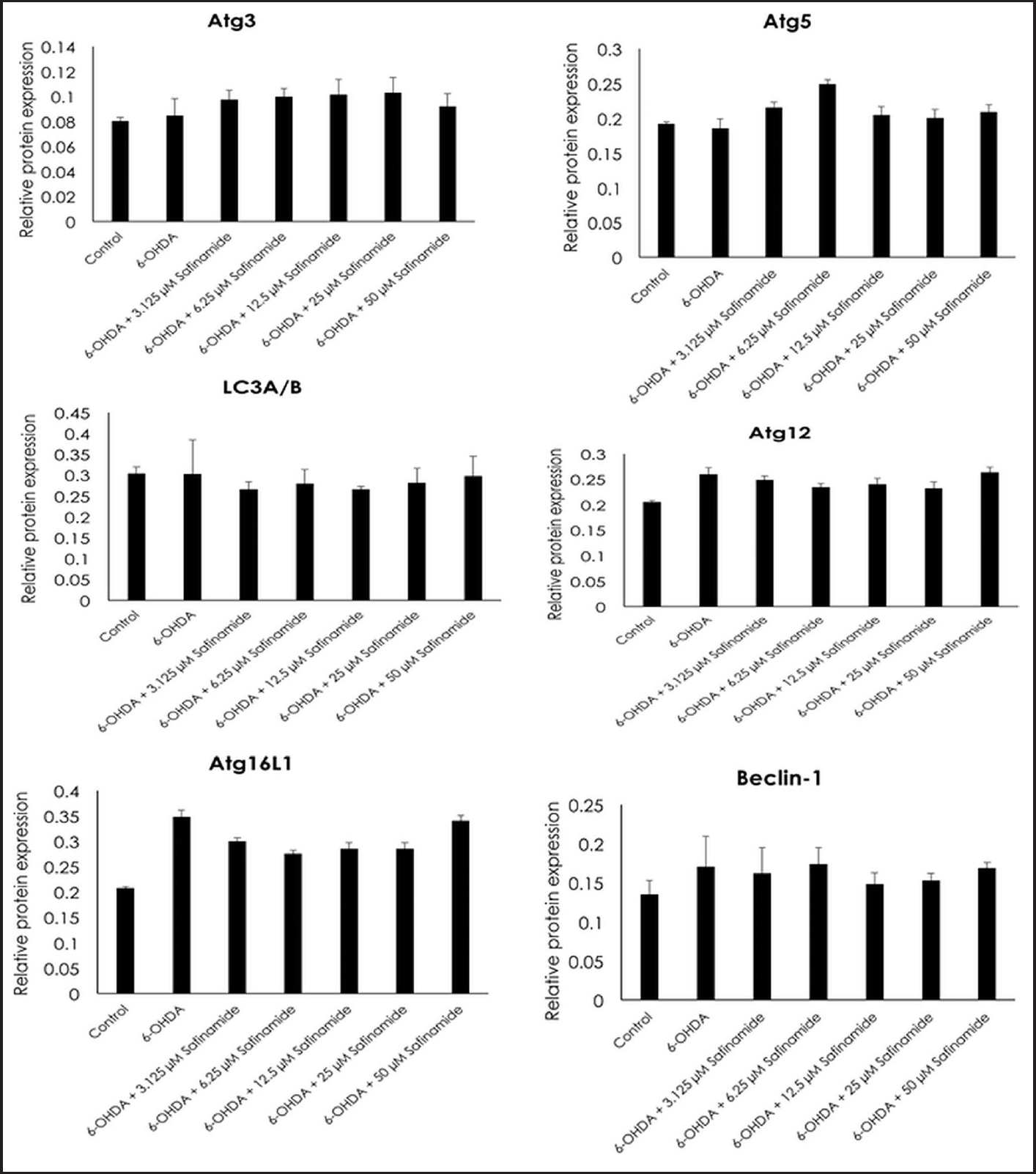 | Figure 7. Effects of safinamide on autophagy signaling pathway (Atg3, Atg5, LC3A/B, Atg12, Atg16L1, and Beclin-1) in 6OHDA-induced SH-SY5Y cells. Mean ± standard deviation of data from at least three independent experiments is shown. [Click here to view] |
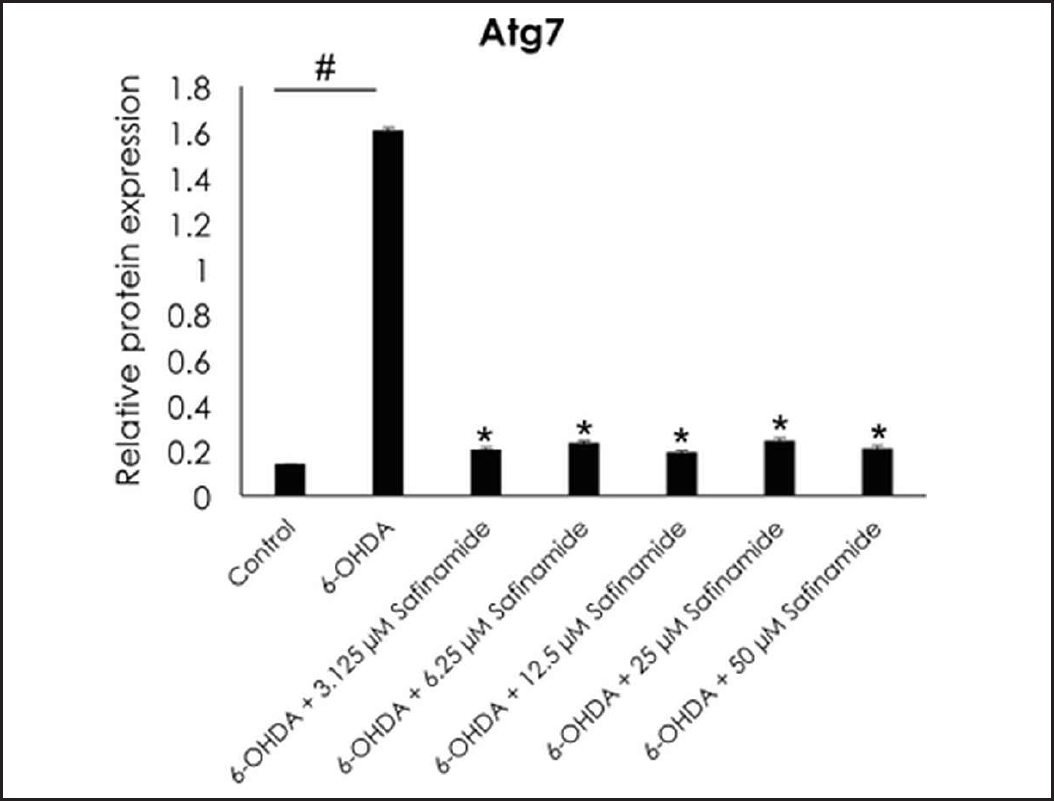 | Figure 8. Effect of safinamide on Atg7 protein in 6OHDA-induced SH-SY5Y cells. Mean ± standard deviation of data from at least three independent experiments is shown. #p < 0.05; *p < 0.05 compared to 6OHDA. [Click here to view] |
Statistical analysis
All values were expressed as mean ± standard deviation from at least three independent experiments. T-test was used for data analysis, and the differences were considered statistically significant at a value of p < 0.05.
RESULTS
Optimization of 6OHDA concentration in SH-SY5Y cells
Acridine orange stain and MTT assay were used to optimize 6OHDA concentration in inducing autophagy and cell death in SH-SY5Y cells, respectively. According to Figure 1A, 6OHDA induces autophagy from 12.5 µM onwards (p < 0.05). As most of the cells underwent autophagy at 25 µM, 25 µM was chosen as the optimum concentration for autophagy induction. A significant decrease in cell viability can be seen in SH-SY5Y cells treated with 12.5, 25, 100, and 200 µM 6OHDA (p < 0.05), as shown in Figure 1B. Most cell death was observed at 200 µM, followed by 25 µM. As 200 µM of 6OHDA induced almost 50% cell death, the concentration is too toxic to the cells. Hence, 25 µM 6OHDA was selected to induce cell death. After comparing both results, 25 µM of 6OHDA was identified as the optimum concentration for further analyses.
Evaluation of cytotoxicity of safinamide in SH-SY5Y cells
MTT assay was performed to determine the cytotoxicity of safinamide on SH-SY5Y cells. Based on the results in Figure 2, the maximum nontoxic dose (MNTD) determined from the graph is about 2 µM. However, the cytotoxicity of safinamide at the highest concentration tested, 50 µM, is about 10%, suggesting that safinamide may not induce harmful effects on neuronal cells. Thus, safinamide at 3.125–50 µM was used for further analyses.
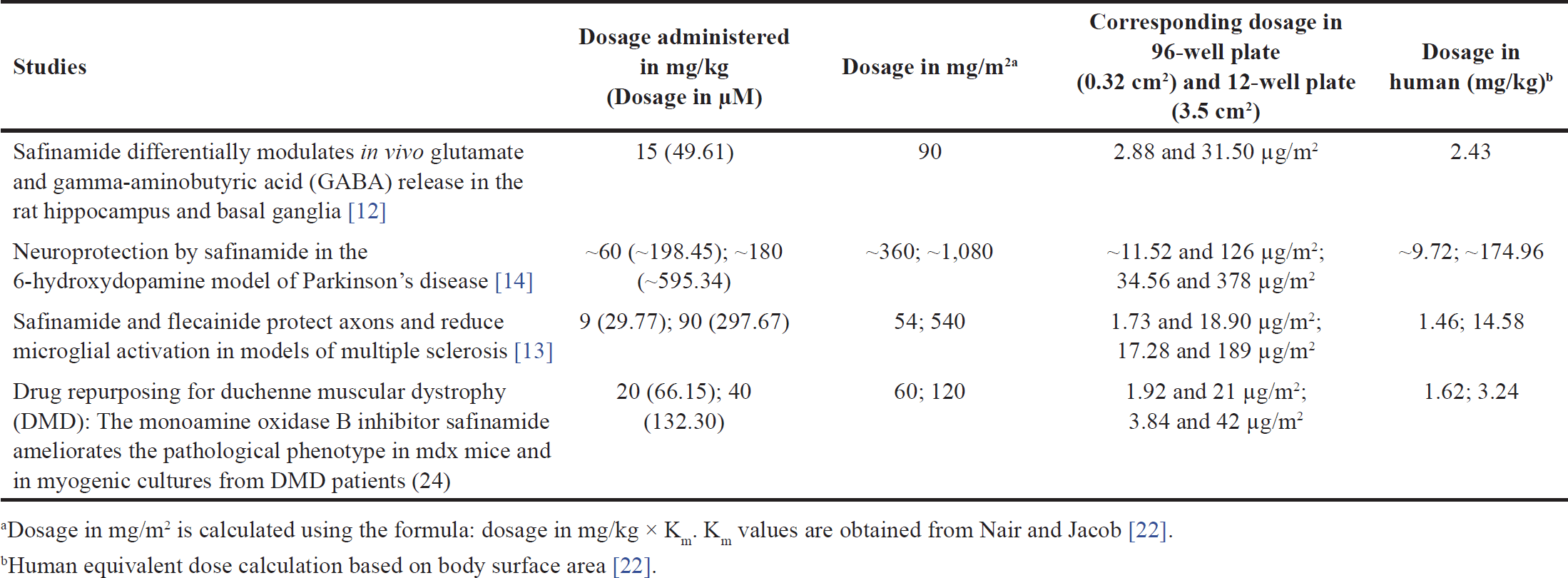 | Table 1. Dosage of safinamide administered to animal models in the previous studies. [Click here to view] |
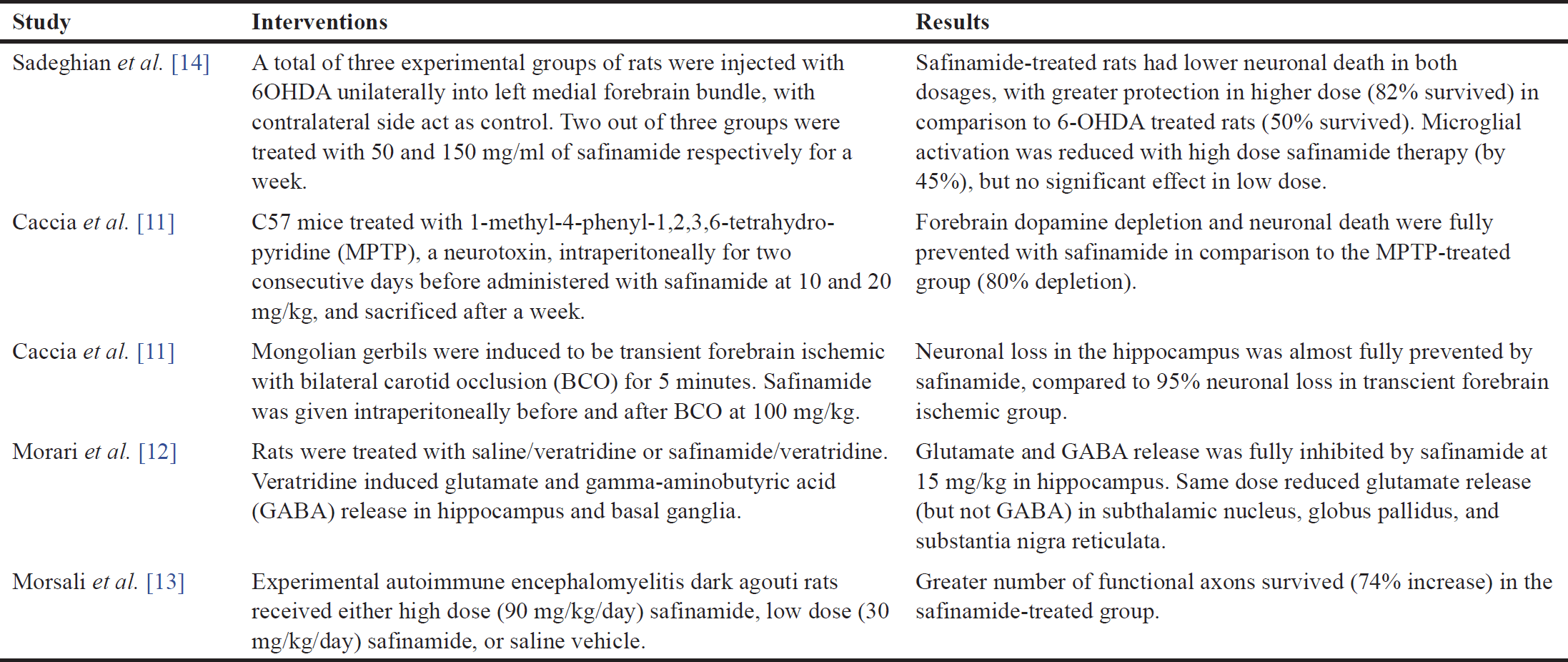 | Table 2. Summary of each study for neuroprotective effects of safinamide. [Click here to view] |
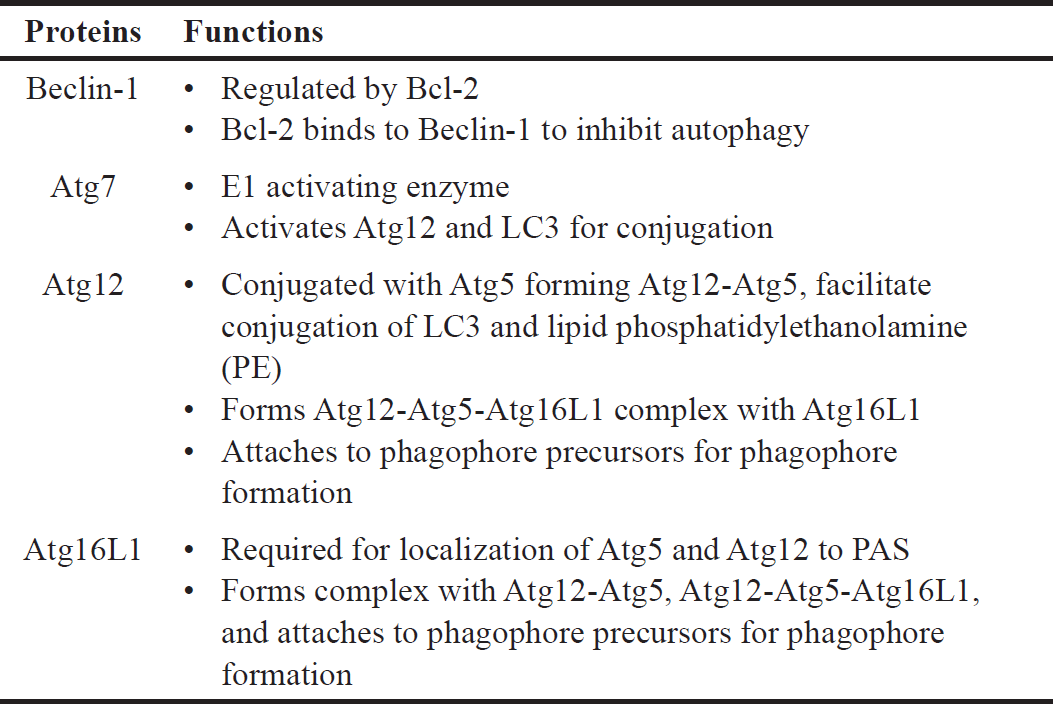 | Table 3. Specific functions of Beclin-1, Atg7, Atg12, and Atg16L1 in autophagy. [Click here to view] |
Determination of neuroprotective effects of safinamide in 6OHDA-induced SH-SY5Y cells
MTT assay, acridine orange stain, and ELISA were used to determine the neuroprotective effects of various concentrations of safinamide in the 25 µM 6OHDA-induced SH-SY5Y cells. MTT assay showed that safinamide slightly increased the cell viability in SH-SY5Y cells (Fig. 3). However, the increase was not statistically significant (p > 0.05).
Based on the staining results, 6OHDA induced autophagy in cells (Fig. 4), and various concentrations of safinamide reduced the percentage of autophagic cells by 23%–40% (Figs. 5 and 6), but the changes were not statistically significant (p > 0.05).
From the ELISA results, protein expressions of Atg3, Atg5, and LC3A/B were not affected by 6OHDA and safinamide treatments (Fig. 7). In contrast, 6OHDA and safinamide treatments affected protein expressions of Atg12, Atg16L1, Beclin-1, and Atg7. 6OHDA slightly increased the expressions of Atg12, Atg16L1, and Beclin-1, and safinamide treatments slightly reduced these proteins’ levels. However, the changes were not statistically significant (p > 0.05). Similarly, 6OHDA significantly increased the Atg7 protein level (p < 0.05), and safinamide treatments significantly reduced the increment of Atg7 protein induced by 6OHDA (p < 0.05) (Fig. 8).
DISCUSSION
The ability of 6OHDA to induce autophagy and cell death was assessed in this study (Fig. 1). 6OHDA, a common neurotoxin used for the neurodegenerative disorder model, is well known for its ability to induce autophagy and cell death. Autophagic markers, LC3B and Beclin-1 (increase with the presence of autophagy) and p62 (degrades during autophagy process) show significant increases and decreases in 6OHDA-treated models, respectively [16–18]. In addition, with monodansylcadaverine, an autophagic vacuoles stain, more green fluorescence (higher number of autophagic vacuoles) was seen in the 6OHDA-treated PC12 cells compared to the control group [19]. Cell viability of PC12 cells decreased in a concentration-dependent manner when treated with 6OHDA, and a lower number of tyrosine hydroxylase (TH)-immunoreactive cell bodies in 6OHDA-treated rats confirms its ability to induce cell death [20,21]. Hence, 6OHDA induces autophagy and cell death in in vitro and in vivo models.
Safinamide, a newly approved drug, has been tested on animal models in past studies. The dosage of safinamide administered to the animal models in the previous studies is converted from mg to µM (calculated based on the molecular weight of safinamide, 302.349 g/mol) for comparison with the present study and summarized in Table 1.
In this study, the cytotoxic effect of safinamide was evaluated, and an MNTD at 2 µM (Fig. 2). The cytotoxicity at the highest concentration tested, 50 µM, is 10%, which is considered negligible according to ISO 10993-5 [23], where it is stated that a percentage of cell viability above 80% is considered noncytotoxicity; within 80%–60% as weak; 60%–40% as moderate and below 40% as strong cytotoxicity. According to Morari et al. [12], the concentration of safinamide tested is approximately 50 µM, and safinamide should be maintained above 10 µM for its neuroprotective effect, as suggested by Sadeghian et al. [14]. In addition, safinamide is considered safe for treatments in PD. Clinical trials demonstrated that there are no obvious signs of toxicity in phase I, and safinamide has a low risk of inducing or exacerbating troublesome dyskinesia. Lower treatment-emergent serious adverse events (6.6%) and death rate (0.4%) were seen in the safinamide group compared to the placebo group (9.5% and 0.7%, respectively), although the death in the safinamide group was unlikely to be related to the medication [8,10]. Thus, in consideration of the past studies and clinical trials, safinamide at 3.125–50 µM was used for further analyses. Cell viability of 6OHDA-induced SH-SY5Y cells is increased slightly with safinamide treatments (Fig. 3). According to previous studies, safinamide may confer a neuroprotective effect. A summary of these studies can be found in Table 2. However, its exact mechanism of action in protecting the neurons is still unclear. Studies show that safinamide suppresses microglial activation and inhibits glutamate release, which might promote cell survival, thus preventing neurodegeneration in PD [11,14].
Findings from this study showed that safinamide treatments reduced the number of autophagic cells induced by 6OHDA (Figs. 4–6). However, the results were not statistically significant. Safinamide is a selective reversible MAO-B inhibitor, with an inhibition constant (Ki) of 0.5 µM (700-fold lower than human MAO-A) [24]. MAO-B, an enzyme that metabolizes dopamine, releases free radicals during the process and leads to oxidative stress and mitochondrial dysfunction. These conditions are believed to initiate autophagy in the dopaminergic neurons in PD individuals. Thus, if MAO-B is inhibited, fewer free radicals will be released, and autophagy may be reduced in PD patients [15].
This study also investigated various proteins (Atg3, Atg5, LC3A/B, Atg12, Atg16L1, Beclin-1, and Atg7) involved in the signaling pathway and demonstrated that Atg12, Atg16L1, Beclin-1, and Atg7 are affected by both 6OHDA and safinamide treatments (Figs. 7 and 8). The specific functions of these four proteins are summarized in Table 3 [4,24,25].
Based on Table 3, Beclin-1 inhibits autophagy when binds to Bcl-2 and will initiate autophagy when it is detached. As for Atg12 and Atg16L1, they are required for phagophore formation [26]. This study shows that safinamide decreased the increment of these protein expressions, thus reducing the autophagy by inhibiting the initiator and autophagosome formation. However, the results are not statistically significant (Fig. 7). Atg7 activates the conjugation of Atg12 and LC3, where both are involved in autophagosome formation. With safinamide treatments, this activating enzyme was decreased in its protein level significantly (Fig. 8), reducing the conjugation of Atg12 and LC3; thus, the formation of autophagosome decreased, leading to a decline in the autophagy process. Therefore, autophagy in PD might be due to the upregulation of Atg7, and this effect should be evaluated in the future.
LIMITATIONS
The present study investigated the effects of safinamide on a panel of autophagy-related proteins including Atg3, Atg5, Atg7, Atg12, Atg16L1, Beclin-1, and LC3A/B. Results showed that safinamide was effective against Atg7, but the specificity of safinamide on Atg7 has yet to be validated. To validate this effect, inhibitors against Atg7 such as pyrazolopyrimidine sulfamates [27] could be used on the 6-OHDA-induced cells, and the effects of safinamide on the cells could be observed. SH-SY5Y cell model, a simple and cost-effective in vitro model for studying PD [28] was used in the current study. As the effects of safinamide via autophagy on SH-SY5Y cells are less significant, hence, validation of the effects using other cell lines such as PC12 is highly recommended.
CONCLUSION
To conclude, with the increasing prevalence of PD, it is imperative to identify treatments that halt disease progression. As past studies demonstrated safinamide may have the potential to protect neurons. Neuroprotective effects of safinamide against autophagy in the 6OHDA-induced PD cell model were investigated. This study showed that safinamide might regulate autophagy by inhibiting Atg7 in the 6OHDA-induced PD cell model, while both 6OHDA and safinamide did not affect Atg3, Atg5, Atg12, Atg16L1, LC3, and Beclin-1.
Atg7 was found suppressed by safinamide in the present study as investigated using the ELISA method; this observation could be further validated by using the polymerase chain reaction technique at the gene level and western blot at the protein level. For future directions, safinamide may be tested at a broader concentration range. In addition, pre-treatment of cells with safinamide may be done to investigate its preventive effect. Moreover, safinamide may be evaluated in combination with other anti-Parkinsonism drugs. The involvement of other pathways for the mechanism of action of safinamide for neuroprotection should also be assessed.
ACKNOWLEDGMENT
The authors are grateful for the financial support from the International Medical University (IMU), Malaysia. The authors wish to thank Prof. Hoe I. Ling of Columbia University (New York, USA) for his editorial input.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
The study protocol was approved by the Joint Committee of Research & Ethics of the International Medical University, Kuala Lumpur, Malaysia (Approval No: KLR 0500119, Project ID No. BMS [/2018 (12), Date: 10 September 2018).
DATA AVAILABILITY
All the data generated and analyzed are included in this report.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Rizek P, Kumar N, Jog MS. An update on the diagnosis and treatment of Parkinson disease. Can Med Assoc J. 2016;188(16):1157–65.
2. Heo JY, Nam MH, Yoon HH, Kim J, Hwang YJ, Won W, et al. Aberrant tonic inhibition of dopaminergic neuronal activity causes motor symptoms in animal models of Parkinson’s disease. Curr Biol. 2020;30(2):276–91.
3. Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76.
4. Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson’s Disease. Cold Spring Harb Perspect Med. 2012;2(4):a009357.
5. Wang B, Abraham N, Gao G, Yang Q. Dysregulation of autophagy and mitochondrial function in Parkinson’s disease. Transl Neurodegener. 2016;5:19.
6. Bette S, Shpiner DS, Singer C, Moore H. Safinamide in the management of patients with Parkinson’s disease not stabilized on levodopa: a review of the current clinical evidence. Ther Clin Risk Manag. 2018;14:1737–45.
7. Kulisevsky J. Emerging role of Safinamide in Parkinson’s disease therapy. Eur Neurol Rev. 2014;9(2):108–12.
8. Perez-Lloret S, Rascol O. The safety and efficacy of safinamide mesylate for the treatment of Parkinson’s disease. Expert Rev Neurother. 2016;16(3):245–58.
9. Safinamide. 2022 [cited 2022 Oct 14]. Available from: https://www.drugbank.ca/drugs/DB06654
10. Schapira AHV, Fox SH, Hauser RA, Jankovic J, Jost WH, Kenney C, et al. Assessment of safety and efficacy of safinamide as a levodopa adjunct in patients with Parkinson disease and motor fluctuations. JAMA Neurol. 2017;74(2):216–24.
11. Caccia C, Maj R, Calabresi M, Maestroni S, Faravelli L, Curatolo L, et al. Safinamide: from molecular targets to a new anti-Parkinson drug. Neurology. 2006;67(7 Suppl 2):S18–23.
12. Morari M, Brugnoli A, Pisanò CA, Novello S, Caccia C, Melloni E, et al. Safinamide differentially modulates in vivo glutamate and gaba release in the rat hippocampus and basal ganglia. J Pharmacol Exp Ther. 2018;364(2):198–206.
13. Morsali D, Bechtold D, Lee W, Chauhdry S, Palchaudhuri U, Hassoon P, et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain, 2013;136(4):1067–82.
14. Sadeghian M, Mullali G, Pocock JM, Piers T, Roach A, Smith KJ. Neuroprotection by safinamide in the 6-hydroxydopamine model of Parkinson’s disease. Neuropathol Appl Neurobiol. 2016;42(5):423–35.
15. Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. J Neural Trans Suppl. 2006;71:53–65.
16. Li J, Ma L, Fan Y, Zhang Y, Sun D, Wu B. Crosstalk between 6-OHDA–induced autophagy and apoptosis in PC12 cells is mediated by phosphorylation of Raf-1/ERK1/2. Pharmazie. 2016;71(8):465–71.
17. Urano Y, Mori C, Fuji A, Konno K, Yamamoto T, Yashirugi S, et al. 6-Hydroxydopamine induces secretion of PARK7/ DJ-1 via autophagy-based unconventional secretory pathway. Autophagy. 2018;14(11);1943–58.
18. Zhang S, Gui X-H, Huang L-P, Deng M-Z, Fang R-M, Ke X-H, et al. Neuroprotective effects of β-asarone against 6-hydroxy dopamine-induced Parkinsonism via JNK/Bcl-2/Beclin-1 pathway. Mol Neurobiol. 2016;53(1):83–94.
19. Yoo H-I, Ahn G-Y, Lee E-J, Kim E-G, Hong S-Y, Park S-J, et al. 6-Hydroxydopamine induces nuclear translocation of apoptosis inducing factor in nigral dopaminergic neurons in rat. Mol Cell Toxicol. 2017;13(3):305–15.
20. Saito Y, Nishio K, Ogawa Y, Kinumi T, Yoshida Y, Masuo Y, et al. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radic Biol Med. 2007;42(5):675–85.
21. Vitiello L, Marabita M, Sorato E, Nogara L, Forestan G, Mouly V, et al. Drug repurposing for Duchenne muscular dystrophy: the monoamine oxidase B inhibitor safinamide ameliorates the pathological phenotype in mdx mice and in myogenic cultures from DMD patients. Front Physiol. 2018;9:1087.
22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31.
23. ISO 10993-5. 2009. Biological evaluation of medical devices–Part 5: tests for in vitro cytotoxicity. Geneva, Switzerland: International Organization for Standardization; 2009.
24. Müller T. Safinamide: an add-on treatment for managing Parkinson’s disease. Clin Pharmacol. 2018;10:31–41.
25. Walczak M, Martens S. Dissecting the role of the Atg12–Atg5-Atg16 complex during autophagosome formation. Autophagy. 2013;9(3):424–5.
26. He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
27. Huang SC, Adhikari S, Brownell JE, Calderwood EF, Chouitar J, D’Amore NR, et al. Discovery and optimization of pyrazolopyrimidine sulfamates as ATG7 inhibitors. Bioorg Med Chem. 2020;28(19):115681.
28. Ioghen OC, Ceafalan LC, Popescu BO. SH-SY5Y cell line in vitro models for Parkinson disease research-old practice for new trends. J Integr Neurosci. 2023;22(1):20.