INTRODUCTION
The adoption of artemisinin-based combination therapy (ACT) by the World Health Organization as the first line treatment for uncomplicated malaria has resulted in increased use of artemisinins (Chen, 2014). In ACT, an artemisinin is combined with one or two other drugs for the treatment of malaria. Compared to the use of a single agent, ACT provides a better treatment outcome, reduces the chance of development of drug resistance, and reduces disease transmission (Pousibet-Puerto et al., 2016).
Solubility and permeability are the two major physicochemical properties determining bioavailability of drugs (Ashford, 2018). All artemisinins have good permeability (Rosenthal, 2004). The major difference in their physicochemical properties is solubility. For instance, the parent compound artemisinin has poor solubility; artemether is lipid-soluble and poorly water-soluble, while artesunate is water-soluble (International Pharmacopoeia, 2006; Rosenthal, 2004).
The low oral bioavailability of artemether (35.0%–43.2%) is due to its poor water-solubility (Laxmi et al., 2015; Tayade and Nagarsenker, 2010). The previous work on the oral delivery of the drug using acacia gum, cashew gum, and prosopis gum (PRG) showed that the PRG caused delayed drug release but enhanced permeation compared to acacia gum, a known standard gum (Olorunsola et al., 2017). Like other drugs having dissolution rate-limited absorption, enhancement of dissolution of artemether from tablet is a way of improving the bioavailability of the drug (Strandgarden et al., 1999).
Prosochit® is a derivative of two biopolymers (PRG and crab shell chitosan). It is presently available in three grades as Prosochit® 201 (PC201), Prosochit® 101 (PC101), and Prosochit® 102 (PC102) (Olorunsola, 2017). Chitosan, which is one of the polymers used for developing Prosochit®, is known to enhance tablet disintegration and drug dissolution (Ritthidej et al., 1994; Sinha et al., 2004). Hence, its combination with PRG for developing Prosochit® for use in delivering artemether could enhance the delivery of the drug.
Dissolution enhancement is one of the methods of improving bioavailability of poorly-soluble drugs (Sinha et al., 2004). This work is aimed at assessing the suitability of the three grades of Prosochit® for enhanced oral delivery of artemether.
MATERIALS AND METHODS
Materials
The materials used include: PRG extracted from the seeds of Prosopis africana; Prosochit® 201, Prosochit® 101, and Prosochit® 102 developed as described in NG Patent 2016/00355 (Olorunsola, 2017). Other materials used are: artemether powder (Afrab Chem. Ltd., Lagos, Nigeria), lactose monohydrate (Surechem Products Ltd., England), maize starch B.P. and talc (BDH Chemicals, Poole, England), and magnesium stearate (Riedel-De Haen, Germany).
Tablet formulation
The three grades of Prosochit® were used as binders at concentrations equivalent to 2.0% w/w PRG for the preparation of granules for tablets of weight 275 mg containing 20 mg artemether. The binders were used as 2% PRG, 3% PC201, 4% PC101, and 6% PC102 to ensure incorporation of 2% PRG in all the formulations, and 0%, 1%, 2%, and 4% chitosan in (Formulations) I, II, III and IV, respectively. Calculations were made for batch sizes of 13.75 g (50 tablets) as shown in Table 1.
The weighed quantities of artemether (the drug), maize starch B.P. (disintegrant), and lactose (diluent) were dry-mixed in a mortar mixing bowl for 5 minutes and then moistened with mucilage of the binder (PRG, PC201, PC101, or PC102). The damp mass was screened through a 2.0-mm size sieve, air-dried for 3 hours, and then dried in a hot air oven (Gallenkamp, Germany) at 60°C for 1 hour. The dried granules were screened through a 1.0-mm size sieve (Olorunsola et al., 2017).
Determination of granule micromeritics
Ten grams sample of granules was placed in a 25 ml capacity measuring cylinder and the bulk volume was taken. The cylinder containing the granules was tapped 100 times after which the volume was retaken. The bulk density (BD) and tapped density (TD) were calculated as the ratio of mass of the granules to the corresponding volume.
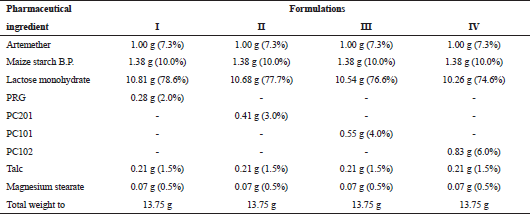 | Table 1. Tablet formulation for 50 units. [Click here to view] |
The Hausner’s ratio (HR) and Carr’s index (CI) of the granules were calculated using the equations below (Aulton, 2018):
Tableting
The required amounts of talc and magnesium stearate (as presented in Table 1) were separately weighed and gently blended with the dried granules. Tablets of targeted weight 275 mg (containing 20 mg artemether) were formed from the resulting mix by compaction at a pressure of 30 KN using a single punch tableting machine (Erweka, Germany) fitted with 8.0-mm punches.
Evaluation of tablet quality
The tablets were kept for a period of 24 hours to allow for stress relaxation before they were subjected to quality control tests (Alebiowu and Itiola, 2003). The tablets were subjected to CS determination (Monsanto hardness tester, Laboratory Tree Co., India), FR test (Roche frabilator, Erweka, Germany), and disintegration test (Disintegration tester ZT3, Erweka, Germany) using the conventional methods (Salih and Niff, 2016).
Different concentrations of artemether (0.05%, 0.10%, 0.15%, 0.20%, and 0.25% w/v) were prepared in 1.0-mol.l−1 ethanolic hydrochloric acid (International Pharmacopoeia, 2006) and the absorbance was taken at 210 nm (Debra et al., 2016). This was used to get the Beer–Lambert’s plot for artemether. The plot was subsequently used for determining the percent drug release from tablets.
Tablet dissolution test was carried out using U.S.P. dissolution apparatus (Panomex Inc., India). One tablet was placed inside the dry basket of the apparatus and lowered inside the glass containing 900 ml of 0.1-mol.l−1 hydrochloric acid thermostatically maintained at 37.0°C ± 0.5°C. The apparatus was set to a rotational speed of 100 rpm for 1 hour. Samples (10-ml volumes) were taken at 10-minute intervals with subsequent replacement with equal volume of the 0.1-mol.l−1 hydrochloric acid. Each withdrawn sample was filtered and the absorbance was taken at 210 nm (Remington et al., 2006) using an ultraviolet spectrophotometer (UNICO Shanghai Instrument, China). The cumulative percent drug release was determined with reference to the Beer–Lambert’s plot and a graph of cumulative percent drug released was plotted against time.
Data analysis
Data (X ± standard error of mean—SEM) were analyzed by applying one-way analysis of variance followed by Turkey–Kramer multiple comparison test using GraphPad Instat-3 software; p-values less than 0.05 were taken as significant.
RESULTS AND DISCUSSION
Granule micromeritics
The micromeritics of the different granules are shown in Table 2. There was no significant difference in the BD of the granules and it ranged from 0.53 to 0.55 g/cm3. Similarly, there was no significant difference in the TD of the granules and it ranged from 0.62 to 0.64 g/cm3. The HR ranged from 1.16 to 1.19, while the CI ranged from 14.06% to 15.88%.
BD is directly related to particle packing. Hence, the packing of the different granules is not significantly different. The insignificant difference in the BD and the insignificant difference in the TD of the granules imply similarity in the consolidation process of the different granules (Aulton, 2018).
According to Aulton (2018), HR from 1.12 to 1.18 (equivalent to CI from 11% to 15%) indicates good flow, while HR from 1.19 to 1.25 (equivalent to CI from 16% to 20%) indicates fair flow. Even though there was no statistically significant difference in the HR and in the CI of the granules, the flow patterns of the granules are different. Based on the two parameters (HR and CI), granules containing PRG (Formulation I) and PC102 (Formulation IV) are characterized by good flow, while those containing PC201 (Formulation II) and PC101 (Formulation III) are characterized by fair flow. To ensure good flow of all the granules during tableting, magnesium stearate (anti-adherent) and talc (glidant) were added to the granules of all the formulations before compression.
Tablet properties
Some tablet properties are shown in Table 3, while the dissolution profile is shown in Figure 1. There was no significant difference in the CS of the tablets, but the FR of tablets containing PRG was significantly higher than those containing Prosochit®.
The DT of tablets was in the order: Formulation I > Formulation II > Formulation III > Formulation IV, while the % drug release at 1 hour was in the inverse order. The dissolution profile showed two-staged dissolution for all the formulations. Stage one spanned the first 20 minutes, while stage two started from time 20 minutes through time 60 minutes for all the preparations in study.
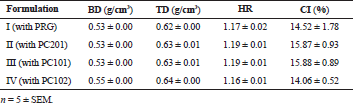 | Table 2. Micromeritics of granules obtained using different binder agents. [Click here to view] |
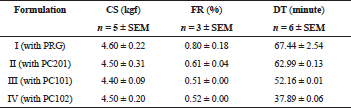 | Table 3. Tablet properties. [Click here to view] |
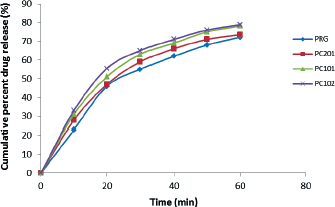 | Figure 1. Plot of cumulative percent drug release versus time. [Click here to view] |
The CS and FR of all the formulations are within the acceptable ranges. A CS of 4–7 kgf and FR of <1% are expected for tablets to pass tests for the strength (Remington et al., 2006). The CS is a measure of the tablet strength, while FR is a measure of the weakness. Both parameters are taken into consideration as measures of the mechanical properties of the tablets.
All the formulations failed the test for DT for immediate release tablets. A DT less than 15 minutes is required for immediate release tablets (United States Pharmacopoeia, 2008). The long DTs correlate with the high binding strength of PRG already reported by Attama et al. (2000). Tablet disintegration is the initial step of drug release from tablets. During disintegration, a tablet in the presence of fluid is broken into aggregates. In some instances, some polymers bring forth gelling of tablet rather than disintegration causing delayed or extended drug release. Both PRG and chitosan have this tendency, especially at high concentrations (Attama et al., 2000; Sonia and Sharma, 2011). This is the reason Prosochit® was used at low concentrations in this work.
The disintegrant and dissolution-enabling properties of the binding agents are in the order: PRG < PC201 < PC101 < PC102. Formulations I, II, III, and IV contain equivalents of 0%, 1%, 2%, and 4% chitosan, respectively. Therefore, there is a direct relationship between the release properties of the formulations and the proportion of chitosan in the binding agents.
The dissolution profile shows that there were two stages of dissolution for each of the formulations. Stage one with greater slope shows that there was an initial fast dissolution rate up to time 20 minutes. Beyond time 20 minutes, there was a slower dissolution rate as revealed by the reduced slope of the plots. Since both PRG and chitosan have swelling and gelling tendencies, the initial fast dissolution rate can be explained as drug release from the surface of the tablet while the slower dissolution rate corresponds to drug release from the swollen gelling mass (Chavanpatil et al., 2005). This observation is different from drug release from chloroquine tablets formulated with corn and yam starches in which there was an initial slow dissolution rate (during tablet disintegration) then a faster dissolution rate (after tablet disintegration) followed by a decline in the drug release (Adetunji et al., 2006).
Tablets containing PRG and PC201 failed dissolution test, while those containing PC101 and PC102 passed the test. A minimum of 75% drug should be released within 1 hour for immediate release tablets (United States Pharmacopoeia, 2008). The good drug dissolution from tablets containing PC101 and PC102 can be linked to the presence of appropriate amount of chitosan in the excipients as chitosan is known to enhance drug delivery (Ritthidej et al., 1994; Sinha et al., 2004; Sonia and Sharma, 2011). There was an inverse relationship between the DT of tablet and the percent dissolved drug in 1 hour. Prosochit® 102 brought forth the best drug delivery, its formulation being characterized by the lowest DT and the highest cumulative percent drug release at 1 hour.
The suitability of Prosochit® in enhancing oral delivery of artemether is in consonance with the work of Ngwuluka et al. (2015) which showed that combination of two polymers is often superior to the individual ingredient. Previous work showed that PRG caused delayed release of artemether but enhanced permeation compared to acacia gum (Olorunsola et al., 2017), whereas dissolution is the rate-limiting step for the absorption of the drug (Rosenthal, 2004). This work has shown that Prosochit® 102 (Formulation IV) is able to enhance the dissolution rate of artemether in comparison with PRG (Formulation I) probably due to the amount of chitosan in the 6.0% binder concentration used to compose the artemether tablet.
CONCLUSION
The flow properties of artemether granules and the mechanical strength of the tablets formulated with Prosochit® are comparable with those of PRG. The use of the new excipient was able to increase the oral delivery of the artemether. It is a better agent compared to PRG for delivering artemether, and the grade Prosochit® 102 offers the best delivery of the drug according to the formulations studied.
FINANCIAL SUPPORT AND SPONSORSHIP
None.
CONFLICTS OF INTEREST
There are no competing interests regarding the publication of this paper.
REFERENCES
Adetunji OA, Odeniyi MA, Itiola OA. Compression, mechanical and release properties of chloroquine phosphate tablets containing corn and trifoliate yam starches as binders. Trop J Pharm Res, 2006; 5(2):589–96. CrossRef
Alebiowu AG, Itiola OA. The effects of starches on mechanical properties of paracetamol tablet formulations I: Pregelatinization of starch binders. Acta Pharm, 2003; 53:231–7.
Ashford M. Bioavailability—physicochemical and dosage form factors. In: Aulton ME, Taylor KMG (eds.). The design and manufacture of medicine. Churchill Livingstone Elsevier, China, pp. 319–38, 2018.
Attama AA, Adikwu MU, Okoli N. Studies in bioadhesive granules 1: Granules formulated with Prosopis africana gum. Chem Pharm Bull, 2000; 48(5):734–7. CrossRef
Aulton ME. Powder flow. In: Aulton ME, Taylor KMG (eds.). The design and manufacture of medicine. Churchill Livingstone Elsevier, China, pp. 189–200, 2018.
Chavanpatil M, Jain P, Chaudhari S, Shear R, Vavia P. Development of sustained release gastroretentive drug delivery system for ofloxacin: In vitro and in vivo evaluation. Int J Pharm, 2005; 304:178–84. CrossRef
Chen C. Development of antimalarial drugs and their application in China: a historical review. Inf Dis Pov, 2014; 3(9):3–9. CrossRef
Debra P, Nettey H, Miltersen KK, Ayeh-Kumi P, Brock B, Sarkodie JA, Akwo-Kretchy I, Owusu-Danso P, Adjei S, Petersen E, Hardlei TF. Artemether-lumefantrine concentrations in tablets and powders from Ghana measured by a new high-performance liquid chromatography method. Am J Trop Med Hyg, 2016; 95(1):158–63. CrossRef
International Pharmacopoeia. World Health Organization Publication, Geneva, pp. 988–94, 2006.
Laxmi M, Bhardway A, Mehta S, Mehta A. Development and characterization of nanoemulsion of carrier for the enhancement of bioavailability of artemether. Art Cells Nanomed Biotech, 2015; 43:334–44. CrossRef
Ngwuluka NC, Nep EI, Ochekpe NA, Odumosu PO, Olorunfemi PO. Eudagrit 100 and polysaccharide polymer blends as matrices for modified-release drug delivery II: swelling and release studies. Trop J Pharm Res, 2015; 14(12):2163–70. CrossRef
Olorunsola EO. Prosochit: a group of multifunctional pharma excipient. Nigerian Patent 2016/00355, 2017.
Olorunsola EO, Bhatia PG, Tytler BA, Adedokun MO, Adikwu MU. Dissolution and permeation characteristics of artemether tablets formulated with two gums of different surface activity. Trop J Pharm Res, 2017; 16(5):981–8. CrossRef
Pousibet-Puerto J, Sals-Coronas J, Sanchez-Crespo A, Molina-Arrebola MA, Soriano-Perez MJ, Gimenez-Lopez MJ, Vazquez-Villegas J, Cabezas-Fernandez MT. Impact of using artemisinin-based combination therapy (ACT) in the treatment of uncomplicated malaria from Plasmodium falciparum in a non-endemic zone. Malaria J, 2016; 15(339):1–7 doi:10.1186/s12936-016-1408-1. CrossRef
Remington JP, Troy DB, Beringer P. Remington—The Science and Practice of Pharmacy. Lippincott Williams and Wilkins, Philadelphia, PA, 2006.
Ritthidej GC, Chomto P, Pummangura S, Menasveta P. Chitin and chitosan as disintegrants in paracetamol tablets. Drug Dev Ind Pharm, 1994; 20(13):2109–34. CrossRef
Rosenthal PJ. Antiprotozoal drugs. In: Katzung BG (ed.). Basic and clinical pharmacology. The McGraw-Hill Companies Inc., Singapore, pp. 864–85, 2004.
Salih OS, Niff RA. Effect of natural and synthetic polymers on the properties of candesartan cilexetil matrix tablet prepared by dry granulation. Asian J Pharm Clin Res, 2016; 9(3):161–70. CrossRef
Sinha VR, Singla AK, Wadhawan S, Kaushik K, Kumria R, Bansal K, Dhawan S. Chitosan microspheres as a potential carrier for drugs. Int J Pharm, 2004; 274(1–2):1–33. CrossRef
Sonia TA, Sharma CP. Chitosan and its derivatives for drug delivery. Adv Polym Sci, 2011; 243:23–54. CrossRef
Strandgarden K, Hoglund P, Nordle O, Wannman J, Gunnarsson PO. Dissolution rate—limited absorption and complete bioavailability of roquinimex in man. Biopharm Drug Dispos, 1999; 20(7):347–54. CrossRef
Tayade NG, Nagarsenker MS. Development and evaluation of artemether parenteral microemulsion. Ind J Pharm Sci, 2010; 32(5):637–40. CrossRef
United States Pharmacopoeia 31—NF 26. 2008. Available via http://www.uspnf.com (Accessed 12 September 2013).