INTRODUCTION
Tadalafil, an oral selective phosphodiesterase-5 inhibitor (PDE-5), recognized officially in the year 2000 and approved by the Food and Drug Administration (FDA) for the management of erectile dysfunction (ED) (Brock et al., 2002). Tadalafil has shown high potency and efficiency in the treatment of ED for patients with varying severity and etiologies. PDE-5 is an enzyme that breaks down cyclic guanosine monophosphate (cGMP), which is a second messenger for nitric oxide-mediated smooth muscle relaxation, thus the PDE inhibitors rely on the accumulation of cGMP which potentiates the vasodilator activity of nitric oxide (Abdel-Aziz et al., 2011). Tadalafil is one of the three marketed PDE-5 inhibitors; vardenafil and sildenafil differs markedly chemically which further reflected on its pharmacokinetic characteristics. The gained merit of the molecular modification enhanced its duration of action up to 36 hours with minimal vision abnormalities (Gresser and Gleiter, 2002). However, tadalafil is categorized as Class II within the biopharmaceutical classification system, maintaining low aqueous solubility and high intestinal permeability. The solubility and dissolution in the gastrointestinal tract play a vital role in the time course of drug distribution which significantly affects its oral bioavailability (Mehanna et al., 2011).
Strategies concerned with the solubility and dissolution enhancement of class II drugs define the borders of promising research area (Vemula et al., 2010). Techniques developed to improve the solubility of tadalafil as an attempt for enhancing its oral bioavailability include, cocrystal (Vinesha et al., 2016), complexation (Badr-Eldin et al., 2008), liquisolid compacts (Lu et al., 2017), solid dispersion (Vyas et al., 2009), and micronization (Ojha and Prabhakar, 2013).
Fast dissolving drug delivery system has become an important strategy for drug delivery application as it enhances drug solubility, onset of action, and bioavailability. Fast dissolving systems are designed to dissolve or disintegrate, thus releasing the drug cargo directly into the mouth. The fast-dissolving drug delivery system is available in various forms such as tablets, buccals, film, sublingual patches and wafers. Several technologies have been achieved for the preparation of this system such as sublimation, compaction, spray drying, and ultra freezing system. Unlike the later mentioned systems, electrospinning systems have emerged in this field due to its nano-sized ultrafine fibrous structure with ease of fabrication (D.G. Yu et al., 2010). The lenience of the fabrication process is achieved through a high distribution of the main components in the filament polymer matrix upon electrospinning. The polymer filament is formed from solution cultivated between two opposite charged electrodes, where the charged solution ejected from the spinneret evaporates to form nanofibers on the charged collector. The collected fibers characteristics depend on various variables including polymers type and properties, solution viscosity, solvent system, distance in between, voltage applied, and humidity (Huang et al., 2003).
Nanofibers bear distinct properties including the controlled diameter of polymeric fibers ranging from few micrometers to several nanometers which reveal a large surface area to volume ratio, high nanoporosity, and a three-dimensional web structure (Shen et al., 2011). Exploiting nanofibers properties achieved applications in various pharmaceutical and biomedical fields as nanofibers have attained desirability in tissue engineering (Vasita and Katti, 2006), vascular graft (Wang et al., 2013), wound dressings (Uppal et al., 2011), as well as drug delivery systems of various active ingredients with a satisfying incorporation and release characteristics including cardiovascular agents (Sipos et al., 2016), antibiotics (Contardi et al., 2017), anti-inflammatories (Jiang et al., 2012), chemotherapeutic agents (Yang and Zhao, 2011), and vitamins (Taepaiboon et al., 2007). The nanofibrous mats large surface area and nanosized diameter obtained by electrospinning preserved the necessary equation for enhancing the limitation of poorly soluble class II drugs.
Nowadays, various polymeric matrices of both natural and synthetic origin have been investigated for their ability to customize electrospun nanofibers, where each polymer is tailored into its own final assembly depending on its unique characteristics (Vrbata et al., 2013). Polymers explored for their stability, biocompatibility, and enhancing release behavior were chitosan (Homayoni et al., 2009), polyvinylpyrollidone (PVP) (D.-G. Yu et al 2010), polylactic acid (Xu et al., 2009), polylactic glycolic acid (Ranjbar-Mohammadi et al., 2016), and polyvinyl alcohol (Kazsoki et al., 2017; Li et al., 2013). Among the synthetic polymers, PVP is used pharmaceutically over the last 60 years and has been utilized in numerous nanofibers as it has good biocompatibility, biodegradability, and recognized by Food and Drug Administration (FDA) as a safe excipient (Yang et al., 2004). In addition, it conquered a wide range of applications as it has complex-formation ability, good adhesion, and being soluble in many solvents. PVP was utilized for solubility and dissolution rate enhancement in several techniques including electrohydrodynamic atomization (Aytimur and Uslu, 2014; Jiang et al., 2012; Xin et al., 2008; Yang et al., 2004).
In light of the above-mentioned merits of the nanofibers, the appraisal of tadalafil-loaded polymeric nanofibers as fast-dissolving drug delivery system is investigated in the current study. Although various reports on the preparation and application of polymeric electrospun nanofibers are published, yet a clear comprehensive understanding of the formulation parameters influencing the electrospun drug-loaded nanofibers characteristics is still limited. Hence, the objective of the present study is the fabrication of nanofibers via electrohydrodynamic technique using the polymer blends. The impact of various formulation parameters, namely, polymers concentrations and their interaction on the prepared fibers are exploited via a 32 full multifactorial design. Further physicochemical characterization of the optimized formulation was carried out.
MATERIALS AND METHODS
Tadalafil was a sample gift from Benta Pharma Industries (Dbayeh, Lebanon). Polyvinylpyrollidone (PVP K60, MW = 360,000) and polyethylene oxide (PEO, MW = 35,000–40,000), Methanol (High Pressure Liquid Chromatography (HPLC) grade), and Hydrochloric acid were purchased from Sigma-Aldrich (Steinheim, Switzerland). All other solvents and excipients used were of analytical grade.
Preparation of composite nanofibers
For the preparation of electrospun fiber formulation, the selection of the solvent system is a critical step for the final fiber characteristics. Methanol was used due to its ability to solubilize tadalafil and the polymers used with the advantage of rapid evaporation during the electrospinning process (Shen et al., 2011). Various concentrations of PVP K60 and PEO were dissolved in methanol under stirring. The desired amount of drug is added to the polymers solution under stirring to assure complete dissolution of tadalafil. The prepared polymeric solution is placed into a plastic standard 20 ml syringe with care to prevent air bubble entrapment. A stainless steel dispensing tip of gauge 22 was connected to the syringe. The dispensing tip was connected to the positive polar emitting electrode from a high voltage DC power supply, while the grounding electrode connected to the collecting plate fixed 15 cm from the needle tip. The applied electrical potential applied over the 15 cm distance is 23 kV. The prepared solution is discharged from the syringe at a feed rate of 1.2 ml/hour. Upon completion of the process, the fiber mats are removed from the collector and stored in a vacuum desiccator for further characterization.
Experimental design
Factorial design is an efficient statistical optimization system carried out by selecting various contributing factors and investigating the response established to obtain reliable data that can be analyzed to attain valid and objective conclusions (Mehanna et al., 2011). The contributing factors also identified as independent variables in different levels resulting in the studied responses known as the dependent variables in order to understand the implications of the factors on the final preparation (Mehanna et al., 2010).
The experimental design is based on two factors at three levels full factorial design that required nine formulations in total. Table 1 demonstrating the independent variables investigated and their corresponding levels. The two factors are PVP and PEO concentrations, with each of the polymers conveyed in low, medium, and high values represented by −1, 0, or 1 sign.
The design utilizing the full nine runs of the prepared formulation (Table 2) is created using design-expert® (version 10.0.7, State-Ease Inc., Minneapolis, MN).
Following the construction of the nine runs, the responses studied including electrospun yield, loading efficiency, drug release at 2 minutes, number of beads/10 μm2, and fiber diameter were subject for analysis in order to optimize the formulation parameters.
Optimization of the composite nanofibers
Electrospun yield
The weight of the solid polymer(s) and active pharmaceutical ingredients present in the solution fed into the electrospinning machine and the weight of the fibers mat collected are determined, then the percentage yield is calculated according to the following Eq. (1). (Mehanna et al., 2015)
Loading efficacy
Loading efficiency is the ratio of the amount of tadalafil loaded in the electrospun fibrous mat to the total amount of drug in the electrospinning solution. The loading efficiency is determined by dissolving an accurately weighed mat in (1:1) methanol:water solution. The tadalafil content is measured at λmax 284 nm using ultraviolet spectrophotometer (Optima, SP-3000 PLUS, Tokyo, Japan) (Mehanna et al., 2010). The loading efficacy is determined by utilizing the following equation:
 | Table 1. Factors and levels for the construction of 32 factorial design experiment. [Click here to view] |
Determination of percentage drug release
The in vitro release profile of tadalafil from various formulations, free drug, and physical mixture is determined using the United State Pharmacopeia I dissolution apparatus (ERWEKA, Heusentamm, Germany) in triplicate. The dissolution medium maintains a complete volume of 900 ml of 0.1 N HCL (pH = 1.2) (Mehanna et al., 2011). Samples equivalent to 15 mg tadalafil of the prepared formulation are filled in hard gelatin capsules (size 0). The baskets are rotated at 50 rpm at 37°C ± 0.5°C. Samples were withdrawn and passed through a 0.22 Millipore filter at preselected time intervals for a period of 60 minutes. The drug concentration was determined spectrophotometrically at λmax 284 nm (Mehanna et al., 2010).
Morphological and topological characterization
The morphology of the various electrospun composite nanofibers formulations was examined by scanning electron microscope (SEM) (SERON technology, AIS2300C, Korea). The resulted samples were fixed on double adhesive tape and vacuum coated using gold Cressington sputter coater 10802 at less than 0.1 millibar pressure and 20 mA. The diameter and distribution of the fibrous mats are scanned at different fields and magnifications. SEM images were analyzed using ImageJ analysis software (Image J, National Institute of Health, USA) for determination of the average diameter of the nanofibers and number of beads (Li et al., 2013).
Solid-state characterization and interaction investigation
Differential scanning calorimetry (DSC)
Differential scanning calorimetry thermograms are evaluated using differential scanning calorimetry (DSC) (DSC60, Shimadzu, MA) to determine the thermal behavior of the formulation components. Samples of optimized formulation, physical mixture, free drug, and the polymers were placed in a standard aluminum pan, with dry nitrogen used as a carrier gas with a flow rate of 25 ml/minute and heated from 21°C to 400°C at a rate of 5°C/minute. An empty aluminum pan is utilized as a reference (Vrbata et al., 2013).
 | Table 2. Formulation of composite nanofibers prepared according to 32 full factorial design and the corresponding responses (n = 3 ± standard deviation). [Click here to view] |
Fourier transform infrared (FTIR) spectroscopy
FTIR is employed to illustrate the possible interactions between tadalafil and the polymers used in the solid state using the PerkinElmer spectrum FTIR (ES version, Massachusetts, United States). Samples of 2 mg were triturated with dry potassium bromide and compacted in a hydraulic press at 10 tones. The spectra of the optimized fiber formulation, polymers, free drug, and physical mixture were scanned over a resolution of 4 cm−1 frequency of range 400–4,000 cm−1 (Mehanna et al., 2011).
X-ray diffractometry
The X-ray diffractometric (XRD) patterns of the nanofiber formulation, physical mixture, free drug, and polymers were traced at room temperature using an automated X-ray powder diffractometer (X-ray diffractometric (XRD) Bruker AXS, D8 focus, Karlsruhe, Germany). A system of diverging and scattering slit of 1º with a nickel filtration were utilized to obtain the monochromatic CuKÉ‘ radiation. The data of sample composite nanofibers, physical mixture, polymers, and free drug were collected in the region of 4° ≤ 2θ ≤ 80° with a step size of 0.02° and dwell time of 0.6 seconds (Mehanna et al., 2011).
Data analysis
The significance of the studied variables and the interaction among the dependent variables on the electrospun fibrous mats were evaluated using design expert software (design expert 10.0.7). The analysis of variance by the design expert for testing of the prevailed model, the analysis depends on basic assumption satisfaction; this assumption is based on the adequate description of the normal distribution of the model and the error within zero mean constant but unknown variance δ2. The residuals (the variation between actual and predicted responses) which are obtained from the regression equation are examined for investigating the assumptions and models attained.
Analysis of variance (ANOVA) available in the design expert software is used for the statistical validation establishment of the equation generated by the software. ANOVA, F-ratios, and correlation coefficients are the principles used for the model validation. To denote statistical significance, p < 0.05 is accepted. To relay on the model statistically evaluated, an assessment between predicted values with experimentally actual observations through checkpoint analysis was carried out. The agreement between the actual and predicted values is probed through the calculation of the bias.
Accelerated stability study
The hygroscopicity of PVP along with the high surface area depicted by the nanofibers necessitates the performance of stability studies for the optimized formulation. Capsules containing the equivalent weight of 15 mg tadalafil electrospun composite fibers are placed in glass vials at 40°C ± 0.5°C and 75% ± 0.5% relative humidity (RH) for 90 days. To confirm the stability of the optimized formulation, SEM images and in vitro release profiles as well as drug content utilizing High Pressure Liquid Chromatography (HPLC) for detection of degradation products (Reddy et al., 2010) were evaluated after the accelerated storage conditions exposure.
RESULTS AND DISCUSSION
Preparation of the composite nanofibers
Selection of polymer composition and solvent are important factors for the successful preparation of drug-loaded nanofibers (Zeng et al., 2005). The solvent utilized should be of low toxicity, compatible, dissolves drug and polymer as well as maintains continuous electrospinning property (Huang et al., 2003). Methanol tends to preserve a good solubility for the pharmaceutically active component as well as various polymers such as PVP, PEO, and Eudragit (Shen et al., 2011). Although literature utilized ethanol for PVP fiber formation but it is of low capability to solubilize tadalafil, nonetheless methanol presents favorable electrospinning properties specifically; rapid evaporation and conductance properties (Chuangchote et al., 2009).
Polyvinylpyrrolidone was utilized as the main matrix-forming polymer due to its excellent physiological compatibility, hydrophilicity, low toxicity, and fiber-forming ability (Folttmann and Quadir, 2008). Moreover, PVP is accepted in pharmaceutical application due to its well-defined molecular weight, mucoadhesive properties, as well as physicochemical properties. In addition, it has been widely used in solid dispersion methodology for dissolution rate improvement of poorly soluble drugs (Saquib Hasnain and Nayak, 2012). Thus, the well-known solubility enhancement ability of PVP is employed for aiding in tadalafil dissolution development in the nanofibers formulation prepared. Despite maintaining most of the desirable characteristics for tadalafil nanofibers, PVP has hygroscopic as well as requires high concentration for full fiber formation; this presents a limitation for its use which necessitates the incorporation of an axillary polymer to balance its drawbacks. In the current research, the secondary polymer used is PEO due to its semi crystallinity which minimizes the hygroscopic nature of the composite nanofibers additionally, fiber-forming capability at low concentration with its hydrophilic property and compatibility. The mixture system has been utilized in various studies to enhance the overall fiber formation as the fabrication of fibrous hydroxyapatite/PVP/PEO nanofibers as a promising scaffold for bone tissue engineering (Zhou et al., 2014) and Vrbata et al. (2014) study for enhancing diosmin solubility using various polymers mixtures including PEO.
Tadalafil-loaded nanofibers forming solutions incorporating different concentrations of PVP and PEO were dissolved in methanol. All formulations presented a completely transparent solution and were able to form nanofibers upon electrospinning.
Optimization of the electrospun fibrous mat
Electrospun fibrous mat yield
Electrospinning is a one-step simple and straightforward process for the generation of nanofibrous mats. The ease of implementation and variability in application through various systems such as drug delivery, cosmetics, and other industrial systems speed up the popularity of the system at hand (Kim and Kim, 2011). Another factor that flourished this system is the convenience in employing various active pharmaceutical ingredients within the processed mats as well as the ability to utilize core-sheath systems which can either improve drugs solubility or control the drug release (Da Costa et al., 2015; Yu et al., 2011). The one-step principle of hydrodynamic atomization technique depends on understanding the interactions involving the process and formulation parameters in order to optimize the final preparation. The final mat characteristics demonstrated by fiber formation, morphology, and diameter influence the product yield, drug loading efficiency, and the in vitro release pattern as well as the physical stability.
The experimental design evaluated two independent variables, namely, the escalating concentrations of both polymers PVP and PEO added into the formulation on the nanofibrous mat yield which is defined as the fraction of the total amount of solid added into the feed which is recovered after the electrospinning process on the collecting disk. The electrospun yield computed from the amount of recovered fibrous mat collected is represented as the percentage of electrospun yield. The yield fluctuated between 70% and 96% as presented in Table 2. ANOVA analysis demonstrates a high R2 (0.9243) revealing that 92% of the variability in the yield is due to the studied factors with a p-value < 0.0158.
The regression Eq. (3) demonstrating the terms of actual factors influence on the percentage yield, illustrated the negative influence of both PVP and PEO concentrations represented as A and B, respectively. Where it must be noted that the PVP concentration poses a more prominent drawback as shown by its regression coefficient (−4.6667).
For more detailed analysis, response surface plots represented by two-dimensional contour lines and a corresponding three-dimensional surface response (Fig. 1). The two-dimensional surface plot (Fig. 2) demonstrates the absence of parallelism revealing interaction between the two independent factors. The peak percentage yield equivalent to 96% is obtained at PVP and PEO concentrations (coded values equal zero). The three-dimensional plot (Fig. 1) assures the presence of interaction between the two factors assessed.
The yield obtained on the collector during electrospinning is highly affected by the independent factors presented in the formula as shown by ANOVA. The decrease in the yield at low PVP and PEO value is attributed to the low viscosity of the solution which minimizes its ability to form fibers, thus prevents the continuous flow out of the electrospinning needle as was observed during experimentation. On the other hand, the increase in the PVP and PEO concentrations in the feed solution negatively affected the mat yield due to the high viscosity which in turn increases the density of the droplet formed, thus increasing the droplet loss allowing leaking of the polymeric solution prior to electrostatic fiber formation.
Drug loading efficiency
A major challenge upon fabrication of a medicated fiber is the drug loading as it may affect the physical and chemical properties of the fibrous mat. In addition, the determination of drug loading is crucial for the determination of the therapeutic efficacy of the nanofibers (Singh et al., 2015).
The loading efficiency of the nine formulations was studied to estimate the incorporation ability of the loaded nanofibers to entrap the drug. The data revealed high loading efficacy in the range of 90%–96% as present in Table 2, which indicated the successful loading of tadalafil into the nanofibers. The independent factors presented statistical significant involvement in the loading criteria (p-value < 0.0035), with a high adjusted R2. The evidence for lack of interaction between the studied factors is observed by the perturbation graph (Fig. 3) and the presence of parallelism in the 3D surface response as well as 2D contour surface response (Figs. 1 and 2).
where A and B are the PVP and PEO concentrations, respectively.
From the regression Eq. (4) it could be inferred that the PVP concentration reflects a negative effect on the loading while PEO incorporation induced a positive influence on the loading of tadalafil into the formed nanofibers. The suggested explanation is attributed to the PVP molecules number which can act as a steric hindrance to the loading of the drug due to the vast increase in molecular interaction, on the other hand, the addition of PEO enhanced tadalafil solubility within the fiber upon formation that can explain its positive influence on the drug loading.
The percentage of drug release within 2 minutes
For a drug in a solid dosage form to reach the systemic circulation, it should undergo dissolution in the specified medium, which is a critical step for its delivery. Hence, whenever a new solid dosage form is formulated, it has to pass through dissolution testing within appropriate conditions to ensure drug release. In vitro studies are thus chosen to interpret and relate oral absorption and bioavailability (Mehanna et al., 2015). Prepared formulations demonstrate a rapid and complete drug release profile.
The statistical model analysis of the in vitro release within 2 minutes fluctuated between 64% and 84% release as presented in Table 2, demonstrating the significant influence of the chosen factors on the drug release (p-value < 0.0175S). The study of each factor highlights the effect of increasing PVP concentration resulting in an increase in the rate of release within 2 minutes; this was not the case for the PEO as the increase of its concentration demonstrated a drawback on the release rate as presented in Figure 4. The regression Eq. (5) after correction of insignificance highlighted that the independent variable positively affecting the drug release was the PVP concertation (A) while the downside of the dissolution rate is related to the PEO concentration (B).
Tadalafil release rate from nanofibers enhancement can be explained by the hydrophilicity as well as the amorphous nature of the PVP (Illangakoon et al., 2014), while the incorporation of the semi-crystalline PEO despite its ability to reduce PVP hygroscopicity brings a minor negative effect on the drug release which can be explained by the interaction between PVP and PEO polymers leading to an increase in viscosity and thickening of the diffusion layer from which the drug is released thus prolonging the time required for primary thrush release (Hong and Oh 2008). Another explanation was reported by Vigh et al. (2013) where the supersaturated concentration of PVP within the nanofibers induced the gelation of the polymer chains after immediate wetting thus hindering the drug release from the nanosystem.
 | Figure 1. Response surface 3D plots showing the effect of PVP concertation (A) and PEO concentration (B) on the different dependent variables. [Click here to view] |
Flaw free nanofibers formation
The hydrodynamic atomization encountering a major obstacle which is the formation of defected nanofibers that is mostly beads. The transition from beads to uniform nanofibers is dependent on both electrospinning conditions and formulation parameters. The main factor preserved for this imperfection is the polymer concentration reflecting on the formulation viscosity. At low concentration of polymer, fiber density is low and thus bead formation occurs at various intervals (Singh et al., 2015).
Photomicroscopical analysis of different formulations was performed to identify fiber morphology, diameter, and surface characteristics as illustrated in Figure 5. Scanning electromicrographs of formulations (1, 2, and 3) show beaded nanofibers of different size and number. The number of beads of each formulation per 10 μm2 surface area of the formed mats present in Table 2 reflecting the importance of the polymers concertation for an optimal nanofibers formation. The number of beads ranges from 0 to 49/ 10 μm2 surface area. The ANOVA evaluation of number of beads (R2 = 0.9494) present response revealed a statistically significant p-value < 0.0087, thus highlighting the importance of the independent factors on the studied response. From the regression Eq. (6), it can be deduced that PVP concertation as well as PEO incorporation denoted as A and B, respectively, maintains a disproportional relationship to the number of beads, where these polymers and to a higher extent PVP, minimizes the bead creation demonstrating the positive influence of each independent variable on the overall beads formation.
 | Figure 2. Response surface 2D plots showing the effect of PVP concertation (A) and PEO concentration (B) on the different dependent variables. [Click here to view] |
The contour plots demonstrate a well-formed interaction between both independent variables as demonstrated by the lack of parallelism of the different contour lines of the bead formation response as seen in Figure 2. The reflected three-dimensional plots, as well confirm the interactions between the two factors (Fig. 1).
The decrease in the beads formation can be solely interpreted by the increase in the formulation viscosity. The addition of the PEO at 2.5% concentration leads to a decrease in the number of beads by half at (−1) coded PVP value, and the addition of 5% PEO furthermore decreased the beads formation. On the other hand, increasing PVP concertation from 10% to 20% minimized the bead formation, with complete absence of beads at (0, 0) coded PVP and PEO concentration. The presence of PEO increased the viscosity and improved surface tension, which may explain the reduction in the beads formation (Huang et al., 2003).
In the current study, it is detected that at low polymer content, disjointed beads of various sizes were formed. As polymers concentrations increased, a reduction in size and number of beads is noticed with a complete disappearance and smooth nanofibers formation. This gradual change is attributed to a competition between surface tension and viscosity. The surface tension overcomes the viscosity in order to reduce the surface area at low polymer concentration causing the formation of beads, until the viscosity surmount this force thus allows the formation of uniform smooth nanofibers (Huang et al., 2003).
 | Figure 3. Perturbation plots. [Click here to view] |
 | Figure 4. The effect of PEO and PEO concentration on the tadalafil release within 2 minutes. [Click here to view] |
Diameter of the composite nanofibers
The fundamental aspect of fiber formation is the translation of polymer solution into fine fiber. In literature, various polymers have been successfully transformed through electrospinning into ultra-fine fibers ranging in diameters from less than 3 nm to over 1 μm. Different targets that display the validity of the polymer fiber formation are the fiber diameter and the ability to be consistent and controllable (Son et al., 2004). Since the nanofibers result from solidification or evaporation of the fluid jet, the fiber diameter is influenced primarily by the jet size as well as the polymer content (Demir et al., 2002).
The experimental design projects the influence of the concentration of both PVP and PEO on the nanofibers diameter. The model presented nanofibers diameter ranged from 382 nm to 1,073 nm as shown in Figure 6. ANOVA revealed that fiber diameter is significantly affected by the concentrations of PVP and PEO (p < 0.0048). The result of the analysis of adjusted R2 was above 0.9663 which proved that more than 96% of the variability is explained by the model. From the regression Eq. (7), it could be deduced that both independent variables present equal positive effect as seen from the regression coefficients.
where A and B represented the PVP and PEO concentration, respectively.
 | Figure 5. Scanning electron micrographs of tadalafil loaded nanofiber formulation, F1 (A), F2 (B), F3 (C). [Click here to view] |
The lack of parallelism in the 2D contour surface plot presented in Figure 2 demonstrates the presence of interaction between the dependent variables in the model which is highlighted in the 3D surface plot shown in Figure 1.
The independent variables prove to be of significant effect on the fiber diameter as resolved from ANOVA analysis. The increase in nanofibers diameters observed at low polymer concentration is due to the bead elongation prior to full fiber formation which is due to low viscosity of polymeric solution which prevents completion of uniform fiber formation, while the increase in polymer concentration at level (0,−1; 0,0) PVP and PEO concentrations coded values, presents optimal diameter range (384 nm, 540 nm). The change in the polymer concentration motivates the change in viscosity which leads to alteration of the electrospinning solution properties mainly its surface tension and conductivity (Pham et al., 2006).
Optimization and checkpoint analysis
Through the utilization of the previous analysis, the optimized tadalafil electrospun composite nanofibers were selected based on setting the following targets; (minimizing the fiber diameter, number of beads and maximizing yield, drug loading, and the in vitro release within 2 minutes). Formula (F5) which represents (0, 0) coded value of dependent variables corresponding to 20% w/v PVP and 2.5% w/v PEO bared the required criteria, as it has a 95% yield with 94% loading efficiency, uniform composite nanofibers with a diameter of 422 nm with no beads, and released 81% of tadalafil within 2 minutes.
To evaluate the validity of the model and capability to maintain the prediction of responses, a checkpoint analysis over three different formulations was performed as presented in Table 3. The values of the responses were determined and matched to the predicted ones in the factorial design. The evaluation of the various independent responses was found to be within the limits of 2.34 and 8.34% bias assuring the agreement between the actual and the predicted values thus the significance of the statistical model.
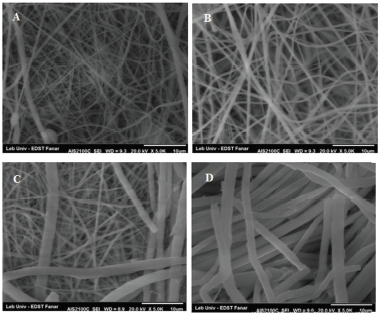 | Figure 6. Scanning electron micrographs of electrospun tadalafil loaded nanofibers formulations showing their diameters, F4 (A), F6 (B), F7 (C), and F9 (D). [Click here to view] |
 | Table 3. Percentage bias values for the dependent responses studied. [Click here to view] |
Physicochemical characterization of the optimized composite nanofibers mat
Electrohydrodynamic atomization technique has been applied for the preparation of drug-loaded nanofibers for many aims, with interest in its utilization for bioavailability enhancement of poorly soluble drugs (Varma and Sravani 2013).
Electromicroscopical examination
Photomicroscopical analysis of the optimized formation was examined to identify nanofibers morphology, diameter, and surface characteristics as illustrated in Figure 7. Scanning electromicrographs of the optimized formulation represent cylindrical smooth surface nanofibers with no visible beads which confirm continuous filament formation. Homogenous nanofibers production for drug-loaded preparations implies that the drug did not affect PVP fiber-forming ability at the used concentrations. The absence of crystalline material on fiber surface with uniform fiber diameter around 422 nm in the optimized formulation indicated incorporation of the drug in the carrier matrix. The absence of tadalafil crystals might be explained by the transformation of the drug into amorphous form or the incorporation of tadalafil molecules as solid solution or solid molecular dispersion, analogous phenomenon was observed by Yu et al. (2010) after the complete absence of ketoprofen crystals on the PVP-based nanofibers.
Differential scanning calorimetrical analysis
DSC was employed for the examination of tadalafil solid state within the electrospun composite nanofibers mats. As illustrated in Figure 8, the free tadalafil is characterized by a single strident endothermic peak at 305.49°C with an enthalpy of fusion of 98.78 J/g, which reveals its crystalline behavior (Mehanna et al., 2011). PVP showed a fusion peak at 62.64°C with an enthalpy of fusion of 11.34 J/g apart from such it represented a broad endotherm which may be due to the dehydration of the polymer chain occurring at 85°C similar to that observed in a previous work (Yu et al., 2011). PEO semi-crystallinity is well illustrated by the DSC thermographs due to the presence of a sharp endothermic peak at 68.18°C with the enthalpy of fusion of 118.94 J/g. The physical mixture obtained represents the characteristic peak corresponding to PEO, while the absence of the characteristic endothermic peak of tadalafil indicated that the drug has been dissolved in the melted polymers upon elevating the temperature. The optimized formulation (F5) thermal analysis presents broadening of the PEO peak and complete absence of tadalafil characteristic endothermic peak which could be explained by the presence of tadalafil in the molecular state dispersed within the carrier or the shifting of tadalafil into the amorphous state both of which possibility can attribute to the drug-enhanced solubility (Vrbata et al., 2014). One factor to enhance bioavailability settles with the manipulation of drug crystallinity into amorphous form. This transformation governs an enhancement in solubility due to high internal energy preserved in the amorphous form (Mehanna et al., 2015).
X-ray Diffraction
The physical state of the drug in the final formulation is critical for achieving the desired release profile (Shen et al., 2011). In the present study, X-ray diffractometric (XRD) analysis participated in realizing the physical characteristics of tadalafil and various polymers in the optimized final preparation. X-ray diffractometric (XRD) reflections present numerous distinct peaks for tadalafil as present in Figure 9, which indicate the crystalline nature of the drug specifically at, 12.20°, 8.35°, 6.10°, 4.81°, and 4.40°. As for PVP polymer, the X-ray diffraction profile shows a diffused background with two halo diffraction indicating that its amorphous nature similar to that represented by Manna et al. (2007). This is not the case in polyethylene oxide which shows various reflections emphasizing the semi-crystalline behavior of the polymer with characteristic peaks at 4.61° and 3.83°. The physical mixture portrays a diminution intensity and broadening of the characteristic tadalafil and PEO peaks (Fig. 9). The difference in the comparative intensity of the reflections is due to the reduction of the crystal particle size as well as the alteration of the different relative abundance of planes exposed to X-ray. This may ensure the partial loss and masking of the tadalafil and PEO by the PVP which was previously reported by Yu et al. (2010).
The X-ray powder diffractometer for the optimized formulation (F5) shows the overall characteristic PVP hump demonstrating the absence of tadalafil crystallinity and full conversion into the amorphous state or molecular state within the mat as shown in Figure 9. The results attained by the X-ray diffractometric (XRD) are in accordance with DSC results.
 | Figure 7. Scanning electron microscopical image (A) and histogram for diameter distribution of the optimized formulation F5 (B). [Click here to view] |
 | Figure 8. DSC thermograms of tadalafil (A), Polyvinylyrollidone (B), poly ethylene oxide (C), optimized composite nanofibers mat (F5) (D). [Click here to view] |
 | Figure 9. X-ray powder of tadalafil free drug (A), PVP (B), PEO (C), their physical mixture (D), and optimized formulation (F5) (E). [Click here to view] |
FTIR spectroscopy
The compatibility among the drug and polymers in the formulation is essential for the production of stable high-quality nanofibers. Often the second-order interaction such as hydrogen bonding, hydrophobic interaction, as well as electrostatic interaction, may influence this compatibility (D.-G. Yu et al 2010). The FTIR spectra of the three components, their physical mixture, and optimal formulation (F5) are depicted in Figure 10. Characteristic tadalafil absorption band at 1,675.3 cm−1 indicating a carbonyl stretch and a sharp band at 3,057.5 cm−1 indicative for a C-H aromatic stretching. In the PVP spectrum, a C-H stretching at 2,954 cm−1 and a stretching vibration of the carbonyl group (C=O) at 1,662 cm−1 are visible. A very broad band attributed to the presence of water confirming the broad endothermic peak in the DSC at 3,415 cm−1 is detected, the same observation was noticed in Yu et al. (2010) study.
The semi-crystalline phase of PEO is definite by the triplet peak C-O-C stretching which is observed at 1,192, 1,095, and 1,059 cm−1 (Noor et al., 2010).
The physical mixture illustrates the characteristic bands of the tadalafil, PVP, and PEO, similarly the optimized formulation (F5) represents the characteristic peaks with no significant differences. Thus, the patterns of the physical mixture and the optimized formulation (F5) indicate the absence of interaction within the formulation which further supports the DSC and X-ray diffractometric (XRD) results.
 | Figure 10. FTIR spectroscopy of tadalafil free drug (A), PVP (B), PEO (C), their physical mixture (D) and optimized formulation (F5) (E). [Click here to view] |
In vitro drug release
In order to evaluate the dissolution characteristics of the optimized tadalafil loaded composite nanofibers (F5), investigation of tadalafil in vitro release from optimized formulation, free drug, and physical mixture have been performed over 60 minutes at 37°C in 900 ml 0.1 N HCL. Tadalafil optimized formulation presented 81% drug release within 2 minutes while the physical mixture demonstrated a mild release of 12.5% and a negligible release of 5.3% for the free drug. Over the period of 60 minutes, the optimized composite nanofibers mat was able to release the drug in a complete rapid manner, while the physical mixture maintained a maximum of 20% while the free drug plateaued with a 5.94% as illustrated in Figure 11.
The improvement in dissolution rate of the tadalafil from the optimized composite nanofibers mat formulation is contributed to many factors, namely, the hydrophilic nature of both PVP and PEO polymers, the massive surface area of the nanofibers which allows increase in wettability and enhancing dissolution; moreover, the transformation of the drug during electrospinning into amorphous form or its present in a molecular state within the nanofibers which further enhances the dissolution and release of the drug. The dissolution improvement observed is analogous to that reported for paracetamol nanofibers formulation which presented 66% release within 30 seconds and up to 87% release within 6 minutes (Illangakoon et al., 2014).
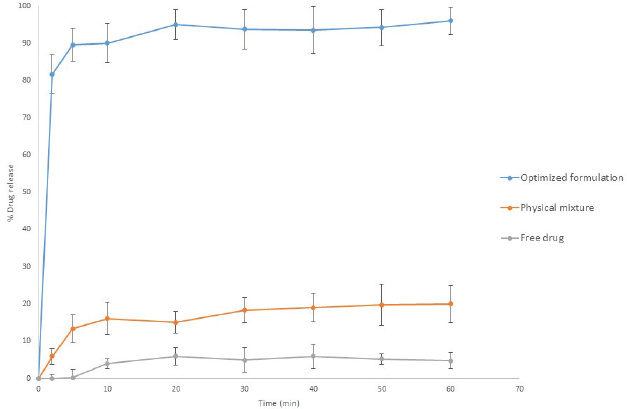 | Figure 11. In vitro release profile of tadalafil optimized composite nanofibers electrospun formulation F5 in comparison to physical mixture and free drug at 37 ±0.5°C in 0.1 N HCL. [Click here to view] |
.png) | Figure 12. In vitro release profile and scanning electron microscopical image of tadalafil-loaded optimized composite nanofibers electrospun formulation (F5) after 3 months storage at 40 ±0.5°C and 75±0.5 % RH. [Click here to view] |
Stability studies
Stability testing for the optimized formulation was carried out using the morphological characteristic and in vitro drug release as indicators for the composite nano-fibers stability at accelerated storage condition of 40°C ± 0.5°C and 75% ± 0.5% relative humidity (RH). The optimized formulation (F5) after 3 months maintained a uniform fiber homogeneity and diameter indicating the physical stability of the composite nanofibers under stress condition (Fig. 12). The drug release of freshly prepared samples presented 81% within 2 minutes while the stored samples demonstrated an average of 78.56% within the first 2 minutes as presented by Figure 12. Drug loading studied through High Pressure Liquid Chromatography (HPLC) presented equivalent results to that of fresh mats of 94.2% ± 1.4% with the absence of degradation product. The results indicate that the optimized formulation is stable and presented no significant loss of drug within the storage condition.
CONCLUSION
Tadalafil loaded composite nanofibers mat was prepared using PVP and PEO mixture via electrohydrodynamic atomization technique. Morphological elucidation indicates uniform nanofibers assembly. 32 full factorial model successfully established a relation between the independent factors (polymers concentrations) and dependent responses (yield, drug loading efficiency, fiber diameter, number of beads, and release rate). Nanofibers with efficient loading, fast drug release, and appropriate diameter were developed. The optimization of the formulation was performed and point analysis confirms the prognostic ability of the developed mathematical model. The optimized formulation elucidates through physicochemical examination; the presence of the drug in an amorphous state or as a molecular dispersion within the nanofibers. The formulation withstands 3 months accelerated stability study. The present study demonstrates the ability for future use of tadalafil nanofibers as an effective drug delivery system for oral delivery and bioavailability enhancement.
ACKNOWLEDGMENT
The authors are grateful for the Lebanese National Council for Scientific Research (CNRS) Grant Award for Lebanese graduates (Awardee; Jana Al wattar)
CONFLICTS OF INTEREST
Mohammed M. Mehanna, Jana K. Alwattar, and Roland Habchi declare that they have no conflict of interest.
REFERENCES
Abdel-Aziz A-AH, Asiri YA, El-Azab A, Al-Omar M a, Kunieda T. Tadalafil. profiles drug subs. Excip Relat Methodol, 2011; 36:287–329.
Aytimur A, Uslu Ä°. Promising materials for wound dressing: PVA/PAA/PVP electrospun nanofibers. Polym Plast Technol Eng, 2014; 53:655–60.
Badr-Eldin SM, Elkheshen SA, Ghorab MM. Inclusion complexes of tadalafil with natural and chemically modified beta-cyclodextrins. I: preparation and in-vitro evaluation. Eur J Pharm Biopharm, 2008; 70:819–27.
Brock GB, McMahon CG, Chen KK, Costigan T, Shen W, Watkins V, Anglin G, Whitaker S. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol, 2002; 168:1332–6.
Chuangchote S, Sagawa T, Yoshikawa S. Electrospinning of poly(vinyl pyrrolidone): effects of solvents on electrospinnability for the fabrication of poly(p-phenylene vinylene) and TiO2 nanofibers. J Appl Polym Sci, 2009; 114:2777–91.
Contardi M, Heredia-Guerrero JA, Perotto G, Valentini P, Pompa PP, Spanò R, Goldoni L, Bertorelli R, Athanassiou A, Bayer IS. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur J Pharm Sci, 2017; 104:133–44.
Da Costa FFP, Araújo ES, Nascimento MLF, De Oliveira HP. Electrospun fibers of enteric polymer for controlled drug delivery. Int J Polym Sci, 2015; 2015:1-8.
Demir MM, Yilgor I, Yilgor E, Erman B. Electrospinning of polyurethane fibers. Polymer (Guildf), 2002; 43:3303–9.
Folttmann BH, Quadir A. Polyvinylpyrrolidone (PVP)––one of the most widely used excipients in pharmaceuticals: an overview. Drug Deliv Technol, 2008; 8:24–27.
Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the pde-5 inhibitors sildenafil, vardenafil and tadalafil review of the literature. Eur J Med Res, 2002; 7:435–446.
Homayoni H, Ravandi SAH, Valizadeh M. Electrospinning of chitosan nanofibers: processing optimization. Carbohydr Polym, 2009; 77:656–61.
Hong SI, Oh SY. Dissolution kinetics and physical characterization of three-layered tablet with poly(ethylene oxide) core matrix capped by Carbopol. Int J Pharm, 2008; 356:121–9.
Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol, 2003; 63:2223–53.
Illangakoon UE, Gill H, Shearman GC, Parhizkar M, Mahalingam S, Chatterton NP, Williams GR. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int J Pharm, 2014; 477:369–79.
Jiang YN, Mo HY, Yu DG. Electrospun drug-loaded core-sheath PVP/zein nanofibers for biphasic drug release. Int J Pharm, 2012; 438:232–9.
Kazsoki A, Szabó P, Zelkó R. Prediction of the hydroxypropyl cellulose—poly(vinyl alcohol) ratio in aqueous solution containing papaverine hydrochloride in terms of drug loaded electrospun fiber formation. J Pharm Biomed Anal, 2017; 138:357–62.
Kim BS, Kim IS. Recent nanofiber technologies. Polym Rev, 2011; 51:235–8.
Li X, Kanjwal MA, Lin L, Chronakis IS. Electrospun polyvinyl-alcohol nanofibers as oral fast-dissolving delivery system of caffeine and riboflavin. Colloids Surf B Biointerfaces, 2013; 103:182–8.
Lu M, Xing H, Yang T, Yu J, Yang Z, Sun Y, Ding P. Dissolution enhancement of tadalafil by liquisolid technique. Pharm Dev Technol, 2017; 22:77–89.
Manna L, Banchero M, Sola D, Ferri A, Ronchetti S, Sicardi S. Impregnation of PVP microparticles with ketoprofen in the presence of supercritical CO2. J Supercrit Fluids, 2007; 42:378–84.
Mehanna MM, Alwattar JK, Elmaradny HA. Optimization, physicochemical characterization and in vivo assessment of spray dried emulsion: a step toward bioavailability augmentation and gastric toxicity minimization. Int J Pharm, 2015; 496:766–79.
Mehanna MM, Motawaa AM, Samaha MW. In sight into tadalafil––block copolymer binary solid dispersion: mechanistic investigation of dissolution enhancement. Int J Pharm, 2010; 402:78–88.
Mehanna MM, Motawaa AM, Samaha MW. Tadalafil inclusion in microporous silica as effective dissolution enhancer: optimization of loading procedure and molecular state characterization. J Pharm Sci, 2011; 100:1805–18.
Noor SAM, Ahmad A, Talib IA, Rahman MYA. Morphology, chemical interaction, and conductivity of a PEO-ENR50 based on solid polymer electrolyte. Ionics (Kiel), 2010; 16:161–70.
Ojha N, Prabhakar B. Advances in solubility enhancement techniques. Int J Pharm Sci Rev Res, 2013; 21:351–8.
Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng, 2006; 12:1197–211.
Ranjbar-Mohammadi M, Zamani M, Prabhakaran MP, Bahrami SH, Ramakrishna S. Electrospinning of PLGA/gum tragacanth nanofibers containing tetracycline hydrochloride for periodontal regeneration. Mater Sci Eng C, 2016; 58:521–31.
Reddy BP, Reddy KA and Reddy MS. Validation and stability indicating RP-HPLC method for the determination of tadalafil API in pharmaceutical formulations.Res In Pharma Biotechnology, 2011; 2: 001-006.
Saquib Hasnain M, Nayak AK. Solubility and dissolution enhancement of ibuprofen by solid dispersion technique using peg 6000-PVP K 30 combination carrier. Chemistry (Easton), 2012; 21: 118–32.
Shen X, Yu D, Zhu L, Branford-White C, White K, Chatterton NP. Electrospun diclofenac sodium loaded Eudragit L 100-55 nanofibers for colon-targeted drug delivery. Int J Pharm, 2011; 408:200–7.
Singh B, Garg T, Goyal AK, Rath G. Development, optimization, and characterization of polymeric electrospun nanofiber: a new attempt in sublingual delivery of nicorandil for the management of angina pectoris. Artif. Cells, Nanomedicine, Biotechnol, 2015;44:1–10.
Sipos E, Szabó ZI, Rédai E, Szabó P, Sebe I, Zelkó R. Preparation and characterization of nanofibrous sheets for enhanced oral dissolution of nebivolol hydrochloride. J Pharm Biomed Anal, 2016; 129:224–8.
Son WK, Youk JH, Lee TS, Park WH. The effects of solution properties and polyelectrolyte on electrospinning of ultrafine poly(ethylene oxide) fibers. Polymer (Guildf), 2004; 45:2959–66.
Taepaiboon P, Rungsardthong U, Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. Eur J Pharm Biopharm, 2007; 67:387–97.
Uppal R, Ramaswamy GN, Arnold C, Goodband R, Wang Y. Hyaluronic acid nanofiber wound dressing-production, characterization, and in vivo behavior. J Biomed Mater Res––Part B Appl Biomater, 2011; 97 B:20–9.
Varma M. Mohan, Sravani V. SP V. Design and evaluation of electrospun nanofibers for the enhancement of dissolution rate of meloxicam. J Bionanoscience, 2013; 7:560–7(8).
Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine 2006; 1:15–30.
Vemula VR, Lagishetty V, Lingala S. Solubility enhancement techniques. Int J Pharm Sci Rev Res, 2010; 5:41–51.
Vigh T, Horváthová T, Balogh A, Sóti PL, Drávavölgyi G, Nagy ZK, Marosi G. Polymer-free and polyvinylpirrolidone-based electrospun solid dosage forms for drug dissolution enhancement. Eur J Pharm Sci, 2013; 49:595–602.
Vinesha V, Sevukarajan M, Rajalakshmi R, Chowdary GT, Haritha K. Enhancement of solubility of tadalafil by cocrystal approach. Int Res J Pharm, 2016; 4:218–23.
Vrbata P, Berka P, Stránská D, Doležal P, LázníÄek M.. Electrospinning of diosmin from aqueous solutions for improved dissolution and oral absorption. Int J Pharm, 2014; 473:407–13.
Vrbata P, Berka P, Stránská D, Doležal P, Musilová M, ÄŒižinská L. Electrospun drug loaded membranes for sublingual administration of sumatriptan and naproxen. Int J Pharm, 2013; 457:168–76.
Vyas V, Sancheti P, Karekar P, Shah M, Pore Y. Physicochemical characterization of solid dispersion systems of tadalafil with poloxamer 407. Acta Pharm, 2009; 59:453–61.
Wang S, Mo XM, Jiang BJ, Gao CJ, Wang HS, Zhuang YG, Qiu LJ. Fabrication of small-diameter vascular scaffolds by heparin-bonded P(LLA-CL) composite nanofibers to improve graft patency. Int J Nanomedicine, 2013; 8:2131–9.
Xin Y, Huang Z, Chen J, Wang C, Tong Y, Liu S. Fabrication of well-aligned PPV/PVP nanofibers by electrospinning. Mater Lett, 2008; 62:991–3.
Xu J, Zhang J, Gao W, Liang H, Wang H, Li J. Preparation of chitosan/PLA blend micro/nanofibers by electrospinning. Mater Lett, 2009; 63:658–60.
Yang Q, Zhenyu LI, Hong Y, Zhao Y, Qiu S, Wang CE, Wei Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J Polym Sci Part B Polym Phys, 2004; 42:3721–6.
Yang Z, Zhao X. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell-scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int J Nanomedicine, 2011; 6:303–10.
Yu D-G, Branford-White C, Shen X-X, Zhang X-F, Zhu L-M. Solid dispersions of ketoprofen in drug-loaded electrospun nanofibers. J Dispers Sci Technol, 2010a; 31:902–8.
Yu DG, Yang JM, Branford-White C, Lu P, Zhang L, Zhu LM. Third generation solid dispersions of ferulic acid in electrospun composite nanofibers. Int J Pharm, 2010b; 400:158–64.
Yu DG, Zhu LM, Branford-White CJ, Yang JH, Wang X, Li Y, Qian W. Solid dispersions in the form of electrospun core-sheath nanofibers. Int J Nanomedicine, 2011; 6:3271–80.
Zeng J, Yang L, Liang Q, Zhang X, Guan H, Xu X, Chen X, Jing X. Influence of the drug compatibility with polymer solution on the release kinetics of electrospun fiber formulation. J Control Release, 2005; 105:43–51.
Zhou Y, Qi P, Zhao Z, Liu Q, Li Z. Fabrication and characterization of fibrous HAP/PVP/PEO composites prepared by sol-electrospinning. RSC Adv, 2014; 4:16731.