INTRODUCTION
Orodispersible films (ODFs) are single or multilayer stamp-sized polymeric thin films that disintegrate or dissolve quickly in the mouth [1]. ODFs are typically made up of hydrophilic synthetic or semi-synthetic polymers such as hydroxypropyl methylcellulose (HPMC), polyvinyl pyrrolidone, and polyvinyl alcohol (PVA) [2]. Currently, natural polymers are gaining considerable attention in the field of ODFs due to their advantageous properties, such as non-toxicity, non-irritation, biocompatibility, biodegradability, and adhesiveness. These inherent characteristics make natural polymers highly promising for various applications in the field of ODFs at present.
Starch made up of amylose and amylopectin has a good film-forming ability and can form biodegradable films with adequate mucosal adhesiveness [3]. However, natural starch is semi-crystallinity, and it may exhibit undesirable properties such as low solubility or poor mechanical strength when used to prepare films. The corn starch combined with HPMC or PVA, the poor film-forming ability of corn starch could be improved to prepare rizatriptan benzoate-loaded films [4]. The two new starches (Bambara nut and yam bean) were blended with an established polymer, HPMC, and were found to have good film-forming properties and good drug release to create amlodipine besylate-loaded ODFs, but the composite films had poor flexibility and disintegrated after 15 minutes [5]. Despite numerous research efforts, the semi-crystalline nature of natural starch remains a significant challenge for formulation scientists, resulting in unsatisfactory ODF formulation design.
Starch can undergo chemical, enzymatic, or physical modifications to enhance its physicochemical properties and functionalities. For instance, the alcohol-alkaline and planetary ball-milling techniques were employed to modify rice starch, demonstrating modified starch with favorable film-forming abilities and a reduced disintegration time. However, the modified starch had poor mechanical properties and had to be combined with other polymers to compensate for a single material’s shortcomings [6]. A novel adhesive film for the delivery of gambier leaf extract for the treatment of periodontitis, and it was discovered that increasing the chitosan/modified tapioca starch ratio increased the composite film’s tensile strength (TS) and elongation ability [7]. By introducing a hydroxypropyl group into the molecular structure of pea starch, LYCOAT® RS 720 was prepared. LYCATAB® DSH is obtained through controlled hydrolysis of corn starch, while Starch 1500® is a partially pre-gelatinized maize starch. Water-soluble starch (WSS) with robust hydration properties can be derived from a low-degree hydrolyzed starch product and starch sugar. Although some research has been conducted on applying modified starch to ODFs, the relationship between the type and ratio of modified starch and the film-forming properties has not been thoroughly investigated.
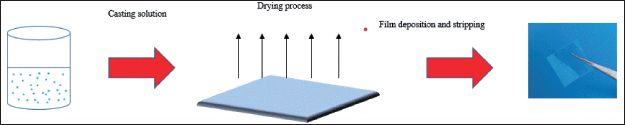 | Figure 1. Schematic diagram of the preparation method of ODFs using solvent casting method. [Click here to view] |
As previously discussed, starch has the advantage of being a non-toxic, non-stimulant, and biodegradable material; thus, a systematic study of modified starch as a matrix material to prepare ODFs is required, as is a further exploration of the potential application of modified starch in ODFs. ODFs were made by combining LYCOAT® RS 720, LYCATAB® DSH, Starch 1500®, and WSS with PVA or pullulan (PU) to prepare ODFs by solvent casting. The effects of polymer type and ratio on ODFs formulation properties were studied, and PVA or PU is used to improve the mechanical properties of modified starch films.
Montelukast sodium is used to treat asthma and allergic rhinitis, but tablets must disintegrate in the stomach to release the drug, making them slow to work and inconvenient to use. Montelukast sodium was selected as active pharmaceutical ingredient (API) to prepare the drug-loaded films. This selection validates the industrial feasibility of utilizing modified-starch polymer composite films and serves as a basis for designing modified starch formulations within the field of ODFs.
MATERIALS AND METHODS
Montelukast sodium was obtained from Hubei Hongyuan Pharmaceutical Group Co., Ltd. (China). LYCOAT® RS 720 (LYCOAT®), LYCATAB® DSH (LYCATAB®), Starch 1500®, WSS, PVA, and PU were obtained from Beijing Fengli Jingqiu Pharmaceutical Co., Ltd. (China), Colorcon (China), Anhui Shanhe Pharmaceutical Excipients Co., Ltd. (China), Jiangxi Alpha Pharmaceutical Group Co., Ltd. (China), Shandong Freda Biotechnology Co., Ltd. (China), respectively. Glycerol (Gl), polyethylene glycol (PEG 400), propylene glycol (PG), sodium dodecyl sulfate (SDS), and sodium phosphate monobasic were purchased from Tianjin Yongda Chemistry Co., Ltd. (China); sucralose, amaranth, sodium hydroxide, acetonitrile, methanol, trifluoroacetic acid were purchased from Ronpharm Technology Co., Ltd. (China), Shandong Qilu Pharmaceutical Group Co., Ltd. (China), Tianjin Hengxing Chemical Reagent Group Co., Ltd. (China). Fisher (USA), and Zhenzhou Weier Biotechnology Co., Ltd. (China), respectively. All other materials used were of analytical grade.
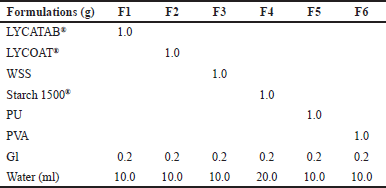 | Table 1. ODFs formulations with a single polymer. [Click here to view] |
Preparation of the blank and drug-loaded ODFs
To prepare ODFs by solvent casting (Fig. 1), LYCATAB®, LYCOAT®, WSS, and Starch 1500® were blended with PVA or PU according to the formulation tables (Tables 1–3). On a hotplate magnetic stirrer, accurately weighed PVA was dissolved in distilled water at 65ºC, and Starch 1500® was dissolved in distilled water at 90ºC (85–2, Zenithlab, China). LYCATAB®, LYCOAT®, WSS, and PU were individually dissolved in distilled water at room temperature. The resulting polymer solutions were combined and stirred for 1 hour. Gl was added to the polymer solution as a plasticizer, and subsequently, the solution was placed in an ultrasonic bath (KQ-500E, Supmile, China) for 30 minutes to eliminate any bubbles. The prepared solutions were cast onto a 10 × 18 cm glass plate with a strip liner and kept in a 25ºC oven (DHG-9070A, Yiheng, China) for 12 hours. The prepared films were cut into 2 × 2 (4 cm2) pieces and stored in a desiccator [8].
After conducting the optimization of the blank formulation, LYCATAB®/PVA (1:4) was chosen as the preferred composite film matrix. Further optimization was performed to determine the appropriate type and quantity of plasticizer and the optimal amount of the API. Excipients were accurately weighed according to Table 4. The polymer solutions were prepared using the same method as described above, different kinds (PG, PEG 400, Gl) and amounts (0.1, 0.2, 0.3 g) of plasticizers were added to the polymer solution (F31–F34), Montelukast sodium was mixed into the polymer solution at a ratio (API/polymer) of 1:2 and 1:5 (N1, N2). The blend solution was cast on a glass plate with a peeling liner and dried at 25ºC for 12 hours.
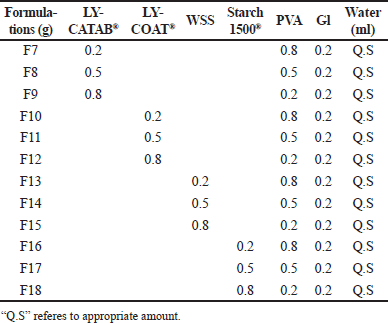 | Table 2. ODFs formulations of modified starch with PVA. [Click here to view] |
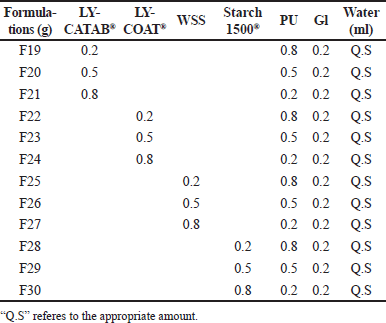 | Table 3. ODFs formulations of modified starch with PU. [Click here to view |
Characterization of ODFs
Appearance
The appearance of prepared ODFs was checked by visual observation [9].
Weight and thickness
The weight of three composite film samples was measured individually, and the average SD was determined. The thickness of four corners and the center points of each ODFs (n = 3) was measured using a micrometer, and the average SD of thickness was calculated [10].
Folding endurance
The folding endurance is a measurement of the integrity and strength of the film. The films (2 × 2 cm) were manually folded 180° repeatedly in the same manner until they broke or folded up, and the average folding number and SD were calculated (n = 3) [5].
Moisture content
The ODFs that were prepared were weighed and placed in a desiccator. After a period of 3 days, the weight of the film remained constant. The films were weighed again to calculate the mean and SD (n = 6) [11]. The moisture content was calculated using the following equation:
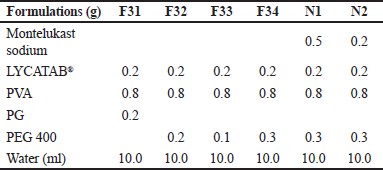 | Table 4. ODFs’ formulations of plasticizer screening and drug-loaded films. [Click here to view] |
pH of composite films
The prepared films were dissolved in 5 ml of water. The pH was determined using a pH meter (PHS-W, Shanghai Lida Instrument Factory, China). The pH value of each film (n = 3) was recorded, and the mean and SD were calculated [5].
Disintegration time
Films were placed in a Petri dish with 5 ml distilled water (37ºC ± 0.5ºC) and shaken gently. The time taken for the films to disintegrate was recorded, and subsequently, the mean and SD of the disintegration time (n = 3) were calculated [12].
Mechanical properties
The mechanical properties of each film were measured using Texture Analyzer (HD.plus, Stable Micro Systems, China). The films (30 × 4 mm) were held between two clamps, and the upper clamp was pulled at a speed of 0.2 mm/second until the films were broken. TS and Elongation at break (%E) were measured according to the following equations, the mean and SD (n = 3) were calculated [9,13].
Scanning electron microscope (SEM)
The microstructure of films was analyzed using a microscope (Gemini 30, ZEISS, Germany). The prepared films underwent gold plating to examine their surface and cross-section. An accelerating voltage of 3 kV was utilized, and the surface was magnified at a 1,000× magnification, while the cross-section was magnified at a 3,000× magnification.
Fourier transform infrared spectroscopy (FTIR)
The prepared films were scanned by infrared spectrum (NICOLET iS10, Thermo Fisher, USA) with a resolution of 2 cm−1, and the wave number region was 400–4,000 cm−1.
X-ray diffraction
An X-ray powder diffractometer (D8 Advance, Bruker, Germany) was employed to study the crystallinity. At a scanning rate of 10°/minute and a wavelength of 15.4 nm, the angular range was 3° to 60°.
Dissolution study
The dissolution of the montelukast sodium ODFs and montelukast sodium chewable tablets (Singulair®, 4 mg, MSD) was performed at 37ºC ± 0.5ºC (USP II apparatus, RC-6, Tianjin Guoming Medical Equipment, China). 900 ml water, 0.1 M HCl, and phosphate buffer solutions (pH 6.8) with 0.5% SDS were used as dissolution media, and the films were secured at the bottom of the dissolution vessel with paper clips.
At specified time intervals of 1, 3, 5, 10, 15, and 30 minutes; samples were obtained and filtered through a 0.22 μm Nylon 66 filter. The analysis of the samples was conducted using the high-performance liquid chromatography (HPLC) method.
The HPLC method was performed using an Eclipse XDB-C18 (4.6 mm, 150 mm, 5 μm) column at a 1.0 ml/minute flow rate. The column temperature was 30ºC, using 0.15% aqueous trifluoroacetic acid (V/V) and 0.15% acetonitrile solution (V/V) (40:60) as mobile phase. The sample size was 10 μl, and the detection wavelength was 398 nm.
The drug quantification was performed using the aforementioned method by constructing a calibration curve. The obtained results demonstrated a linear equation of y = 15.012x–9.4651, with a correlation coefficient R2 = 0.9995. The linear relationship between the area of the main peak and the concentration of Montelukast sodium was found to be excellent, within the range of 1.0 to 15.0 μg/ml.
RESULTS
ODFs prepared with one type of polymer
ODFs should ideally disintegrate or dissolve quickly in the mouth while retaining sufficient mechanical strength for handling and storage. Based on the data presented in Table 5, it can be observed that ODFs formulated solely with a single type of modified starch exhibited limitations in simultaneously achieving desirable characteristics such as favorable appearance, adequate mechanical strength, rapid disintegration time, and good flexibility. LYCOAT®, LYCATAB®, and WSS films were too brittle to peel off, while Starch 1500® films ripped easily but remained white. PVA films were transparent and flexible but easily stretched during molding, whereas PU films were hygroscopic and took a long time to dry.
ODFs prepared with modified starch and PVA
ODFs containing only one type of modified starch frequently lack the balanced properties needed to successfully deliver APIs, such as mechanical strength, disintegration time, and flexibility. It is possible to incorporate two or more polymers along with the modified starch to address the limitation of using a single type of modified starch in ODFs. This combination enables the production of ODFs with tailored mechanical properties and desired drug delivery rates, facilitating formulation design.
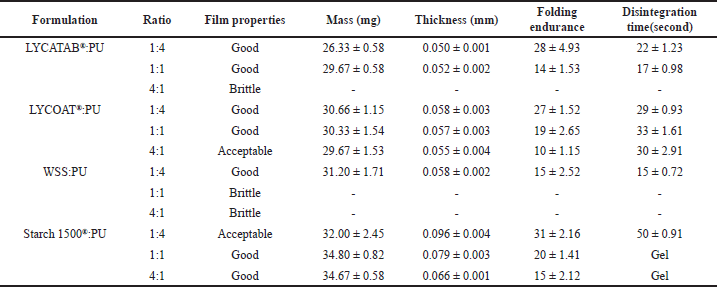
LYCATAB®/PVA composite films spread and peeled off easily. The morphology of the composite films changed from uniformly transparent to unevenly and opaque when the LYCATAB®/PVA ratio was increased from 1:4 to 4:1, the disintegration time decreased from 39 to 8 seconds, and the folding endurance decreased from 300 to 248 folds (Table 6) from transparent and uniform to opaque and uneven (each phase existed in the form of aggregation and interleaving). When the ratio was 4:1, the composite films were opaque and microphase separated.
The combination of LYCOAT® and PVA composite films (in ratios of 1:4, 1:1, and 4:1) exhibited challenges in terms of ease of casting and spreading, leading to phase separation. Consequently, this particular combination was deemed unsuitable for ODFs and was not subjected to further investigation.
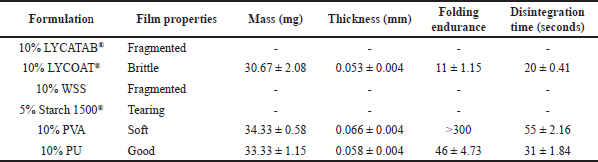 | Table 5. Physical properties of films made of single polymer (Mean ± S.D., n = 3). [Click here to view |
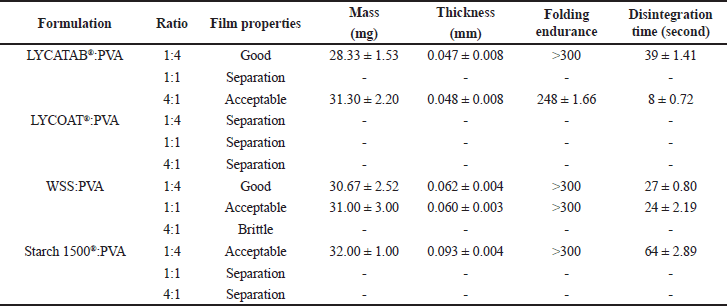 | Table 6. Physical properties of composite films (modified starch and PVA) (Mean ± S.D., n = 3). [Click here to view] |
The spreading and peeling properties of WSS/PVA composite films were excellent. Composite films of WSS/PVA (1:4) were transparent, uniform, and flexible. When the WSS/PVA ratio was increased from 1:4 to 1:1, the four corners of the films showed slight phase separation, disintegration times ranged from 27 to 24 seconds, and folding endurance was greater than 300. WSS/PVA (4:1) composite films were broken during the peeling process and could not be cut.
Because of the high viscosity of Starch 1500®, the solution of Starch 1,500 at 10% w/v could not be cast onto the glass plate; instead, the composite films were prepared using a 5% w/v solution of Starch 1500®. The composite films exhibited a white and opaque appearance with a textured grain-like sensation. Additionally, they demonstrated a folding endurance exceeding 300 folds. However, the Starch 1500®/PVA composite films (in ratios of 4:1 and 1:1) displayed limited stretch and peeling capabilities, resulting in opaque films with significant phase separation (Fig. 2). The phase separation between the two polymers may be exacerbated because the molecular chain of modified starch becomes more ordered after gelatinization.
The long chains of oxygen Hexa-ring macromolecules LYCOAT® and Starch 1500® have poor interpenetration with PVA, and changing the ratio (modified starch/PVA) did not improve phase separation [14,15]. The experimental findings indicated that combinations of LYCOAT® or Starch 1500® with PVA were not suitable as carriers for ODFs. However, when LYCATAB® or WSS were blended with PVA at a 1:4 ratio, the resulting film exhibited satisfactory performance and could be effectively utilized as ODFs.
ODFs prepared with modified starch and PU
The LYCATAB® and PU composite films had a transparent appearance, good spreading ability, and adhesiveness. When the LYCATAB®/PU ratio was increased from 1:4 to 1:1, the composite films appeared transparent and adhesive, the disintegration time was reduced from 22 to 17 seconds, and the folding endurance decreased from 28 to 14 folds (Table 7). The composite films LYCATAB®/PU (4:1) were brittle and peeled poorly.
The physical properties of LYCOAT®/PU composite films were appropriate in terms of transparency, flexibility, spreading ability, and ease of peeling. As the LYCOAT®/PU ratio was increased from 1:4 to 4:1, notable changes were observed in the composite films. They exhibited improved transparency and smoothness, with a disintegration time of approximately 30 seconds. However, this increase in ratio resulted in a decrease in folding endurance from 27 to 10 folds.
The spreading ability of WSS/PU composite films was good, but the peeling ability was poor. WSS/PU (1:4) composite films were transparent, uniform, and adhesive, with a folding endurance of 15 folds and a disintegration time of 15 seconds. Brittleness prevented the film made of WSS/PU (1:1 and 4:1) from being completely peeled off.
Starch 1500® gelatinization, which destroyed the original chemical bonds between starch molecules, resulted in molecular expansion and increased the thickness of the composite films. The Starch 1500®/PU composite films exhibited a white and opaque appearance with satisfactory spreading ability. The disintegration time for the Starch 1500®/PU (1:4) composite films was measured at 50 seconds, while the folding endurance reached 31 folds. These films displayed uniformity but had a rough surface texture. The folding endurance of the Starch 1500®/PU (1:1 and 4:1) composite films was between 15 and 20 folds, and they were white and opaque with a smooth surface and had certain anti-deformation ability.
The structures of LYCATAB®, LYCOAT®, WSS, Starch 1500®, and PU were highly similar, and they could form uniform composite films with sufficient adhesiveness. The PU was hygroscopic and took a long time to dry. Following careful consideration, it was determined that LYCOAT®/PU (1:4) and Starch 1500®/PU (1:1) composite films with potential industrial applications would be investigated further.
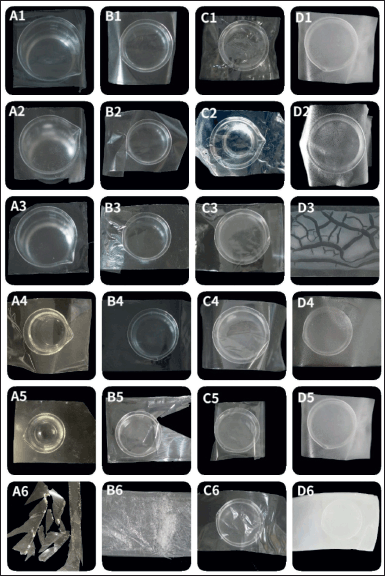 | Figure 2. Appearance of composite films. A1–6. The appearance of LYCATAB®/PVA (1:4, 1:1, 4:1) and LYCATAB®/PU (1:4, 1:1, 4:1) composite films. B1–6. The appearance of WSS/PVA (1:4, 1:1, 4:1) and WSS/PU (1:4, 1:1, 4:1) composite films. C1–6. The appearance of LYCOAT®/PVA (1:4, 1:1, 4:1) and LYCOAT®/PU (1:4, 1:1, 4:1) composite films. D1–6. The appearance of Starch 1500®/PVA (1:4, 1:1, 4:1) and Starch 1500®/PU (1:4, 1:1, 4:1) composite films. [Click here to view] |
Moisture content
High moisture content in ODFs can diminish the mechanical strength and compromise the stability of the API. Conversely, low moisture content during film drying can lead to polymer chain tightening and impede water penetration [16,17]. The moisture contents of the LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) composite films were 2.98%, 4.45%, 4.57%, and 1.58%, respectively, less than 5%, meeting the requirements for the industrial production of ODFs [18].
pH of composite films
The pH of saliva ranges from 6.2 to 7.6 [19]. A neutral pH of ODFs is generally considered favorable to avoid oral irritation. The pH of the LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) composite films were 6.89, 7.10, 6.95, and 7.05, respectively, without the potential risk of oral mucosal irritation.
Disintegration time
Three disintegration patterns were observed in all of the composite films (Fig. 3). LYCATAB®, LYCOAT®, and WSS with PVA or PU composite films dissolved in water (Fig. 3D1–3). Composite films of Starch 1500®/PU (1:4) and Starch 1500®/PVA (1:4) dispersed in water (Fig. 3B1–2). In water, the composite films Starch 1500®/PU and Starch 1500®/PVA (1:1 and 4:1) form gels (Fig. 3C1–2).
The experiment results showed that composite films of modified starch (LYCATAB®, WSS, and LYCOAT®) with PVA or PU disintegrated rapidly in water, decreasing the disintegration time as the proportion of modified starch increased. The propensity of gel formation in Starch 1500®/PVA and Starch 1500®/PU composite films exhibited an upward trend with the increase in the Starch 1500® ratio. Depending on the type of modified starch used, the composite films could display either rapid or slow drug release profiles.
Mechanical properties
The texture analyzer revealed the TS and fracture behaviors of composite films to ensure that the ODFs have sufficient mechanical strength to avoid damage during handling, packaging, and storage. The stress-strain curves (Fig. 4) revealed that the film samples underwent elastic deformation, plastic deformation, strain hardening, and finally fractured at the fracture point [20]. TSs of LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) composite films were 123.20, 100.23, 23.18, and 12.81 MPa, respectively, and the elongation was 164.41%, 204.72%, 3.95%, and 1.58%, respectively.
The TS, ductility, and flexibility of LYCATAB®/PVA (1:4) and WSS/PVA (1:4) composite films were excellent. The high strength and ductility of the films were primarily attributed to the inherent structural characteristics of PVA. The incorporation of Gl into the polymer matrix enhanced the flexibility of the molecular chains. In addition, the hydroxyl groups present in LYCATAB® and WSS formed hydrogen bonds with the hydroxyl groups PVA, thereby augmenting the tensile properties of the composite films. The difference in TS and elongation between LYCATAB®/PVA (1:4) and WSS/PVA (1:4) composite films could be explained by the length of the LYCATAB® and WSS molecular chains, as well as the number of hydrogen bonds formed with PVA.
The composite films LYCOAT®/PU (1:4) and Starch 1500®/PU (1:1) were rigid with low TS and ductility. The PU molecule had a longer-chain oxygen Hexa-ring structure with greater steric hindrance than PVA. Lower TS and elongation values could be attributed to the long chain of LYCOAT® and Starch 1500® molecules.
In general, high TS and elongation were regarded as positive characteristics of ODFs [17]. The LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) composite films demonstrated stress and strain resistance to external forces as a result of damage caused by external forces during preparation such as cutting, packaging, and transportation.
 | Table 7. Physical properties of composite films (modified starch and PU) (Mean ± S.D., n = 3). [Click here to view] |
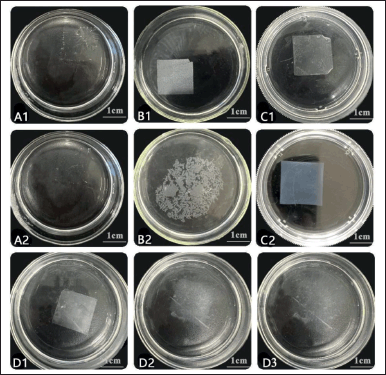 | Figure 3. The disintegration diagrams of composite films. A1–2. LYCATAB®/PVA (1:4) films (0 second, 30 seconds). B1–2. Starch 1500®/PU (1:4) films (0 second, 50 seconds). C1–2. Starch 1500®/PU (1:1) films (0 second, 15 minutes). D1–3. LYCOAT®/PU (1:4) films (0, 15, 30 seconds). [Click here to view] |
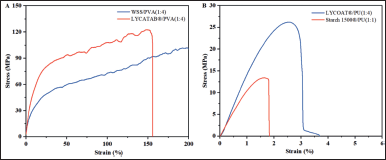 | Figure 4. Stress-strain curves of composite films. [Click here to view] |
Scanning electron microscope
LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PVA (1:1) composite films showed uniform integrity and consistent color under macroscopic observation. SEM analysis (Fig. 5) revealed the presence of fragmented areas on the surface of LYCATAB®/PVA (1:4) and WSS/PVA (1:4) composite films, potentially resulting from minor phase separation between LYCATAB® and WSS with PVA. Notably, this phenomenon could vary depending on the ratio of modified starch to PVA. However, the cross-sectional texture appeared well-defined and densely packed.
The surface of LYCOAT®/PU (1:4) composite films was smooth and uniform, the cross-section was slightly rough, and no stratification was found. The surface of Starch 1500®/PU (1:1) composite films was uneven, which may be caused by the incomplete plasticization of starch particles. The cross-section was compact and had high structural integrity.
Fourier transform infrared spectroscopy
Infrared spectra of LYCATAB®/PVA and WSS/PVA composite films (Fig. 6) revealed a peak at 3,500~3,200 cm−1 corresponding to the stretching vibration of −OH of free hydroxyl (O-H) groups [8], and the wavelength of 3,000–2,900 cm−1 was stretching vibration of the methyl C-H bond [21]. The C-O-C stretching vibration generated the peak at 1,020 cm−1, 924 cm−1 (C-H bending and C-C ring bending of the glycosidic bond), and 851 cm−1 (the stretching vibration of the glucose ring), which represent characteristic absorption peaks of starch [22].
The stretching vibration peaks of O-H and O-C-O stretching were observed in the infrared spectra of LYCOAT®/PU and Starch 1500®/PU composite films at 3,206 cm−1 and 1,650 cm−1 [23]. The vibrational absorption peak of CH and CH2 was observed in the range of 1,500–1,300 cm−1. Starch exhibited characteristic peaks at 930 cm−1 (α-(1,6) glycosidic bond), 850 cm−1 (α-glycosidic bond), and 757 cm−1 (α-(1,4) glycosidic bond) [24]. Because the basic molecular structures of LYCOAT®, Starch 1500®, and PU were similar, the majority of the peaks were caused by a superposition of the two polymers.
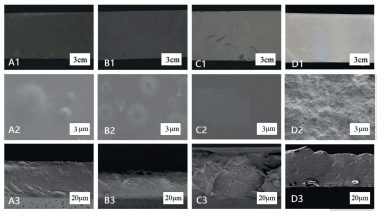 | Figure 5. SEM images of composite films. A1–3. LYCATAB®/PVA (1:4) (film, surface, section). B1–3. WSS/PVA (1:4) (film, surface, section). C1–3. LYCOAT®/PU (1:4) (film, surface, section). D1–3. Starch 1500®/PU (1:1) (film, surface, section). [Click here to view] |
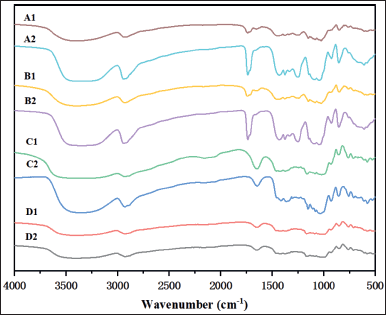 | Figure 6. FT-IR spectra of composite films and physical mixtures of polymers. A1–2. LYCATAB®/PVA (1:4) physical mixture and films. B1–2. WSS/PVA (1:4) physical mixture and films. C1–2. Starch 1500®/PU (1:1) physical mixture and films. D1–2. LYCOAT®/PU (1:4) physical mixture and films. [Click here to view] |
The peaks of LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) composite films were shifted nearby when compared to physical mixtures, indicating the formation of intermolecular hydrogen bonds. Each characteristic bond was clearly absorbed, and no new characteristic peaks appeared, indicating that there was no chemical interaction between modified starches and PVA or PU.
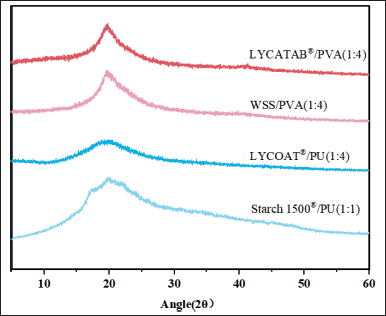 | Figure 7. XRD spectra of composite films. [Click here to view] |
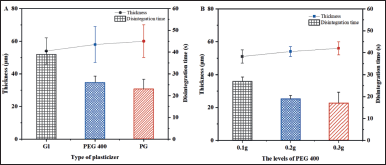 | Figure 8. Thickness and disintegration time of the prepared films with different plasticizers. [Click here to view] |
 | Figure 9. The appearance of API:LYCATAB®/PVA (1:5) films. A. Before cutting. B. After cutting. [Click here to view] |
X-ray diffraction (XRD)
The X-RD results of the composite film are shown in Figure 7. The crystal peak was found in LYCATAB®/PVA (1:4) and WSS/PVA (1:4) composite films and was located near 20°. Due to the presence of intramolecular hydrogen bonds on the hydroxyl group of the PVA molecular chain, which was a typical strong diffraction crystallization peak [25].
LYCOAT®/PU (1:4) and Starch 1500®/PU (1:1) composite films showed a wide diffraction peak at 2θ = 20°, and no crystallization peak was observed. The degree of crystallinity was found to have a strong correlation with the mechanical properties of the composite film. Specifically, the higher degree of crystallinity resulted in improved mechanical strength [26]. The XRD results were consistent with the results of mechanical properties.
Drug-loaded ODFs
Montelukast sodium, which is utilized for the treatment of asthma and allergic rhinitis, was chosen as the API for the preparation of drug-loaded films. This selection aimed to validate the industrial applicability of modified-starch polymer composite ODF. Considering blank film appearance, mechanical properties, and disintegration properties, LYCATAB®/PVA (1:4) was chosen as the composite film matrix, and three types of plasticizers were screened: Gl, PEG 400, and PG (Fig. 8). The films prepared by PG were too soft and easily deformed, while the films prepared by Gl disintegrated too slowly. PEG 400 films with a suitable thickness and fast disintegration time were chosen for further investigation.
PEG 400 levels were optimized. All films were transparent, smooth, uniform, and flexible. The higher the PEG 400 content, the thicker the films and the faster they disintegrated. The variety and dosage of plasticizer had no significant effect on the folding endurance of composite film, which is above 300 folds.
The drug-loaded films were made with an API/film-forming polymer ratio of 1:2 (N1) and 1:5 (N2). The drug-loaded films became white and opaque at a ratio of 1:2 (N1), possibly due to the precipitation of the drug at a higher API concentration. The drug-loaded films became transparent and uniform (Fig. 9) when the ratio (API:LYCATAB®/PVA) reached 1:5 (N2), the folding endurance was more than 300 folds, the disintegration time was about 25 seconds, the TS was 10.86 MPa, and the elongation was up to 226.65%.
The dissolution results of the prepared drug-load films (N2) and Singulair® Chewable Tablets were shown below under three different dissolution media. The drug release of the prepared films was faster than those of Singulair® chewable tablets in the dissolution media of 0.1M HCL and water solutions, and both were similar at phosphate buffer (pH = 6.8) solutions. Under the three dissolution conditions (Fig. 10), the prepared films achieved more than 80% drug release within 15 minutes, similar to that of Singulair® chewable tablets.
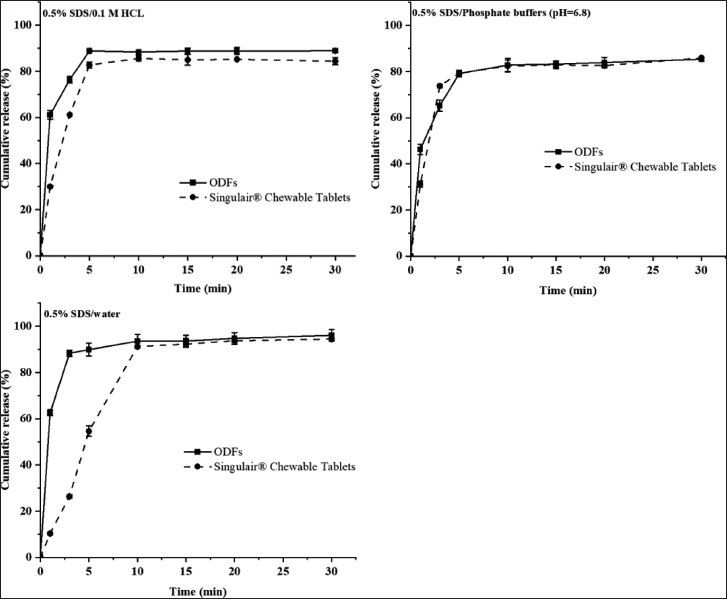 | Figure 10. Dissolution of the Montelukast ODFs and Singulair® Chewable tablets in different dissolution media. [Click here to view] |
DISCUSSION
When compounded with other polymers, modified starch could form composite films with good mechanical properties, fast disintegration, and satisfactory adhesiveness, which could be suitable for ODFs’ industrial applications. This study aimed to examine the influence of various types and compounding ratios of modified starches mixed with PVA or PU on the properties of ODFs. The objective was to systematically investigate the rule of modified starches on the characteristics of the composite films.
The appearance of ODFs is affected by the type of polymer. The morphology of the modified starch film is poor when applied alone, and can be improved when combined with PVA/PU. As the proportion of modified starch in the composite film increases, the disintegration time of the film will decrease, and as the proportion of PVA/PU increases, the appearance and mechanical properties of the composite film will be significantly improved.
The compatibility between polymers plays a crucial role in determining the appearance, mechanical properties, and drug-release characteristics of ODFs. Starch-based natural polymers and synthetic polymers such as PVA are known to be thermodynamically incompatible systems. Consequently, when these two polymers are combined, phase separation can occur [14]. It has been found that the compounding of modified starches (LYCATAB® and WSS) with PVA could make satisfactory ODFs by carefully adjusting the compounding ratio. Research has found [8] that phase separation when combining modified starch with HPMC; however, the authors found that by adjusting the ratio of the two polymers, the appearance of the film could be improved, which demonstrates the feasibility of enhancing the film’s appearance through the manipulation of the polymer composite ratio. The modified starch has better compatibility with PU, and the composite film based on LYCATAB® or LYCOAT® with PU has large room to prepare satisfactory ODFs, which could give formulation scientists more choices to design ODFs’ formulation to fulfill the formulation design.
The disintegration experiments showed that different types of modified starches (LYCATAB®, LYCOAT®, and WSS) were laminated with PVA or PU, and the laminates dissolved in water, with a disintegration time of about 30 seconds. Starch 1500®/PVA (1:4) and Starch 1500®/PU (1:4) laminates dispersed, while Starch 1500®/PU and Starch 1500®/PVA laminates (1:1 or 4:1) formed gels in water. Depending on the disintegration patterns of the composite films, a suitable matrix material can be selected as a carrier to fulfill various therapeutic objectives.
A fast dissolution rate of drugs is critical for the formulation design of ODFs. LYCATAB®/PVA (1:4) was used as the matrix material because of the good mechanical properties, appearance, and rapid disintegration to prepare montelukast sodium-loaded films. The prepared films achieved more than 80% drug release within 15 minutes. With the increase of modified starch content, the hydrophilicity of the composite film is enhanced and the dissolution is accelerated. The experiment data showed that the composite films prepared in the study were able to act as carriers to deliver APIs successfully, which could provide new polymer combinations for various APIs to achieve efficient drug release profiles.
CONCLUSION
This study aimed to develop and evaluate ODFs based on modified starches. The composite matrix materials LYCATAB®/PVA (1:4), WSS/PVA (1:4), LYCOAT®/PU (1:4), and Starch 1500®/PU (1:1) showed good physical properties and fast disintegration time. Moreover, the weight uniformity, thickness, pH, and moisture content of the prepared ODFs were all in line with the pharmaceutical requirements of ODFs. SEM showed structural integrity and good compatibility with the composite films. XRD showed that LYCOAT®/PU and Starch 1500®/PU composites were present in a dispersed form, and the LYCATAB®/PVA and WSS/PVA composites were in a crystalline form. In addition, FTIR showed no interaction among these excipients.
ODFs formulated with modified starches exhibit favorable flexibility, conforming effectively to the oral contours, and their release profiles can be modulated by choice of polymer type and the ratio of modified starch. The films studied are thin, non-irritating, and odorless, which helps achieve high patient compliance and provides a blueprint and framework for developing ODFs in the pediatric and geriatric drug delivery fields. The systematic study of the ratio variation of modified starch to polymer composite provides a promising technological platform for industrial applications.
ABBREVIATIONS
API, Active pharmaceutical ingredient; FT-IR, Fourier-transform infrared; Gl, Glycerol; HPLC, High-performance liquid chromatography; LYCATAB®, LYCATAB® DSH; LYCOAT®, LYCOAT® RS 720; ODFs, orodispersible films; PEG, polyethylene glycol; PG, propylene glycol; PU, pullulan; PVA, polyvinyl alcohol; SDS, sodium dodecyl sulfate; SEM, scanning electron microscopy; WSS, water-soluble starch; XRD, X-ray diffraction.
ACKNOWLEDGMENT
Gratitude is due to the pharmaceutical excipients company for samples. Gratitude is also due to the platform of Zhengzhou University.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; Drafting the work or revising it critically for important intellectual and revising it critically for important intellectual content. All the authors agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Krampe R, Visser JC, Frijlink HW, Breitkreutz J, Woerdenbag HJ, Preis M. Oromucosal film preparations: points to consider for patient centricity and manufacturing processes. Expert Opin Drug Deliv. 2016;13(4):493–506. CrossRef
2. Sevinc Ozakar R, Ozakar E. Current overview of oral thin films. Turk J Pharm Sci. 2021;18(1):111–21. CrossRef
3. Pacheco MS, Barbieri D, da Silva CF, de Moraes MA. A review on orally disintegrating films (ODFs) made from natural polymers such as pullulan, maltodextrin, starch, and others. Int J Biol Macromol. 2021;178:504–13. CrossRef
4. Wang B, Zhang A, Chen F. Preparation and in vitro/in vivo evaluation of rizatriptan benzoate fast dissolving films. Chin J Pharm. 2016;47(11):1398–403.
5. Ayorinde JO, Odeniyi MA, Balogun-Agbaje O. Formulation and evaluation of oral dissolving films of amlodipine besylate using blends of starches with hydroxypropyl methyl cellulose. Polim Med. 2016;46(1):45–51. CrossRef
6. Limpongsa E, Jaipakdee N. Physical modification of Thai rice starch and its application as orodispersible film former. Carbohydr Polym. 2020;239:1–10. CrossRef
7. Miksusanti, Fithri AN, Herlina, Wijaya DP, Taher T. Optimization of chitosan-tapioca starch composite as polymer in the formulation of gingival mucoadhesive patch film for delivery of gambier (Uncaria gambir Roxb) leaf extract. Int J Biol Macromol. 2020;144:289–95. CrossRef
8. Bodini RB, Guimarães JdGL, Monaco-Lourenço CA, Aparecida de Carvalho R. Effect of starch and hydroxypropyl methylcellulose polymers on the properties of orally disintegrating films. J Drug Deliv Sci Technol. 2019;51:403–10. CrossRef
9. Wang B, Yang L, Wang B, Luo C, Wang Y, Wang H, et al. Development, in vitro and in vivo evaluation of racecadotril orodispersible films for pediatric use. AAPS PharmSciTech. 2021;22(15):1–13. CrossRef
10. Sumaiyah S, Mentari J, Suryanto S. The effect of crospovidone on the dissolution profile of amlodipine besylate from fast orally dissolving film. Open Access Maced J Med Sci. 2019;7(22):3811–5. CrossRef
11. Verma M, Yasir M, Chauhan I. Oral delivery of zolmitriptan loaded fast disintegrating film: formulation development, statistical optimization, in-vitro and in-vivo evaluation. J Appl Pharm Sci Res. 2019;2(1):13 -22. CrossRef
12. Speer I, Steiner D, Thabet Y, Breitkreutz J, Kwade A. Comparative study on disintegration methods for oral film preparations. Eur J Pharm Biopharm. 2018;132:50–61. CrossRef
13. Walicová V, Gajdziok J, Pavloková S, Vetchý D. Design and evaluation of mucoadhesive oral films containing sodium hyaluronate using multivariate data analysis. Pharm Dev Technol. 2016;22(2):229–36. CrossRef
14. Zhu Z. Research progress on miscibility of starch and PVA Journal of Basic Science of Textile University. 2003;16(4):350–6.
15. Chen L, Huang B. Study on the miscibility of starch and PVA. Cotton Text Technol. 1996;24(2):87–9.
16. Takeuchi Y, Hayakawa F, Tahara K, Takeuchi H. Orally disintegrating films: the effects of water content on disintegration and mechanical properties. J Drug Deliv Sci Technol. 2021;66:1–9. CrossRef
17. Draškovi? M, Turkovi? E, Vasiljevi? I, Trifkovi? K, Cviji? S, Vasiljevi? D, et al. Comprehensive evaluation of formulation factors affecting critical quality attributes of casted orally disintegrating films. J Drug Deliv Sci Technol. 2020;56:1–14. CrossRef
18. Visser JC, Woerdenbag HJ, Crediet S, Gerrits E, Lesschen MA, Hinrichs WLJ, et al. Orodispersible films in individualized pharmacotherapy: the development of a formulation for pharmacy preparations. Int J Pharm. 2015;478(1):155–63. CrossRef
19. Pezik E, Gulsun T, Sahin S, Vural I. Development and characterization of pullulan-based orally disintegrating films containing amlodipine besylate. Eur J Pharm Sci. 2021;156:1–42. CrossRef
20. Li Z, Hu Y, Chen T, Wang X, Liu P, Lu Y. Microstructural evolution and mechanical behavior of thermally aged cast duplex stainless steel. Materials (Basel). 2020;13(24):1–13. CrossRef
21. Zhao Y, Wang J, Jin L, Ding Y. Properties of starch-chitosan composite films based on different starches. Packag Eng. 2021;42(7):151–8.
22. Zaman M, Hassan R, Razzaq S, Mahmood A, Amjad MW, Raja MAG, et al. Fabrication of polyvinyl alcohol based fast dissolving oral strips of sumatriptan succinate and metoclopramide HCL. Sci Prog. 2020;103(4):1–21. CrossRef
23. Li S, Yi J, Yu X, Wang Z, Wang L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int J Biol Macromol. 2020;148:258–64. CrossRef
24. Vuddanda PR, Montenegro-Nicolini M, Morales JO, Velaga S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur J Pharm Sci. 2017;96:290–8. CrossRef
25. Teodorescu M, Bercea M, Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol Adv. 2019;37(1):109–31. CrossRef
26. Pourjavaher S, Almasi H, Meshkini S, Pirsa S, Parandi E. Development of a colorimetric pH indicator based on bacterial cellulose nanofibers and red cabbage (Brassica oleraceae) extract. Carbohydr Polym. 2017;156:193–201. CrossRef