An unlimited scope for novel formulations as orally disintegrating systems: Present and future prospects
Reeta Rani Thakur, Mridul Kashi
Pages: 13-19

Development and validation of dissolution procedures
Bhavesh Vaghela, Rajan Kayastha, Nayana Bhatt, Nimish Pathak, Dashrath Rathod
Pages: 50-56

Enhancement of Dissolution Rate and Formulation Development of Efavirenz Tablets Employing Starch Citrate-A New Modified Starch
K.P.R. Chowdary and Veeraiah Enturi
Pages: 119-123

Process optimization of efavirenz film coated tablet
K. Bala Krishna, M. Saritha
Pages: 146-149

Use of anhydrous calcium phosphate and selected binders in the tablet formulation of a deliquescent crude plant extract: Vernonia galamensis (Asteraceae)
M. Autamashih, A. B. Isah, T. S. Allagh, M. A. Ibrahim
Pages: 118-122

Formulation and optimization of solid dispersion of Clopidogrel with PEG 6000
Shailendra Kumar Singh, Soukarya Som, Upender Shankhwar
Pages: 217-226

Comparative in vitro dissolution study of Aceclofenac Marketed Tablets in Two Different Dissolution Media by Validated Analytical Method
S.M. Ashraful Islam, Sharmi Islam, Mohammad Shahriar, Irin Dewan
Pages: 87-92

Study of binary and ternary solid dispersion of ibuprofen for the enhancement of oral bioavailability
Mohammad Fahim Kadir, Muhammad Shahdaat Bin Sayeed, Rajibul Islam Khan, Tahiatul Shams, Md. Saiful Islam
Pages: 103-107

Comparative Evaluation of Physicochemical Properties of Some Commercially Available Brands of Metformin Hcl Tablets in Lagos, Nigeria
Akinleye M. Olusola, Adelaja I. Adekoya, Odulaja J. Olanrewaju
Pages: 41-44

Review of in vitro drug release test method's statistical evaluation to compare dissolution profile of semisolid dosage forms - Part I
Eva Petro, Istvan Eros, Ildiko Csoka
DOI: 10.7324/JAPS.2012.2329Pages: 180-181

A comparative study of the in-vitro dissolution profiles of paracetamol and caffeine combination in different formulations using HPLC
M.E.M. Hassouna, Y.M. Issa, A.G. Zayed
DOI: 10.7324/JAPS.2012.2531Pages: 52-59

Liquisolid Compacts: An Approach to Enhance the Dissolution Rate of Nimesulide
Srinivas Vaskula, Sateesh Kumar Vemula, Vijaya Kumar Bontha, Prasad Garrepally
DOI: 10.7324/JAPS.2012.2520Pages: 115-121

In-vitro bioequivalence study of 8 brands of metformin tablets in Iran market
Parvin Zakeri-Milani, Peyman Nayyeri-Maleki, Saeed Ghanbarzadeh, Mahboob Nemati, Hadi Valizadeh
DOI: 10.7324/JAPS.2012.2834Pages: 194-197

Study of Binary and Ternary Solid Dispersion of Spironolactone Prepared By Co-Precipitation Method for the Enhancement of Oral Bioavailability
Mohammad Fahim Kadir, Md. Rashedul Alam, Akib Bin Rahman, Yeakuty Marzan Jhanker, Tahiatul Shams, Rajibul Islam Khan
DOI: 10.7324/JAPS.2012.21023Pages: 117-122

Effect of Recrystallization on the Pharmaceutical Properties of Valsartan for Improved Therapeutic Efficacy
Buchi N. Nalluri, Ramya Krishna M, Rao TP, Peter A. Crooks
DOI: 10.7324/JAPS.2012.21025Pages: 126-132

Development and In-Vitro Evaluation of Vinpocetine Loaded Bi-Layer Tablet Using Different Polymers
Mithilesh Kumar Jha, Md. Zakaria Faruki, Md. Habibur Rahman, Md. Mofizur Rahman, Md. Mesbah Uddin Talukder
DOI: 10.7324/JAPS.2012.21227Pages: 149-157

Solid Dispersion: Methods and Polymers to increase the solubility of poorly soluble drugs
Ladan Akbarpour Nikghalb, Gurinder Singh, Gaurav Singh and Kimia Fazaeli Kahkeshan
DOI: 10.7324/JAPS.2012.21031Pages: 170-175

Development of extended release matrices of rifampicin using hot melt extrusion technique
Vanita J. Sharma and Purnima D. Amin
DOI: 10.7324/JAPS.2013.31006Pages: 030-038

Formulation Development and Evaluation of Ticagrelor Tablet for Regulatory Market
Md. Shafayat Hossain, Md. Anisuzzaman, Md. Anwar Hossain and Vikash Kumar Shah
DOI: 10.7324/JAPS.2013.31020Pages: 114-118

Formulation and in vitro evaluation of flurbiprofen-polyethylene glycol 20000 solid dispersions
Bhaskar Daravath and Rama Rao Tadikonda
DOI: 10.7324/JAPS.2014.40713Pages: 076-081

Evaluation and study of mebendazole polymorphs present in raw materials and tablets available in the Brazilian pharmaceutical market
Aline Quelian Penna Garbuio, Tássia Hanashiro, Blanca Elena Ortega Markman, Fernando Luiz Affonso Fonseca, Fábio Ferreira Perazzo, Paulo Cesar Pires Rosa
DOI: 10.7324/JAPS.2014.4111Pages: 001-007

Noveon AA1 as enhancer of HPMC as a direct compression matrix for controlled release
Sara Laguna-López, Leopoldo Villafuerte-Robles
DOI: 10.7324/JAPS.2014.41111Pages: 062-068

Chromatographic Separation and in Vitro Dissolution Assessment of Tenofovir disoproxil fumarate, Emtricitabine and Nevirapine in a Fixed Dose Combination of Antiretrovirals
Kalpana Jayapalu, Himaja Malipeddi, Anbarasu Chinnasamy
DOI: 10.7324/JAPS.2014.41113Pages: 076-080

Enhancing Solubility and Dissolution of Olanzapine by Spray Drying Using β- Cyclodextrin Polymer
Mudit Dixit, R. Narayana Charyulu, Anupama Shetty, Narayana Charyalu, Meghana Rao, Pallavi Bengre, Sharin Thomas
DOI: 10.7324/JAPS.2014.41114Pages: 081-086

RP-HPLC-PDA method development and validation for the analysis of Tadalafil in bulk, pharmaceutical dosage forms and in-vitro dissolution samples
Aziz Unnisa, Yogesh Babu, Santosh kumar suggu, Siva Chaitanya
DOI: 10.7324/JAPS.2014.41213Pages: 072-076

Study of the in vitro release profile of sesquiterpenes from a vaginal cream containing Copaifera duckei Dwyer (Fabaceae) oleoresin
Helison Oliveira Carvalho, Clarissa Silva Lima, Anderson Almeida Sanches , Jocivania Oliveira da Silva, Caio Pinho Fernandes, Jose Carlos Tavares Carvalho
DOI: 10.7324/JAPS.2015.50401Pages: 001-006

Enhancement of Simvastatin dissolution by surface solid dispersion: effect of carriers and wetting agents
Ebtessam Ahmed Essa, Mai Dwaikat
DOI: 10.7324/JAPS.2015.54.S8Pages: 046-053

Comparative in vitro dissolution of commercially available sustained release nifedipine tablet brands in the Kumasi Metropolis, Ghana
Christina Osei-Asare, Samuel Lugrie Kipo, Kwabena Ofori-Kwakye, Mariam El Boakye-Gyasi
DOI: 10.7324/JAPS.2015.50809Pages: 054-060

Investigations of polyethylene glycol mediated ternary molecular inclusion complexes of silymarin with beta cyclodextrins
Mohammad Ansari
DOI: 10.7324/JAPS.2015.50905Pages: 026-031

Influence of different types of lactose on powder flow and tablets dissolution
Karen Velázquez-González, Eduardo Ramírez-Flores, Leopoldo Villafuerte-Robles
DOI: 10.7324/JAPS.2015.50916Pages: 089-096

Development and validation of a stability indicating HPLC-diode array-fluorescence method for the determination of meclofenoxate hydrochloride and p-chlorophenoxyacetic acid
Marwa Said Moneeb, Feda Elgammal, Suzy Mohamed Sabry
DOI: 10.7324/JAPS.2016.60701Pages: 001-011

Self microemulsefying and non-self microemulsefying liquisolid tablet of felodipine
Gehad S. Khorshed, Gamal M. El-Maghraby
DOI: 10.7324/JAPS.2016.60719Pages: 125-132

Improvement of Domperidone Solubility and Dissolution Rate by Dispersion in Various Hydrophilic Carriers
Ahmed E. Aboutaleb, Sayed I. Abdel-Rahman, Mahrous O. Ahmed, Mahmoud A. Younis
DOI: 10.7324/JAPS.2016.60720Pages: 133-139

Functionality of Benecel K4M/Carbopol 971P NF matrices in direct compression tablets for controlled release
Leopoldo Villafuerte-Robles, Zeltzin Michelet Aguilar-Hernández
DOI: 10.7324/JAPS.2016.60901Pages: 001-008

Formulation Development of Solid Dispersions of Bosentan using Gelucire 50/13 and Poloxamer 188
Tapan Kumar Panda, Debajyoti Das, Lalatendu Panigrahi
DOI: 10.7324/JAPS.2016.60904Pages: 027-033

In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia
Ahmed F. Hanafy
DOI: 10.7324/JAPS.2016.60909Pages: 063-068

Development of Validated Stability Indicating RP-HPLC-PDA Method for Camptothecin Analysis
Buchi N. Nalluri, Saisrianusha Valluru, Chandrapriyanka Bonthu
DOI: 10.7324/JAPS.2016.60921Pages: 140-146

Physical stabilization of amorphous itraconazole in solid dispersions for improved dissolution profile
Yogesh Vilas Pore, Vikram Ramchandra Shinde, J. Venkateswara Rao
DOI: 10.7324/JAPS.2016.601005Pages: 037-044

Fast disintegrating tablets of amiodarone for intra-oral administration
Ebtessam Essa, Marwa Negm, Esmat Zin Eldin, Gamal El Maghraby
DOI: 10.7324/JAPS.2017.70109Pages: 064-072

Physicochemical Characterization of Physical Mixture and Solid Dispersion of Diclofenac Potassium with Mannitol
Yong K. Han, Sonia N. Faudone, Gustavo Zitto, Silvina L. Bonafede, María A. Rosasco, Adriana I. Segall
DOI: 10.7324/JAPS.2017.70130Pages: 204-208

Enhanced Solubility and Dissolution Rate of Clopidogrel by Nanosuspension: Formulation via High Pressure Homogenization Technique and Optimization Using Box Behnken Design Response Surface Methodology
Mohd Javed Qureshi, Fung Fuie Phin, Sreenivas Patro
DOI: 10.7324/JAPS.2017.70213Pages: 106-113

Xanthine Oxidase Inhibitor Febuxostat: Quality Comparisons and Release Kinetic Profile
Saquib M. Qureshi, Farya Zafar, Huma Ali, Shazia Alam, Yusra Shafiq, Sohail Khan, Saba A. Baloch, Kashif Maroof
DOI: 10.7324/JAPS.2017.70231Pages: 223-227

In vitro and In vivo Evaluation of Tablets Containing Meloxicam- PEG 6000 Ball-Milled Co-Ground Mixture
Mohamed Etman, Mustafa Shekedef, Aly Nada, Assem Ismail
DOI: 10.7324/JAPS.2017.70306Pages: 031-039

Self-Nanoemulsifying Drug Delivery Systems of Poorly Soluble Drug Dutasteride: Formulation and In-Vitro characterization
Poonguzhali Subramanian, Rajinikant Siddalingam
DOI: 10.7324/JAPS.2017.70402Pages: 011-022

Design and evaluation of Carvedilol nanocrystals sustained release tablets
Ashok Kumar Janakiraman, Balan Sumathi, T. Mohamed Saleem, S. Ramkanth, P. Odaya Kumar, Gopal Venkatachalam
DOI: 10.7324/JAPS.2017.70408Pages: 061-068

The Flow-Through Cell as an In Vitro Dissolution Discriminative Tool for Evaluation of Gliclazide Solid Dispersions
Laila H. Emara, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Nadia M. Morsi
DOI: 10.7324/JAPS.2017.70513Pages: 070-077

Dissolution Enhancement of Gendarusin A by Poloxamer 188 Addition in Justicia gendarussa Burm. f Ethanolic Extract Granule Matrix
Weka Sidha Bhagawan, Bambang Prajogo, Achmad Radjaram
DOI: 10.7324/JAPS.2017.70628Pages: 194-196

Effect of Self-Microemulsifying Lipid Formulations on the Dissolution and Compaction Profiles of Tablets Containing Theophylline; A BCS Class I Compound
Naser M.Y. Hasan, Mohammad A. Khaleel, Abdullah S. Altwairqi, Abdulmajeed G. Alqurashi, Abduallah H. Altwairqi
DOI: 10.7324/JAPS.2018.8605Pages: 030-038

Formulation and Evaluation of Gliclazide in Vegetable Oil-Based Self Emulsifying Delivery System
Gehan F. Balata
DOI: 10.7324/JAPS.2018.8905Pages: 023-033

Dissolution profiles of partially purified bromelain from pineapple cores [Ananas comosus (L.) Merr] encapsulated in glutaraldehyde-crosslinked chitosan
Siswati Setiasih, Hegi Adi Prabowo, Emil Budianto, Sumi Hudiyono
DOI: 10.7324/JAPS.2018.81003Pages: 017-024

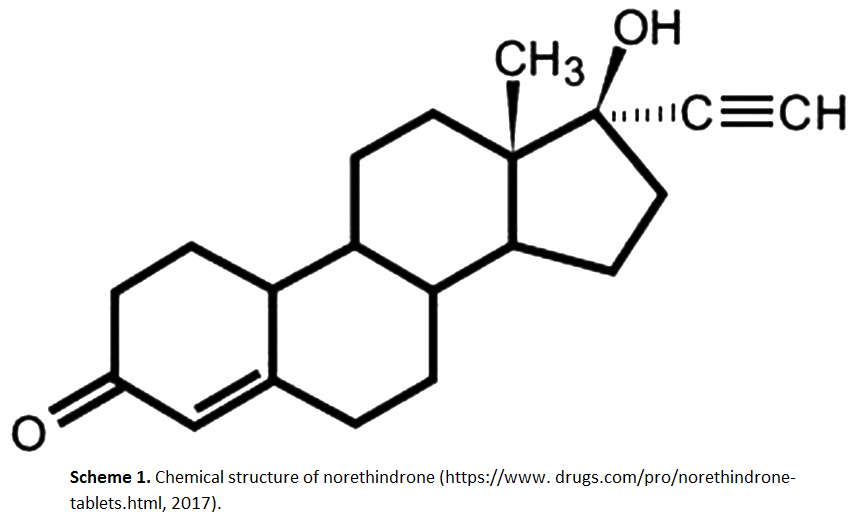
RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation
Suhair S. Al-Nimry, Bashar M. Altaani, Razan H. Haddad
DOI: 10.7324/JAPS.2019.90211Pages: 079-086
A post-market quality assessment of first-line, fixed-dose combination antiretrovirals in South Africa
Kim Ward, Reem Suleiman, Yunus Kippie, Admire Dube
DOI: 10.7324/JAPS.2019.90213Pages: 097-104

Enhanced oral delivery of artemether: An application of Prosochit®
Emmanuel O. Olorunsola, Adedayo A. Tologbonse, Ngozi A. Onwuka, Michael U. Adikwu
DOI: 10.7324/JAPS.2019.90417Pages: 133-136

Inclusion complexes of atorvastatin calcium–sulfobutyl ether β cyclodextrin with enhanced hypolipidemic activity
Anureet Arora, Geeta Aggarwal, Thakur Gurjeet Singh, Manjinder Singh, Gitika Arora, Manju Nagpal
DOI: 10.7324/JAPS.2019.91108Pages: 060-068

Electrohydrodynamic atomization, a promising avenue for fast-dissolving drug delivery system: Lessons from tadalafil-loaded composite nanofibers
Mohammed M. Mehanna, Jana K. Alwattar, Roland Habchi
DOI: 10.7324/JAPS.2020.101005Pages: 033-045

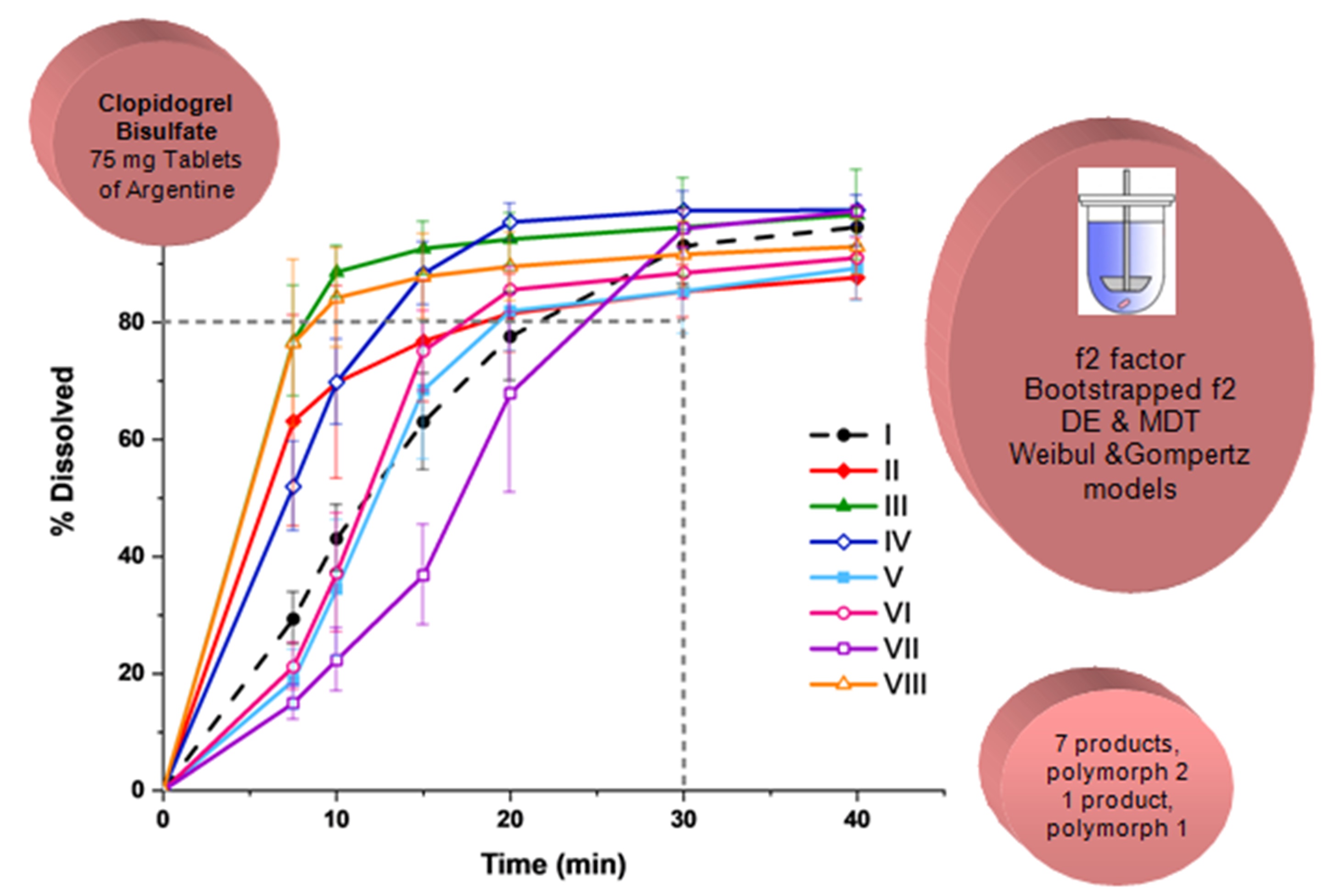
Comparative dissolution and polymorphism study of clopidogrel bisulfate tablets available in Argentine
Silvia Farfan, Marina Marcos Valdez, Octavio Fandino, Norma Sperandeo, Sonia Faudone
DOI: 10.7324/JAPS.2020.10107Pages: 062-071
Validation of a high-performance liquid chromatographic method for the assay and dissolution of captopril in mucoadhesive tablet formulation
Ratna Budhi Pebriana, Olivia Damayanti, Yunda Dewi Agustin, Endang Lukitaningsih, Angi Nadya Bestari
DOI: 10.7324/JAPS.2021.110209Pages: 066-074

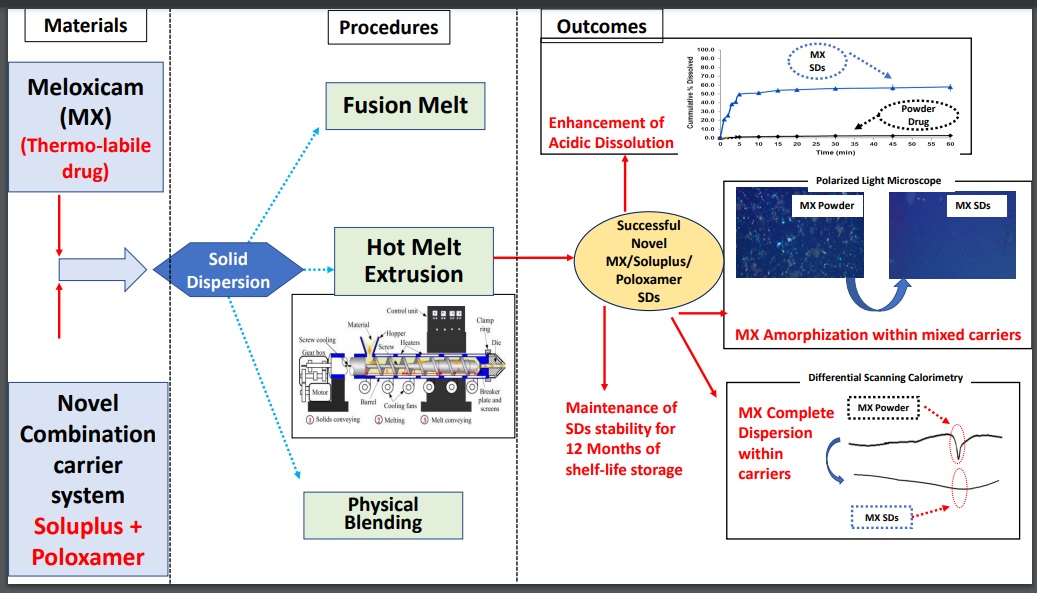
A novel combination of Soluplus® and Poloxamer for Meloxicam solid dispersions via hot melt extrusion for rapid onset of action—part 1: dissolution and stability studies
Maha F. Emam, Nesrin F. Taha, Laila H. Emara
DOI: 10.7324/JAPS.2021.110218Pages: 141-150
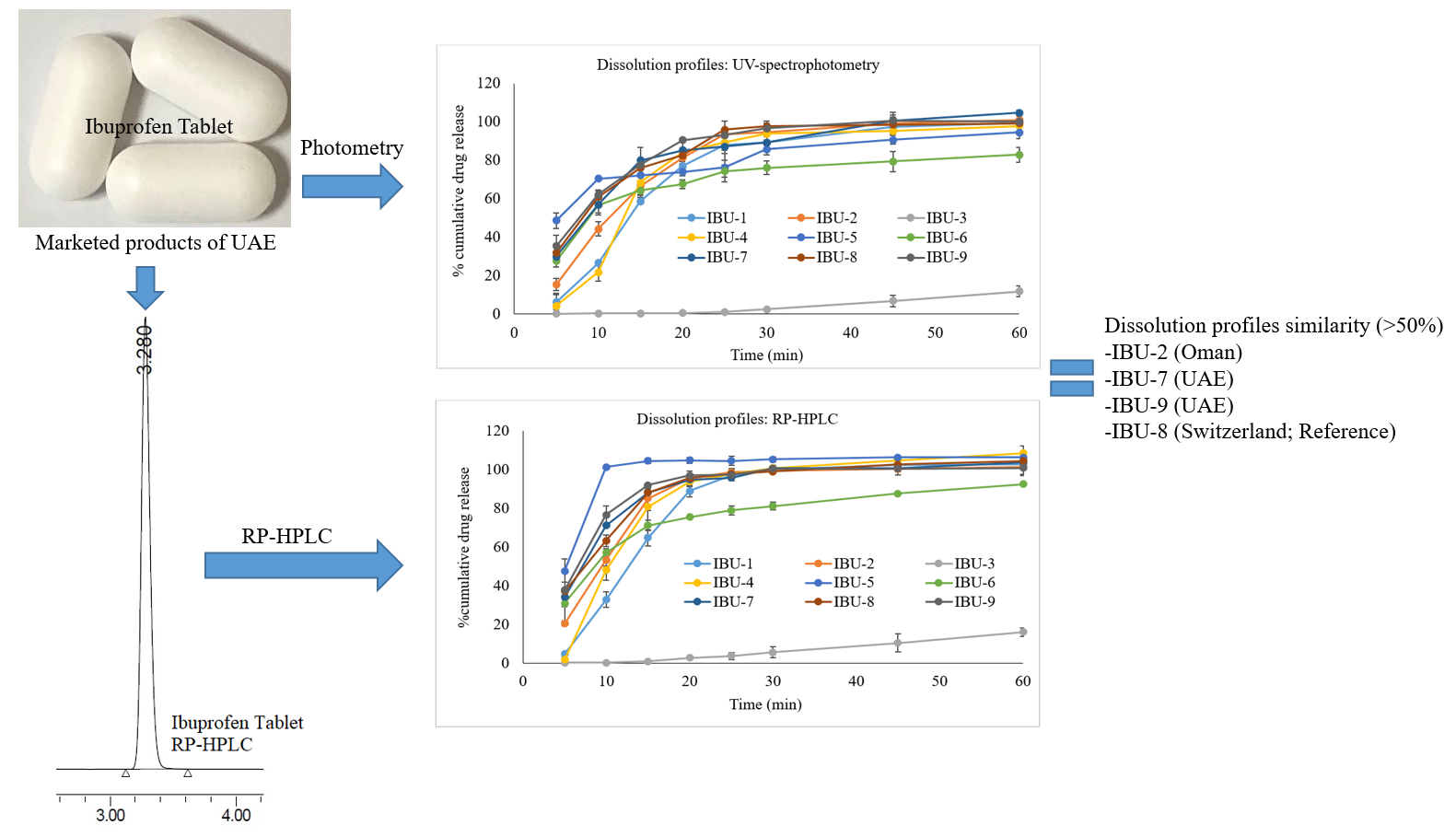
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
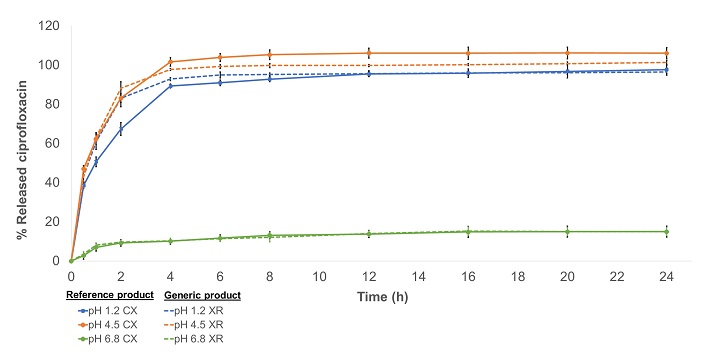
Comparison between the dissolution profiles of prolonged-release ciprofloxacin tablets available in the Colombian market
Andrés Vicente De la Cruz Gómez, Raynni Marcela Ramos Iglesias, Tatiana Sugey Ruiz Afanador, Indira Beatriz Pájaro Bolívar, Gina Paola Domínguez Moré
DOI: 10.7324/JAPS.2022.120322Pages: 209–217
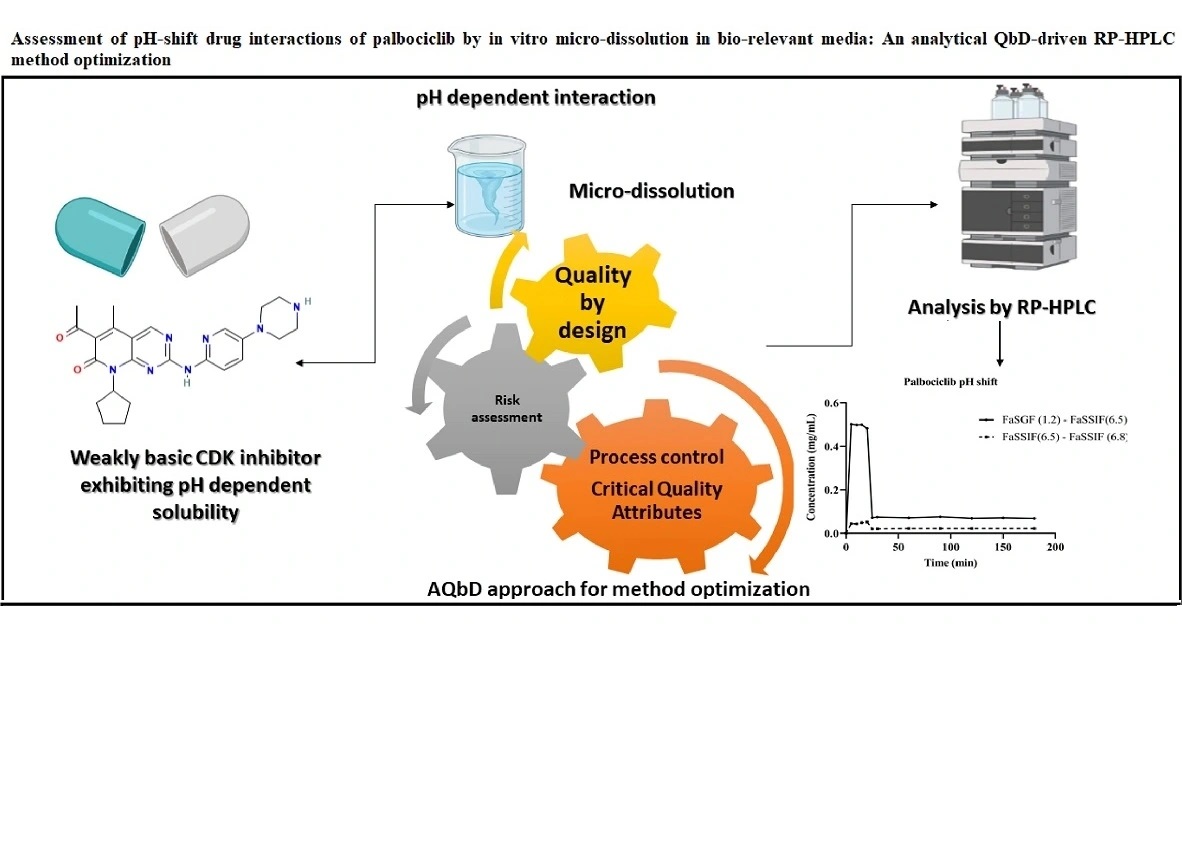
Assessment of pH-shift drug interactions of palbociclib by in vitro micro-dissolution in bio relevant media: An analytical QbD-driven RP-HPLC method optimization
Prajakta Harish Patil, Mrunal Desai, Rajat Radhakrishna Rao, Srinivas Mutalik, Gurupur Gautham Shenoy, Mahadev Rao, Puralae Channabasavaiah Jagadish
DOI: 10.7324/JAPS.2022.120505Pages: 078-087
Preparation and properties characterization of composite modified starch-based orodispersible films
Yaoyao Zhao, Yuqi Feng, Xiaoqing Ren, Yuru Hou, Fangyuan Wang, Runze Wang, Mintong Guo
DOI: 10.7324/JAPS.2024.153167Pages: 080-090

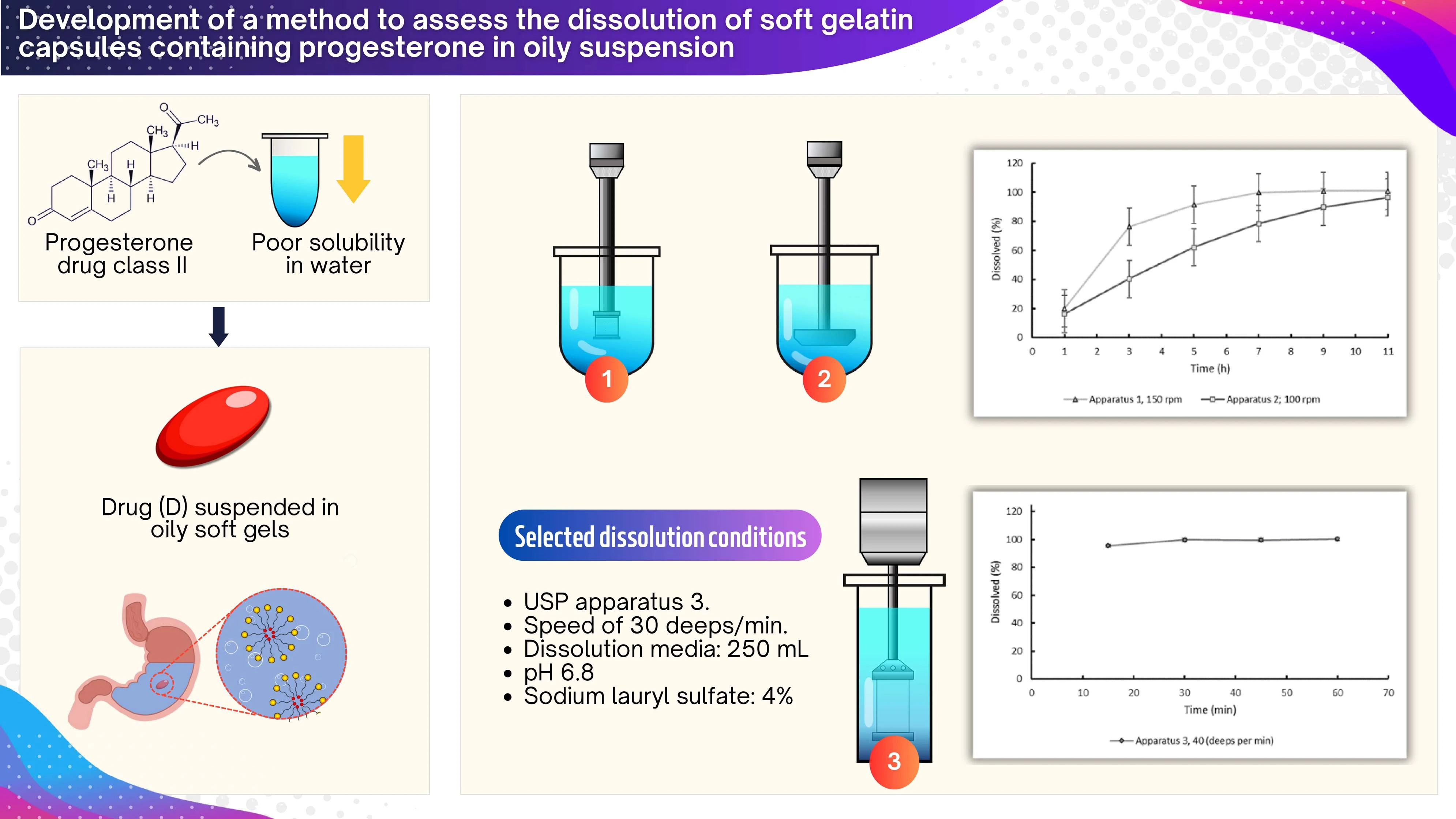
Development of a method to assess the dissolution of soft gelatin capsules containing progesterone in oily suspension
Diego R. Monterroza, Claudia M. Baena-Aristizábal, Luisa Fernanda Ponce D´león, Yolima Baena
DOI: 10.7324/JAPS.2024.158285Pages: 163-170
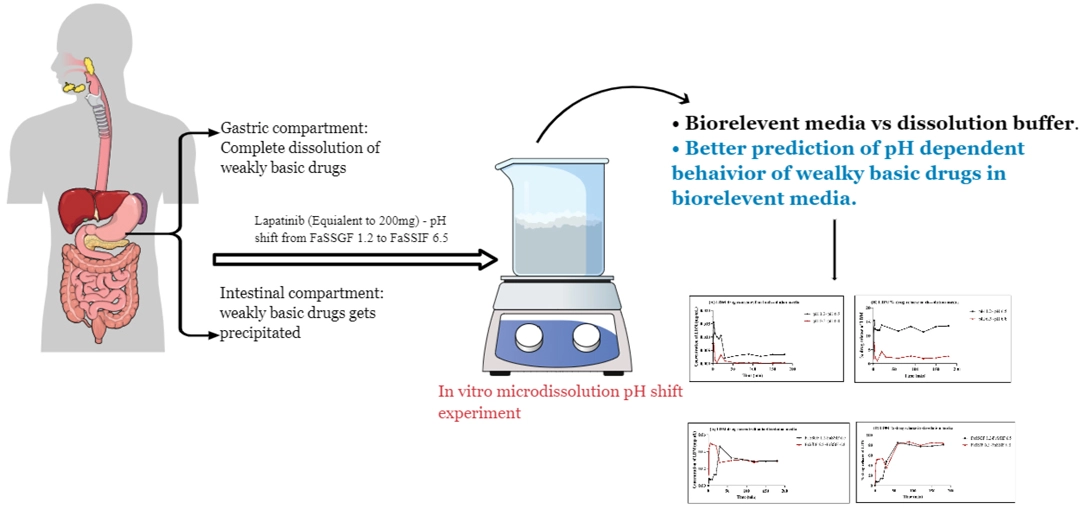
The prediction of pH-dependent interaction using micro-dissolution approach in bio-relevant media: Insights from model drug study of lapatinib
Anithakumari Uttam Singh Rajpurohit, Prajakta Harish Patil, Mrunal Desai, Jagadish Puralae Channabasavaiah
DOI: 10.7324/JAPS.2024.170413Pages: 105-115
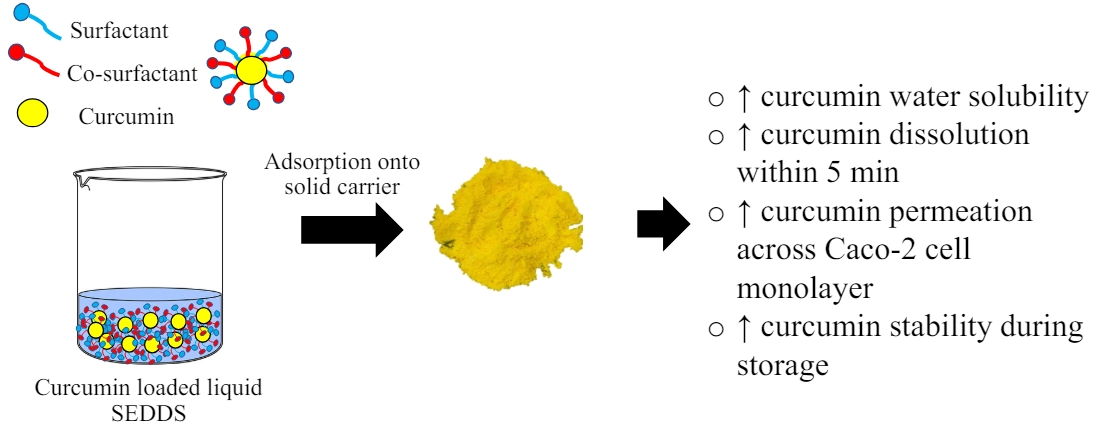
Development of curcumin-loaded solid SEDDS using solid self-emulsifying drug delivery systems to enhance oral delivery
Suchiwa Pan-On, Duy Toan Pham, Waree Tiyaboonchai
DOI: 10.7324/JAPS.2024.168338Pages: 111-119
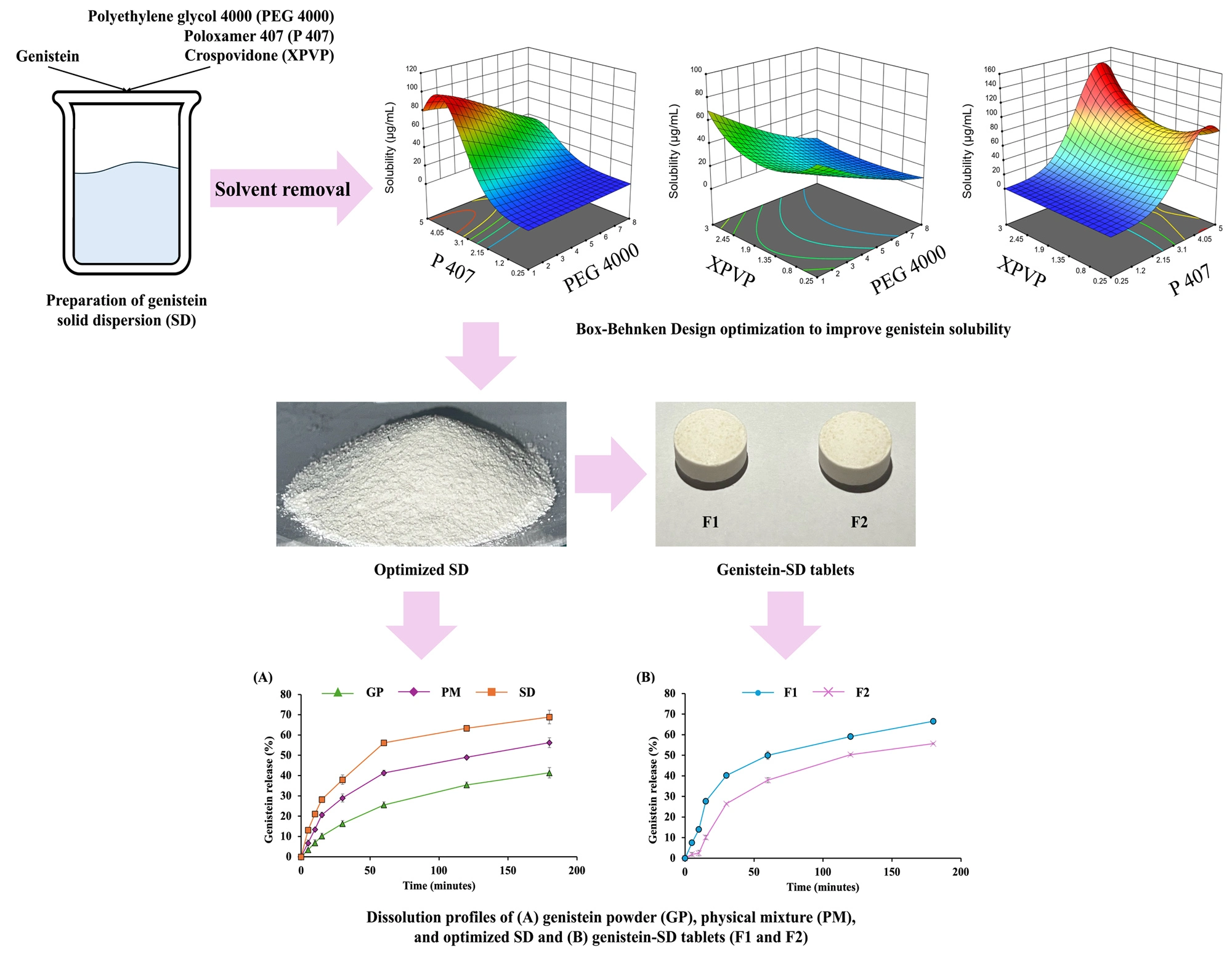
Development of tablet formulations containing genistein solid dispersion optimized using Box-Behnken design for enhanced solubility
Suranate Phanapithakkun, Gorawit Yusakul, Chanakan Sitthisak, Thipapun Plyduang
DOI: 10.7324/JAPS.2025.222224Pages: 053-064
Model integrated evidence approach for rational and safe formulation development: case of alfuzosin prolonged-release tablets
Sivacharan Kollipara, Rajkumar Boddu, Suribabu Bonda, Hariharan Venugopal, Chandra Deb, Pavan Kumar Mittapalli, Anand Arumugam, Sohel Mohammed Khan, Venkat Ramana Naidu
DOI: 10.7324/JAPS.2025.231215Pages: 253-264
_.webp)
Formulation development of diclofenac sodium extended-release tablet using hydrophobic and hydrophilic matrix systems
Truc-ly Thi Duong, Duyen Thi My Huynh, Tu-Uyen Thi Nguyen, Quoc-Dung Tran Huynh, Thuy-Tien Thi Phan, Thu-Thi Tran, Ha Van Nguyen, Benni Iskandar, Dang-Khoa Nguyen
DOI: 10.7324/JAPS.2026.253028Pages: