INTRODUCTION
Curcumin, a highly lipophilic compound commonly obtained from the Curcuma longa L. (turmeric) rhizomes [1], has been widely explored as an interesting candidate to treat central nervous system disorders possibly due to its anti-inflammation, antioxidant, and neurotransmitter modulating properties [2]. It has been reported that curcumin protects neuronal cells in Alzheimer’s disease from beta-amyloid-induced oxidative stress [3]. In in vivo settings, curcumin decreases the invasion of inflammatory cells in the brain by inhibiting differentiation and the development of cell expression [4]. Moreover, at a dose of 100 mg/kg, curcumin possesses antidepressant-like action in mice by increasing the brain’s dopamine, norepinephrine, and serotonin levels, comparable to the oral administrations of fluoxetine and imipramine [5]. In addition, curcumin has proven its safeness in humans, since the long-term administrations (i.e., 18 months) of curcumin doses of as high as 8 g/day do not cause any potential toxicity in clinical trials [6]. However, the medical use of curcumin is restricted due to its low oral bioavailability, correlating to its poor aqueous solubility, high intestinal/hepatic metabolisms, and rapid elimination [7,8]. In addition, a number of studies have reported various factors affecting the decomposition process of curcumin, such as exposure to ultraviolet and visible light, which could be a challenging task for long-term preservation [9]. Therefore, it is important to develop novel oral delivery system for curcumin.
Due to the benefits of lipid-based nanodrug delivery systems over other systems, these systems have been increasingly developed for curcumin delivery [10,11]. The two most potential advantages of these systems are (1) the utilization of biocompatible and biodegradable natural nontoxic lipids as carriers, and (2) the ability to selectively improve the absorption of lipophilic drugs in the gastrointestinal (GI) tract via lymphatic uptake pathway [12]. Among various well-known systems, self-emulsifying drug delivery systems (SEDDS) are attractive approaches to enhancing curcumin water solubility and oral bioavailability [13–15]. SEDDS, commonly classified as self-microemulsifying/-nanoemulsifying drug delivery systems (SMEDDS/SNEDDS), are isotropic combinations of surfactants, oil, and active ingredient that could re-constitute to become an emulsion upon contact with the GI fluid after oral administration. Owing to their tiny particle sizes and high solubilization potential, SEDDS is a technology that is anticipated to improve the lipophilic drug water solubility. These properties allow for enhancing drug permeation through the GI membrane, leading to an increase in drug bioavailability [16,17]. However, as a liquid state, SEDDS may cause the drug to be physicochemical unstable (i.e., precipitation and leakage) at ambient temperature due to the incompatibilities between the gelatinous capsules and the volatile excipients. This disadvantage could be overcome without affecting self-emulsifying properties by preparing these systems in a solid form using an absorbent [18–20].
Therefore, the present research developed curcumin-loaded solid-SEDDS (C-SSEDDS) with improved oral bioavailability. The system physicochemical properties of droplet size, morphology, zeta potential, drug loading capacity, and drug recovery were investigated. The particles in vitro release pro?les in the simulated GI fluids were then examined. In addition, the in vitro cytotoxicity and Caco-2 cell permeability of the systems were also investigated. Finally, the system’s long-term storage stability was determined at ambient temperature (25°C ± 0.5°C).
MATERIALS AND METHODS
Materials
Curcumin (purity of ≥80%) was bought from the Thai-China Flavours and Fragrances Industry (Bangkok, Thailand). The standard curcumin was imported from Sigma–Aldrich (MO, USA). Transcutol® HP (diethylene glycol monoethyl ether) and Labrasol® (PEG-8 caprylic/capric glycerides, LS) were obtained from Gattefosse (Cedex, France). Oleic acid, castor oil, and Lexol® (medium-chain triglyceride) were bought from Namsiang Trading (Bangkok, Thailand). Neusilin®UFL2 was imported from Fuji Chemical Industry Co., Ltd. (Toyama, Japan). All other utilized chemicals were of reagent grade or higher.
Caco-2 cells (HTB-37™) were imported from the American Type Culture Collection (VA, USA). Dulbecco’s modi?ed Eagle’s medium (DMEM F-12), fetal bovine serum, trypsin, antibiotic/antifungal, and other relevant cell culture chemicals were bought from Sigma–Aldrich (MO, USA). The 12-mm-transwell plates were obtained from Corning Costar (NY, USA). The 2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)-2H-Tetrazolium-5-Carboxanilide (XTT) assay kits were bought from Roche Diagnostics Corporation (Thermo Fisher Scientific, Thailand).
Development of curcumin-loaded solid SEDDS
Solubility studies
To evaluate the curcumin solubility, an excess curcumin amount was subjected to 1 ml of several vehicles as indicated in Table 1, magnetically stirred (900 rpm) for 1 hour, and the samples were centrifuged (14,000 rpm, 30 minutes) after equilibrium (Mikro 120 Hettich, Burladingen, Germany). The supernatant was then diluted with an appropriate mobile phase and the curcumin content was quantified by high-performance liquid chromatography (HPLC) (LD10A, Shimadzu, Kyoto, Japan), with a C18 column (Vertisep, 250 × 4.6 × 5 μm), a mobile phase consisted of acetonitrile and acetate buffer (50:50, v/v), a flow rate of 1.2 ml/minute, and an UV detection wavelength of 425 nm. The curcumin concentrations were then calculated based on a calibrated standard curve (y = 123,817.5x + 39,589.1, R2 = 0.9994) with a linear range of 0.1–100 μg/ml.
Pseudo-ternary phase diagram
The pseudo-ternary phase diagrams were systematically observed in the absence of curcumin at ambient temperature (25°C ± 0.5°C) by the water titration method and created utilizing the CHEMIX School software (version 3.51) [13–15]. The surfactant and co-surfactants (Smix) were established in various ratios of 1:0.25, 1:0.5, 1:1, and 2:1 (v/v). The oil and Smix were then thoroughly blended, with the oil and Smix ratios varied from 9:1 to 1:9 (v/v). To establish an equilibrium condition, the distilled water was subjected dropwise to a clear solution with mild agitation. Next, the mixture’s transparency was visually observed and evaluated. Finally, the mixtures were titrated with water until turbidity. The optimal ratios were chosen from samples with isotropic and transparency properties, indicating that they were in the areas of a micro/nanoemulsion.
Preparation of curcumin-loaded liquid SEDDS
The SEDDS at the selected optimal ratios were fabricated for the curcumin incorporation. Curcumin was dissolved in SEDDS and mixed by stirring at 900 rpm to obtain a ?nal curcumin content of 100 mg/ml [13–15].
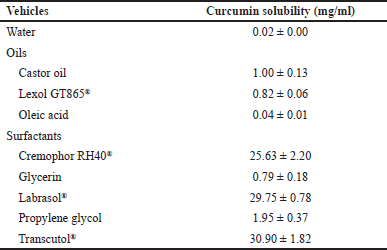 | Table 1. Solubility of curcumin in various vehicles. [Click here to view] |
Preparation of curcumin-loaded solid SEDDS
The C-SEDDS was transformed into its respective powder form, C-SSEDDS, utilizing the simple mixing method. This conversion was achieved through the mortar-and-pestle technique, employing a solid inert carrier, Neusilin®UFL2. The optimized liquid C-SEDDS was mixed with Neusilin®UFL2 at a ratio of 1:1 (w/v), and the final products were placed in glass bottles and subsequently stored in a desiccator for further experiments.
Characterizations of C-SSEDDS
Curcumin loading and recovery
The curcumin loading and recovery of C-SSEDDS formulations were analyzed immediately after preparations to confirm appropriate drug incorporation. Accurate weight of C-SSEDDS, 10 mg, was mixed with methanol, and the curcumin amounts were measured using HPLC as described in the “Solubility studies” section. The curcumin loading/recovery percentages were determined by Equations (1) and (2).
Mean droplet size and polydispersity index
The mean droplet sizes and polydispersity indexes of C-SSEDDS were measured utilizing the photon correlation spectroscopy technique (ZetaPALS® zeta-analyzer, Brookhaven Instrument Corporation, Holtsville, USA). For this, 10 mg of samples were diluted with 15 ml of three different media, namely water, HCl (pH 1.2), and phosphate buffer (pH 6.8). The machine was run at a 90° angle for six measurement cycles.
Morphology
The morphology of the C-SSEDDS formulations was observed by transmission electron microscopy (TEM, Tecnai G TF20, Philips, USA). The samples were diluted with water at the ratio of C-SSEDDS:water at 1:1.5 w/v. Then, the C-SSEDDS solution was dropped onto a film-coated copper grid to generate a thin film, followed by negatively stained with 2% uranyl acetate and air-dried. Finally, TEM micrographs of the samples were photographed.
In vitro dissolution studies
The dissolution studies were conducted with USP apparatus II (Model UDT-804, Logan Instrument Corp., NJ, USA) at 100-rpm paddle speed. A fixed amount of free curcumin (the control) and C-SSEDDS, equivalent to 50 mg curcumin, was weighed and dispersed in the dissolution media composed of 300 ml of HCl (pH 1.2) (simulating the stomach condition) or phosphate buffer solution (pH 6.8) (simulating the small intestine condition) and maintained at 37°C ± 0.5°C in the dark for the entire tests. Samples were withdrawn at 5, 15, 30, and 60 minutes and media refilled. The samples were then ?ltered and analyzed using HPLC as previously described. The emulsification time of micro/nano-emulsions was also visually assessed.
Determination of C-SSEDDS absorption in Caco-2 cells
In vitro cytotoxicity studies
Caco-2 cells (passage number 35–45) were cultured on a 96-well plate at a seeding amount of 1 × 104 cells/well at 37°C, 5% CO2, and 95% humidity. Then, the C-SSEDDS, equivalent to the curcumin concentrations of 5–25 μg/ml, were dispersed in a serum-free medium and subjected to each separate well, followed by a 4-hour incubation. After that, the medium was withdrawn and XTT solution (200 μl) was put into each well and incubated for another 4 hours. Finally, the cell viability was quanti?ed at a measuring wavelength of 490 nm employing the microplate reader (DTX880, Beckmancoulter, CA). The control, representing 100% viability, was the cell-free culture medium. The cell viability (%) was determined by the following Equation (3).
Curcumin transport across Caco-2 monolayers
For the in vitro permeation studies of C-SSEDDS, Caco-2 cells (1 × 105 cells/well) were cultured on the apical side of the Transwell® plate (1.12 cm2 surface area, 12 wells/plate), and incubated at 37°C ± 0.5°C and 5% CO2 for the entire experiments. Cells were cultured for 21 days with media changes every even day. The formation and integrity of Caco-2 monolayers and tight junctions were checked by transepithelial electrical resistance (TEER) with a volt–ohm meter (Millicell® RERS-2, Millipore Corporation, MA). Monolayers with TEER below 400 Ω?cm2 were discarded. After cell differentiation, the apical compartment solution was replaced with 500 μl of the serum-free medium containing C-SSEDDS (equivalent to 7.5 μg curcumin), and the basolateral compartment solution was replaced with a serum-free medium. A sample volume of 0.5 μl was taken from the basolateral side at 0.5, 1, 2, 3, and 4 hours, and fresh medium was refilled. The curcumin amount in the apical side and in the cell monolayer was determined after 4 hours. The apical and basolateral samples were concentrated by a vacuum concentrator (Labconco Corporation, Missouri, USA) at 45°C for 1 hour, and the solid mass was re-dissolved in 200 μl methanol before injection into HPLC for quantification of curcumin. The cell monolayers were washed thrice with 0.5 ml of phosphate buffer saline (PBS) (pH 7.4), dispersed in 0.5 ml of acetonitrile, and mixed with methanol. The samples were then concentrated, re-dissolved, and subjected to HPLC as described above.
The curcumin transport from the apical side to the basolateral side was shown as permeation amount in the basolateral compartment against time. The apparent permeability coef?cient (Papp, cm/second) of curcumin was determined based on Equation (4).
Papp = (dQ/dt)/(C0 × A) (4)
dQ/dt is the curve slope (mg/hour), C0 is the initial curcumin concentration on the apical side (15 μg/ml), and A is the diffusion area (1.12 cm2).
Stability of C-SSEDDS
The curcumin-loaded solid self-microemulsifying drug delivery system (C-SSMEDDS) and curcumin-loaded solid self-nanoemulsifying drug delivery system (C-SSNEDDS) were placed in glass bottles, stored in a desiccator, and kept in the dark for 1 year at ambient temperature (25°C ± 0.5°C). After each time interval of 3, 6, and 12 months, the physical appearance, self-emulsifying time, and droplet size were evaluated. Using HPLC, as indicated above, the curcumin remaining percentages were determined.
Statistical analysis
The results were demonstrated as mean ± standard deviation (SD) from at least three separate measurements. For statistical comparisons, one-way analysis of variance and Tukey’s post hoc tests were utilized, with significant differences set at p < 0.05.
RESULTS AND DISCUSSIONS
SEDDS are isotropic combinations of surfactants/co-surfactants and oils that could emulsify spontaneously and form homogenous oil-in-water emulsions when being contacted with an aqueous phase with gentle agitation by gastric mobility [16]. In terms of formulation factors, oil is the most crucial component because it can dissolve lipophilic drugs or facilitate self-emulsification at a certain dose. It also enhances the transportation of these lipophilic drugs via the intestinal lymphatic route, thus improving drug absorption from the GI system [17]. Furthermore, they help increase the microemulsion thermodynamic stability by reducing the interfacial energy via absorption at the interface. After C-SSEDDS are diluted in an aqueous solution, they immediately generate oil-in-water particles that rapidly spread in the medium. The most commonly utilized surfactants are the nonionic molecules with a reasonably high hydrophilic–lipophilic balance (HLB), as they are less toxic and typically have a low critical micelle concentration [16].
Development of curcumin-loaded solid SEDDS
Solubility studies
SEDDS is a potential strategy to increase the water solubility of curcumin, a lipophilic drug with an aqueous solubility of ~0.02 mg/ml. Thus, the types of oils and surfactants were chosen based on the curcumin solubility in each vehicle. The castor oil and Lexol® showed higher curcumin solubility compared to that of the oleic acid, and therefore, were selected as the oily vehicle. Regarding the surfactants, nonionic surfactants of Cremophor RH40® (HLB ~12–14), Labrasol® (HLB ~14), and Transcutol® (HLB ~4) yielded good curcumin solubility (~25–30 mg/ml) (Table 1).
Pseudo-ternary phase diagrams
To identify the micro/nanoemulsion area, pseudo-ternary phase diagrams were constructed in the absence of curcumin based on the results of solubility tests. Consequently, the optimal oil: surfactant: co-surfactant ratio was estimated.
Castor oil and Lexol® were used as oil in these systems. The phase diagrams were built for the 03 systems of (1) Labrasol® and Transcutol®, (2) Labrasol® and Cremophor RH40®, and (3) Transcutol® and Cremophor RH40®, which represented as Smix (LT), Smix (LC), and Smix (TC), correspondingly. The maximum region of SEDDS was obtained with Smix in the ratio of 1:1. Then, the effect of Smix on phase diagrams was further investigated. Regardless of the oil types, the phase diagrams of the system with Smix (LT) were turbid, indicating unstable systems, and hence, were not employed. The phase diagrams of Lexol® and Smix (TC) at the ratio of 1:9 showed the largest region of SNEDDS (Fig. 1a), similar to the phase diagram of Lexol® and Smix (LC) at the ratio of 2:8 (Fig. 1b). In addition, the phase diagram of castor oil and Smix (TC) at the ratio of 1:9 showed a large region of SMEDDS (Fig. 1c), similarly to the phase diagrams of Lexol® and Smix (LC) at the ratio of 1:9 (Fig. 1b). Based on the results, 04 formulations of SEDDS were used for further experiments.
The phase diagrams also indicated that the micro/nanoemulsion area was achieved when using Transcutol® or Labrasol® with Cremophor RH40® due to the effect of the longer hydrocarbon chain length of Cremophor RH40® (C57) compared to Transcutol® (C6) and Labrasol® (C8–C10), which leads to increased oil dissolution and nano-size droplets [16,21].
Preparation of curcumin-loaded liquid SEDDS
As previously discussed, 04 SEDDS formulations were chosen to fabricate C-SEDDS. The effect of Smix on curcumin solubility was investigated by dissolving curcumin in 1 ml of oil: Smix mixture at the ratio of 1:9. Regardless of the oil types, the SEDDS formulation with Smix (TC) exhibited curcumin solubility of 100 mg/ml. On the contrary, the SEDDS formulation of Lexol® and Smix (LC) showed curcumin solubility of 70 mg/ml. Conclusively, 02 formulations composed of castor oil with Smix (TC), and Lexol® with Smix (TC) at the ratio of 1:9 were classified as C-SMEDDS and C-SNEDDS, respectively.
Transcutol®, Labrasol®, and Cremophor RH40® are nonionic surfactants with HLB values of 4, 14, and ~12–14, respectively [16]. The fact that curcumin possesses higher solubility in Smix (TC), a lipophilic–hydrophilic surfactant mixture, than in Smix (LC), a hydrophilic–hydrophilic surfactant mixture, could be attributed to the lipophilic surfactants that dissolve curcumin more efficiently than hydrophilic ones [22].
Unfortunately, we found that after 1-month storage, the C-SEDDS showed only ~70% of the curcumin remaining. This discouraging effect might be due to Cremophor RH40®, which possesses conjugated unsaturated bonding that is strongly vulnerable to oxidation, leading to the peroxyl radicals formation, and resulting in curcumin degradation [23,24].
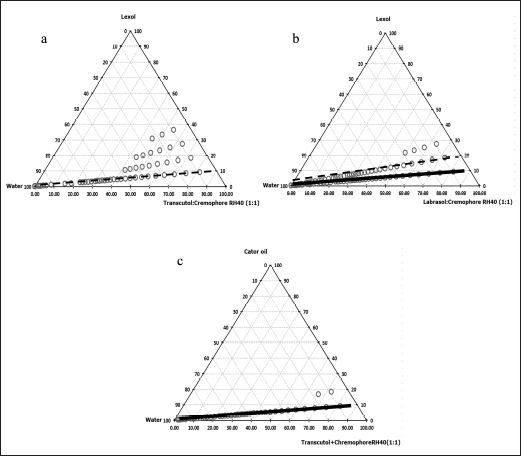 | Figure 1. Pseudo-ternary phase diagrams of SEDDS formulations. (a) lexol: Smix (Transcutol® and Cremophor RH40®), (b) lexol: Smix (Labrasol® and Cremophor RH40®), and (c) castor oil: Smix (Transcutol® and Cremophor RH40®). The black line ( ) represents SMEDDS and the black dash line (-------) represents SNEDDS. [Click here to view] |
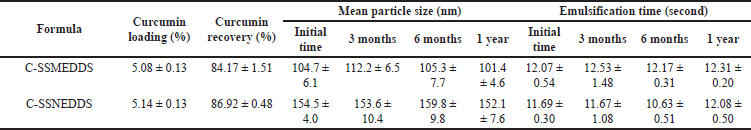 | Table 2. Physicochemical characterizations of C-SSMEDDS and C-SSNEDDS (n = 3). [Click here to view] |
Preparation of curcumin-loaded solid SEDDS
To increase the chemical stability of curcumin during storage, C-SMEDDS and C-SNEDDS were transformed into solid C-SMEDDS (C-SSMEDDS) and solid C-SNEDDS (C-SSNEDDS) using Neusilin®UFL2 as an adsorbent. All C-SSEDDS, prepared at the C-SEDDS:Neusilin®UFL2 ratio of 1:1 (w/w), demonstrated fine powder with a free-flowing property. Curcumin loading was ~5% for each C-SSEDDS formulation, whereas curcumin recovery was >80%, indicating that this technique was suitable for preparing C-SSEDDS (Table 2).
Mean droplet size, morphology, and emulsification time
To improve oral bioavailability, the nano-size of drug delivery carriers is one crucial property [10]. In this study, deionized water, HCl (pH 1.2), and PBS (pH 6.8) were used as a dispersion medium. In all tested media, C-SMEDDS and C-SNEDDS showed similar droplet sizes, ~100 and ~150 nm, respectively. SMEDDS are formulations that result in the production of transparent microemulsions with droplet sizes of 1–100 nm. Whereas, SNEDDS form emulsions with sizes ranging from 100 to 300 nm [16]. Thus, the present particle sizes fall within the parameters of typical SEDDS. In addition, the C-SSMEDDS and C-SSNEDDS prepared with Neusilin®UFL2 gave mean droplet sizes similar to those of C-SEDDS in the liquid form (Table 2). The polydispersity indexes were in the range of 0.175–0.280, indicating a narrow size distribution. This result confirmed that the droplet size remained stable, without aggregation, in the GI fluid. Furthermore, regardless of the formulation factors, TEM micrographs of C-SSMEDDS and C-SSNEDDS showed spherical micelles with an average size of ~100 and ~150 nm, respectively (Fig. 2), which was consistent with data obtained by photon correlation spectroscopy technique
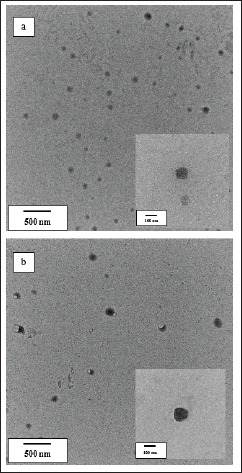 | Figure 2. TEM micrographs of (a) C-SSMEDDS and (b) C-SSNEDDS after dilution with water. [Click here to view] |
In vitro dissolution studies
Before the dissolution tests, the emulsification time was assessed as an important factor for evaluating the self-emulsification efficiency. Upon mixing with all mediums, C-SSMEDDS and C-SSNEDDS demonstrated instant self-emulsification with an emulsification time of 10–15 seconds (Table 2) without phase separation or aggregation.
The in vitro release patterns of C-SSMEDDS and C-SSNEDDS in pH 1.2 and 6.8 are demonstrated in Figure 3a and b, respectively. Expectedly, the free curcumin powder could not be quantified by HPLC in both mediums because of its low solubility and dissolution. In contrast, SSEDDS could significantly improve the dissolution rates of curcumin. Specifically, curcumin was burstly released from C-SSMEDDS and C-SSNEDDS at pH 1.2, with % release of ~70% and ~60% within 5 minutes, respectively. Similarly, in pH 6.8, within 5 minutes, ~60% and ~70% of curcumin were released for C-SSMEDDS and C-SSNEDDS, respectively. Since the dissolution process governs the subsequent availability of the drug for absorption within the body, a rapid curcumin release could enhance its absorption and, ultimately, its oral bioavailability.
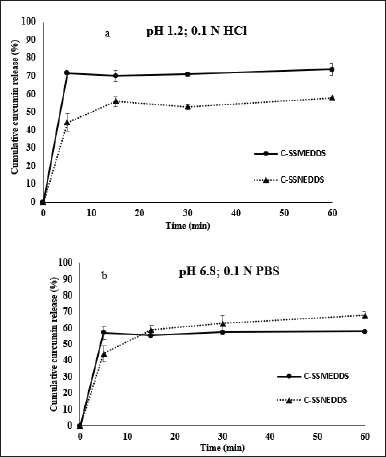 | Figure 3. Cumulative release percentages of curcumin from C-SSMEDDS and C-SSNEDDS in (a) HCl (pH 1.2) and (b) phosphate buffer solution (pH 6.8). Each value represents the mean ± SD (n = 3). [Click here to view] |
Rapid drug dissolution from the C-SSEDDS could be attributed to the nano-carriers obtained by means of rapid self-emulsification through establishing an interface between the dissolution medium and the oil [25]. The self-emulsi?cation efficiency of (co)-surfactants is closely associated with their respective HLB values. Cremophor RH40® (HLB 12–14) is generally considered as a surfactant with good self-emulsi?cation ef?ciency [26]. However, the released drug amount was <100% due to the drug-Neusilin®UFL2 complex formation, possibly via hydrogen bonding [27]. Neusilin®UFL2, an amorphous powder of synthetic magnesium aluminosilicate, has a mean particle size of approximately 2.94 μm, an average pore size of about 17 nm (high porosity), a pore volume of 1.37 cm3/g, and a specific surface area of 300 m2/g. Consequently, it offers great adsorption capacity [28].
Determination of C-SSMEDDS and C-SSNEDDS absorption in Caco-2 cells
The potential cytotoxicity of C-SSMEDDS and C-SSNEDDS on differentiated Caco-2 cells was investigated using the XTT assay before permeation studies. In accordance with the International Organization for Standardization (ISO 10993-5) (ISO 2009), a cell viability of more than 70% is seen as nontoxic. At dosages of 25 μg/ml curcumin, the viability of cells decreased to 50%, suggesting toxicity at this high concentration. On the other hand, all formulations with low curcumin amounts (5, 10, and 15 μg/ml) showed cell viabilities of >70%, demonstrating no potential cytotoxicity. Thus, the amounts of C-SSMEDDS and C-SSNEDDS, equivalent to 15 μg/ml curcumin, were selected for the permeation experiments.
 | Table 3. The percentages of curcumin in the apical side, basolateral side, and cell monolayer; and the apparent permeability coef?cients across Caco-2 monolayer after 4-hour of incubation (n = 3). [Click here to view] |
 | Table 4. The TEER value of the Caco-2 cells monolayer before and after in vitro permeation studies (n = 3). [Click here to view] |
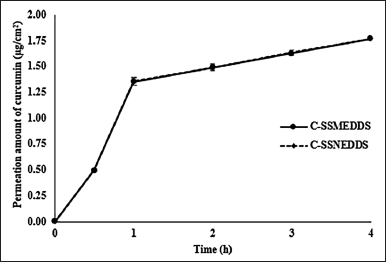 | Figure 4. Permeation amounts of curcumin across Caco-2 cells monolayer. Each value represents means ± SD (n = 3). [Click here to view] |
Curcumin is not a P-glycoprotein (P-gp) efflux transporter substrate because it has the same Papp value in both directions, from the apical to the basolateral side and from the basolateral to the apical side. Therefore, only curcumin transported from the apical to the basolateral side was performed. After 4-hour incubation, the free curcumin dispersion showed ~24% curcumin recovery (Table 3), which was found only in the apical side, while curcumin could not be detected in the Caco-2 cell monolayer and basolateral compartment, indicating restricted curcumin absorption. This is due to the curcumin’s rapid hydrolytic degradation at neutral conditions [29]. On the contrary, the curcumin distribution of C-SSMEDDS and C-SSNEDDS in the apical side, the basolateral side, and the cell monolayer showed no significant differences with ~100% curcumin recovery (Table 3). Moreover, Figure 4 shows that both formulations could help curcumin permeate through the cell monolayer as soon as 30 minutes after incubation, and showed linear kinetics up to 4 hours.
The corresponding Papp values were determined based on the curcumin’s ability to cross the cell monolayers. The C-SSMEDDS and C-SSNEDDS showed no significant difference in Papp value (Table 3). The associations between the permeability through the Caco-2 monolayer and the absorbed fraction in humans have been evaluated in many studies [30,31]. A compound with Papp of <1 × 10−6, 1–10 × 10−6, and >10 × 10−6 cm/second is considered as in vivo low absorption (0%–20%), moderate absorption (20%–70%), and high absorption (70%–100%), correspondingly [31]. Thus, according to the Papp value, C-SSMEDDS and C-SSNEDDS were classified as moderate permeability systems, ~5.11 × 10−6 cm/second (Table 3).
It is worth noting that the cell monolayer TEER values after 4 hours of incubation was decreased from ~400 to ~340 Ω?cm2 (Table 4). This is due to the effects of surfactants that disrupt the cell membrane [32]. Nonetheless, the Caco-2 monolayer integrity has been stated with TEER values of 150–400 Ω?cm2 [33]. Therefore, the current study TEER values indicated that the monolayer integrity was sustained throughout the in vitro permeability experiments.
Overall, the results clearly showed that the degradation of curcumin in the cell culture medium (pH 7.4) could be hindered by the C-SSMEDDS and C-SSNEDDS formulations. In addition, SSEDDS could significantly enhance the curcumin absorption efficiency because of several advantages of these systems. First, SSEDDS increase the curcumin solubility. Based on Fick’s first law, the compound permeation rate is generally influenced by the surface areas and the drug concentrations in the intestinal lumen. An increase in the drug concentrations at the epithelial cell surfaces leads to a higher drug absorption by the transcellular pathway [34]. In addition, nanoparticles with sizes of <200 nm could be directly taken up by cells via endocytosis [35]. Third, surfactant, in SSEDDS, could transiently disrupt cell membrane integrity, which facilitates curcumin penetration through the cell membranes via transcellular transport [32]. Finally, the lipid core of the nanoparticles could stimulate the formation of chylomicrons and facilitate uptake into the lymph, bypassing hepatic first-pass metabolism [36].
Stability of C-SSEDDS
After 1-year storage at ambient temperature (25°C ± 0.5°C) and without sunlight, the physicochemical properties of both C-SSEDDS were maintained. Both samples remained in the powder form with no physical changes. Upon dilution with water, a rapid self-emulsification time of 12 seconds was observed and the droplet sizes of C-SSMEDDS and C-SNEDDS were ~100 and ~150 nm, respectively, similar to their freshly prepared counterparts (Table 2). In addition, compared to the freshly prepared formulations, no significant differences (p > 0.05) were noted in the percentages of remaining curcumin, which was ~100%. Thus, the finding indicated that C-SSEDDS demonstrated physical and chemical stability for at least 1 year at ambient temperature.
CONCLUSION
This study demonstrated the possibility of C-SSMEDDS and C-SSNEDDS as promising delivery systems for curcumin. These nano-carrier systems could improve curcumin oral bioavailability by encapsulating and protecting curcumin degradation in the GI tract, and enhancing curcumin dissolution and absorption through intestinal monolayer. Upon dilution in water, the obtained C-SSMEDDS and C-SSNEDDS demonstrated fast and complete self-emulsification within 15 seconds, and possessed mean droplet sizes of ~100 and ~150 nm, respectively, with a narrow size distribution. Furthermore, the droplet sizes of C-SSMEDDS and C-SSNEDDS were not affected by the pH variation in the GI tract. Curcumin dissolved and released completely in dissolution media within 15 minutes. In addition, the developed formulations showed a greater curcumin absorption in the Caco-2 cell monolayer than the free curcumin, suggesting an improvement in the curcumin oral bioavailability. Nevertheless, these assumptions could be further investigated in animal studies to confirm safety and efficacy, followed by a full clinical evaluation, to facilitate the commercialization of these systems.
ACKNOWLEDGMENTS
The authors would like to sincerely acknowledge the Faculty of Pharmaceutical Sciences, Naresuan University, for supporting research facilities. The authors also wish to thank Mr. Roy I. Morien of the Naresuan University Graduate School for his efforts in editing and checking the English grammar and expression in this paper.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research work was financially assisted by the Agricultural Research Development Agency, Thailand, the Center of Excellence for Innovation in Chemistry (PERCH-CIC), the Commission on Higher Education, and the Ministry of Education, Thailand.
CONFLICTS OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019 Aug 13;24(16):2930.
2. Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005 Apr;2(2):131–6.
3. Kulkarni S, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. ScientificWorldJournal. 2009 Nov 1;9:1233–41.
4. Xie L, Li XK, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, et al. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol. 2009 May;9(5):575–81.
5. Sanmukhani J, Anovadiya A, Tripathi CB. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: an acute and chronic study. Acta Pol Pharm. 2011 Sep–Oct;68(5):769–75.
6. Kurien BT, D’Souza A, Scofield RH. Heat-solubilized curry spice curcumin inhibits antibody-antigen interaction in in vitro studies: a possible therapy to alleviate autoimmune disorders. Mol Nutr Food Res. 2010 Aug;54(8):1202–9.
7. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007 Nov–Dec;4(6):807–18.
8. Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z Lebensm Unters Forsch. 1985 May;180(5):402–4.
9. Tønnesen HH, Másson M, Loftsson T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int J Pharm. 2002 Sep 5;244(1–2):127–35.
10. Sadegh Malvajerd S, Azadi A, Izadi Z, Kurd M, Dara T, Dibaei M, et al. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: preparation, optimization, and pharmacokinetic evaluation. ACS Chem Neurosci. 2019 Jan 16;10(1):728–9.
11. Karimi N, Ghanbarzadeh B, Hamishehkar H, Mehramuz B, Kafil HS. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloid Interface Sci Commun. 2018 Jan 1;22:18–24.
12. Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 2017 Dec 1;6:37–56.
13. Sermkaew N, Ketjinda W, Boonme P, Phadoongsombut N, Wiwattanapatapee R. Liquid and solid self-microemulsifying drug delivery systems for improving the oral bioavailability of andrographolide from a crude extract of Andrographis paniculata. Eur J Pharm Sci. 2013 Nov 20;50(3–4):459–66.
14. Kang BK, Lee JS, Chon SK, Jeong SY, Yuk SH, Khang G, et al. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004 Apr 15;274(1–2):65–73.
15. Zhang P, Liu Y, Feng N, Xu J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int J Pharm. 2008 May 1;355(1–2):269–76.
16. Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004 Apr;58(3):173–82.
17. Chatterjee B, Hamed Almurisi S, Ahmed Mahdi Dukhan A, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016 Nov;23(9):3639–52.
18. Tuleu C, Newton M, Rose J, Euler D, Saklatvala R, Clarke A, et al. Comparative bioavailability study in dogs of a self-emulsifying formulation of progesterone presented in a pellet and liquid form compared with an aqueous suspension of progesterone. J Pharm Sci. 2004 Jun;93(6):1495–502.
19. Franceschinis E, Voinovich D, Grassi M, Perissutti B, Filipovic-Grcic J, Martinac A, et al. Self-emulsifying pellets prepared by wet granulation in high-shear mixer: influence of formulation variables and preliminary study on the in vitro absorption. Int J Pharm. 2005 Mar 3;291(1–2):87–97.
20. Beg S, Jena SS, Patra CN, Rizwan M, Swain S, Sruti J, et al. Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloids Surf B Biointerfaces. 2013 Jan 1;101:414–23.
21. Acharya B, Guru PS, Dash S. Tween-80–n-butanol–diesel–water microemulsion system—a class of alternative diesel fuel. J Dispers Sci Technol. 2014 Oct 3;35(10):1492–501.
22. Bhandari S, Rana V, Tiwary AK. Antimalarial solid self-emulsifying system for oral use: in vitro investigation. Ther Deliv. 2017 Apr;8(4):201–13.
23. Kharat M, Zhang G, McClements DJ. Stability of curcumin in oil-in-water emulsions: impact of emulsifier type and concentration on chemical degradation. Food Res Int. 2018 Sep 1;111:178–86.
24. Sinha Babu SP, Sarkar D, Ghosh NK, Saha A, Sukul NC, Bhattacharya S. Enhancement of membrane damage by saponins isolated from Acacia auriculiformis. Jpn J Pharmacol. 1997 Dec;75(4):451–4.
25. Craig DQ, Barker SA, Banning D, Booth SW. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm. 1995 Jan 31;114(1):103–10.
26. Constantinides PP, Lancaster CM, Marcello J, Chiossone DC, Orner D, Hidalgo I, et al. Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J Controlled Release. 1995 May 1;34(2):109–16.
27. Gupta MK, Vanwert A, Bogner RH. Formation of physically stable amorphous drugs by milling with Neusilin. J Pharm Sci. 2003 Mar 1;92(3):536–51.
28. Fuji Chemical Industries. Neusilin®. [cited 2020 Apr 28]. Available from: http://www.neusilin.com/product/pharmacopoeia.php
29. Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, et al. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997 Aug 1;15(12):1867–76.
30. Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun. 1991 Mar 29;175(3):880–5.
31. Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm Res. 1997 Jun;14(6):763–6.
32. Maher S, Heade J, McCartney F, Waters S, Bleiel SB, Brayden DJ. Effects of surfactant-based permeation enhancers on mannitol permeability, histology, and electrogenic ion transport responses in excised rat colonic mucosae. Int J Pharm. 2018 Mar 25;539(1–2):11–22.
33. Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015 Apr;20(2):107–26.
34. Dahan A, Lennernäs H, Amidon GL. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol Pharm. 2012 Jun 4;9(6):1847–51.
35. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001 Apr 25;47(2–3):165–96.
36. Aji Alex MR, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011 Jan 18;42(1–2):11–8.