Comparison between Microplate Spectrometry and LC/MS Chromato-graphy for Facile Pilot Pharmacokinetics and Biodistribution Studies of Doxorubicin-loaded Nanoparticle Drug Carriers
Pengxiao Cao and Younsoo Bae
DOI: 10.7324/JAPS.2012.2901Pages: 001-009

A Study of Potential Drug-Drug Interactions in Indoor Patients of Medicine Department at a Tertiary Care Hospital
Jigar Kapadia, Dhaval Thakor, Chetna Desai and R. K. Dikshit
DOI: 10.7324/JAPS.2013.31015Pages: 089-096

Bioavailability of karanjin from Pongamia pinnata L. in Sprague dawley rats using validated RP-HPLC method
Naresh Shejawal, Sasikumar Menon, Sunita Shailajan
DOI: 10.7324/JAPS.2014.40303Pages: 010-014

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Estimation of Pharmacokinetic Parameters Using Nonlinear Fixed Effects One Compartment Open Model
Mithun Kumar Acharjee, Uttom Kumar, Sitesh Chandra Bachar, Wasimul Bari
DOI: 10.7324/JAPS.2014.41210Pages: 056-059

Pharmacokinetics interaction of dapoxetine with different doses of green tea extract in male healthy volunteers using midazolam as CYP3A4 enzyme probe
Khaled Sobhy Abdelkawy, Ahmed M Donia, Mahmoud S Abdallah
DOI: 10.7324/JAPS.2015.501201Pages: 001-007

A Simple and Sensitive HPLC/UV Method for Determination of Meloxicam in Human Plasma for Bioavailability and Bioequivalence Studies
Laila H. Emara, Maha F. Emam, Nesrin F. Taha, Hala M. Raslan, Ahmed A. El-Ashmawy
DOI: 10.7324/JAPS.2016.60702Pages: 012-019

Liquid Chromatographic Assay for the Analysis of Kanamycin sulphate nanoparticles in Rat after intramuscular administration: Application to a Pharmacokinetic Study
Sanaul Mustafa, V. Kusum Devi
DOI: 10.7324/JAPS.2016.60809Pages: 057-066

Investigation of possible pharmacokinetic interaction of metformin with sugar replacement sweeteners in rats
Riad Awad, Eyad Mallah, Israa Al-Ani, Wael Abu Dayyih, Zainab Zakarya, Tawfiq Arafat
DOI: 10.7324/JAPS.2016.601029Pages: 210-215

Discovery of two diacetylene glycosides as human uridine-cytidine kinase 2 inhibitors: an in silico approach
Magdi A. Mohamed, Amina I. Dirar, Sami Hamdoun
DOI: 10.7324/JAPS.2016.601106Pages: 034-039

Chromatogram profiles of andrographolide in A23187-induced New Zealand rabbit’s urine and faeces
Jutti Levita, Tanti Juwita, Selma Ramadhani, Nyi Mekar Saptarini, Mutakin Mutakin
DOI: 10.7324/JAPS.2017.70121Pages: 156-159

Pharmacokinetics of a new imidazoline receptor agonist in rat plasma after intragastric and intravenous administration
Kulikov Aleksandr, Avtina Tatyana, Pokrovsky Mikhail, Korokin Mikhail
DOI: 10.7324/JAPS.2017.70302Pages: 006-008

The natural anti-tubercular agents: In silico study of physicochemical, pharmacokinetic and toxicological properties
Mohammad Firoz Khan, Md. Abdul Bari, Md. Kamrul Islam, Md. Shariful Islam, Md. Shahidulla Kayser, Nusrat Nahar, Md. Al Faruk, Mohammad A. Rashid
DOI: 10.7324/JAPS.2017.70506Pages: 034-038

A validated LC-MS/MS method for the pharmacokinetic study of alogliptin in healthy rabbits
Yatha Ravi, Bigala B. Rajkamal
DOI: 10.7324/JAPS.2019.90204Pages: 029-037
Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties
Eric Wei Chiang Chan, Chen Wai Wong, Yong Hui Tan, Jenny Pei Yan Foo, Siu Kuin Wong, Hung Tuck Chan
DOI: 10.7324/JAPS.2019.90717Pages: 124-129

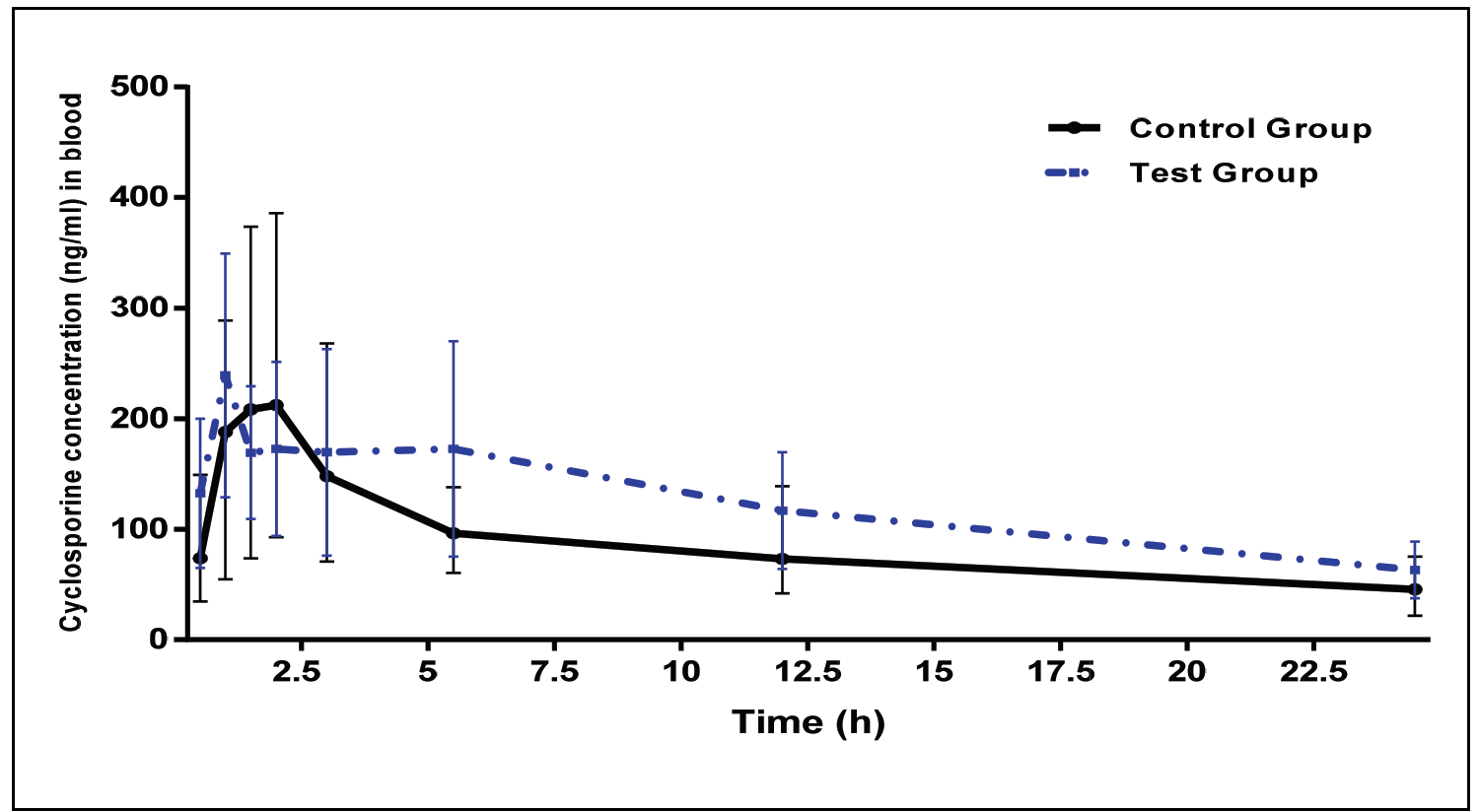
Pharmacokinetic drug–drug interaction study between clindamycin and cyclosporin in rabbits
Issam Mohammed Abushammala, Lama Attef El Gussein, Badea Elzaman Zomlot, Kamal Fakher Abushammalleh, Mohammed Mahmoud Taha, Mohammed Yousef Miqdad
DOI: 10.7324/JAPS.2020.102016Pages: 108-111
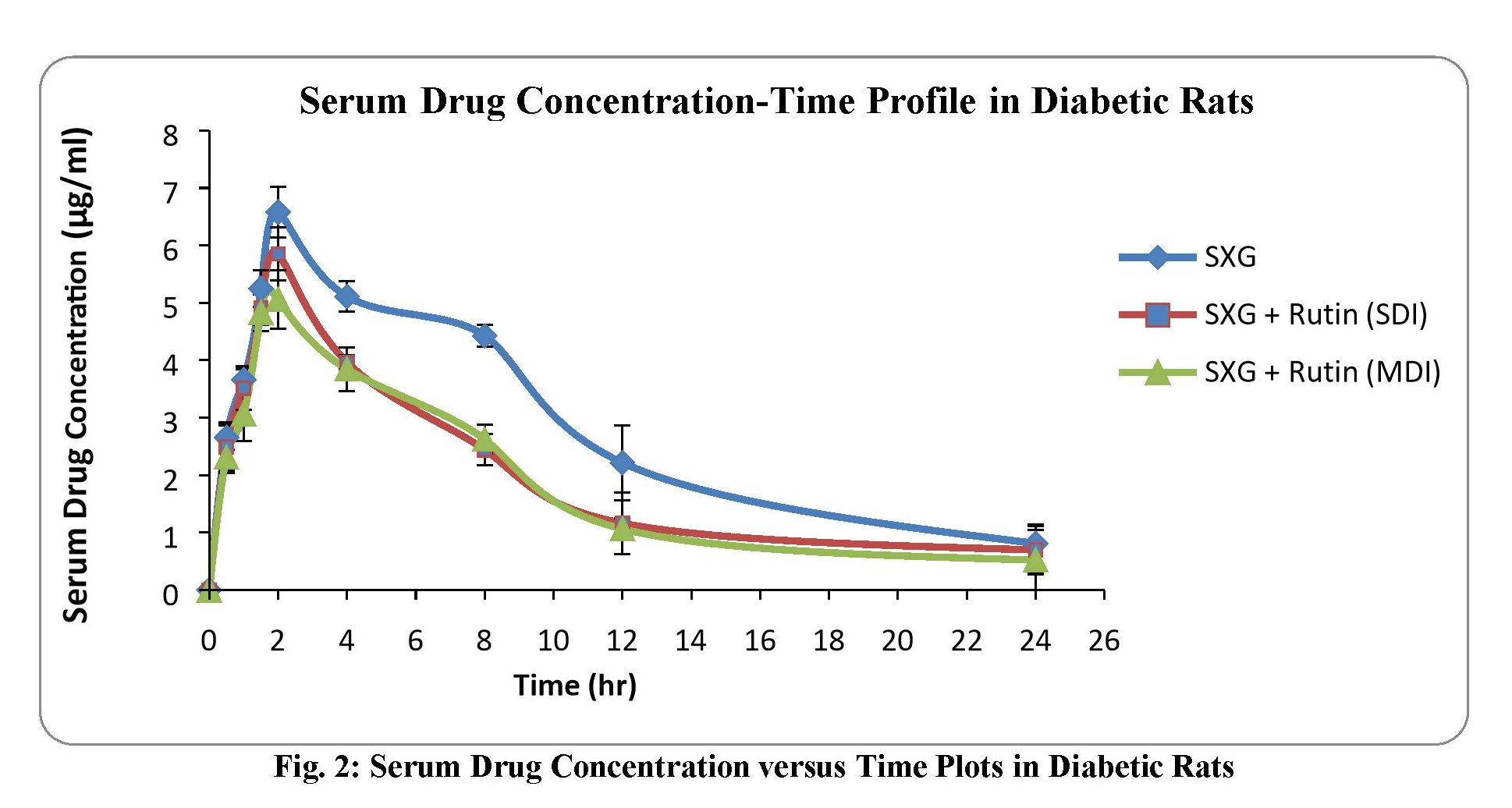
Study of alterations in pharmacokinetics and pharmacodynamics of Saxagliptin in the presence of Rutin: An interaction study in rats
Naga Raju Kandukoori, Pavani Uppu, Narsimha Reddy Yellu
DOI: 10.7324/JAPS.2020.101111Pages: 081-086
Effect of piperine and its analogs on pharmacokinetic properties of sorafenib tosylate: Bioanalytical method development and validation
Anshuly Tiwari, Kakasaheb R. Mahadik, Satish Y. Gabhe
DOI: 10.7324/JAPS.2020.101201Pages: 001-012
Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053

Method development and validation of LC–ESI–MS/MS method for the quantification of sonidegib in healthy rabbits
Vankayala Devendiran Sundar, Kumar Raja Jayavarapu, Parimala Krishnan
DOI: 10.7324/JAPS.2021.110510Pages: 071-078

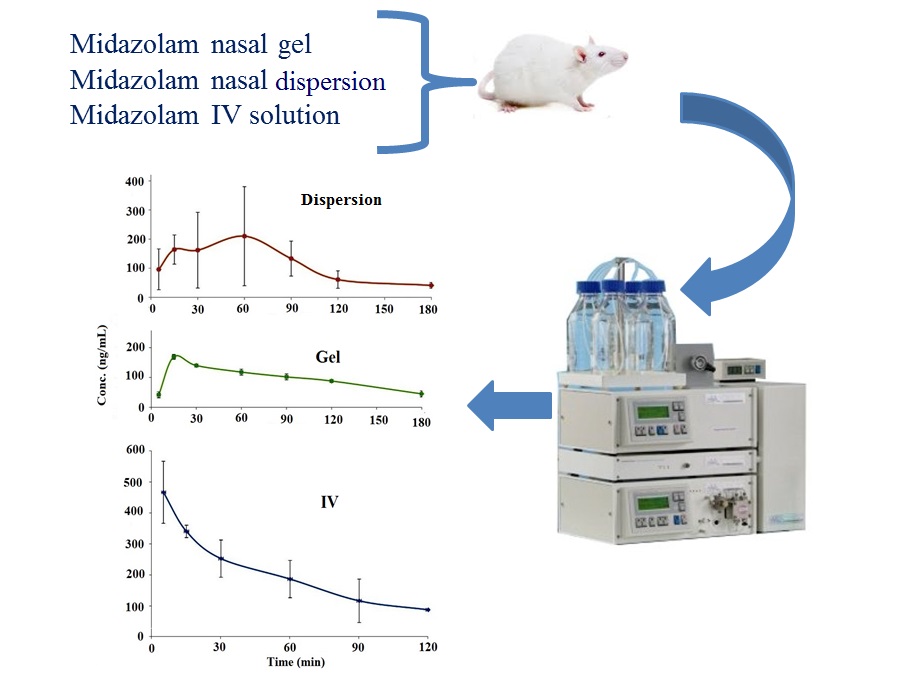
Pharmacokinetics and bioavailability of midazolam in rats following single dose administration of intravenous solution, intranasal dispersion, and in situ nasal gel
Elahehnaz Parhizkar, Saba Movaffagh, Shohreh Alipour
DOI: 10.7324/JAPS.2021.1101109Pages: 070–075
Clinical awareness of therapeutic drug monitoring among medical students—A descriptive cross-sectional study
Amberkar Mohanbabu Vittalrao, Aditya Kumar Adhikarla, Sadhana N. Holla, Meena Kumari Kamalkishore, Seema Kumari Kamal Kishore
DOI: 10.7324/JAPS.2021.1101105Pages: 034–045

Gentamicin pharmacokinetics and pharmacodynamic correlation in pediatrics - A systematic review
Keerthana Chandrasekar, Vahini B, Vijay V, Shalini R, Arun KP
DOI: 10.7324/JAPS.2021.1101102Pages: 011-017

Chrysin or mannitol for treatment of acute kidney injury: Evidence for pharmacokinetic interaction
Heba M. I. Abdallah, Sally A. El Awdan, Salma A. El-Marasy, Omar A. Ahmed-Farid, Azza Hassan
DOI: 10.7324/JAPS.2021.1101213Pages: 139–150

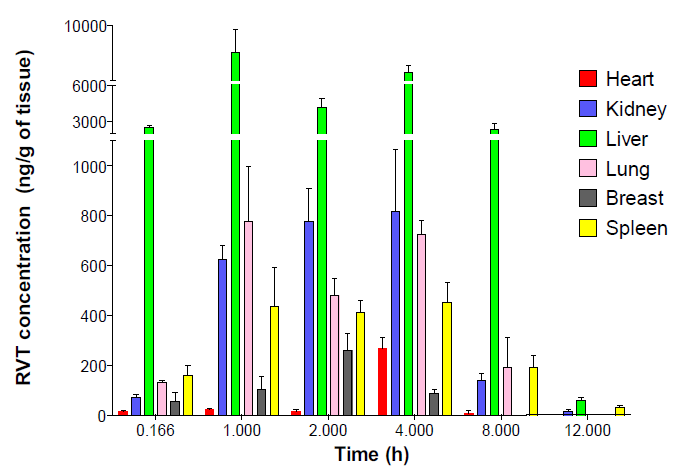
Bioanalytical RP-HPLC method validation for resveratrol and its application to pharmacokinetic and drug distribution studies
Shivaprasad Gadag, Reema Narayan, Yogendra Nayak, Usha Yogendra Nayak
DOI: 10.7324/JAPS.2021.120216Pages: 158-164
In silico screening of hispolon and its analogs: Pharmacokinetics and molecular docking studies with cyclooxygenase-2 enzyme
Mohadese Mohammadi, Mohammad Firoz Khan, Ridwan Bin Rashid, Sina Mirzaie Nokhostin, Mohammad A. Rashid
DOI: 10.7324/JAPS.2022.120509Pages: 120-128

N-Heterocycle derivatives: An update on the biological activity in correlation with computational predictions
Sahar Saleh Alghamdi, Rasha Saad Suliman, Rawan Awadh Alshehri, Razan Suleiman Almahmoud, Renad Ibrahim Alhujirey
DOI: 10.7324/JAPS.2022.120504Pages: 059-077
QbD-driven HPLC method for the quantification of rivastigmine in rat plasma and brain for pharmacokinetics study
Divya Gopalan, Prajakta H. Patil, Puralae Channabasavaiah Jagadish, Suvarna G. Kini, Angel Treasa Alex, Nayanabhirama Udupa, Srinivas Mutalik
DOI: 10.7324/JAPS.2022.120606Pages: 056-067

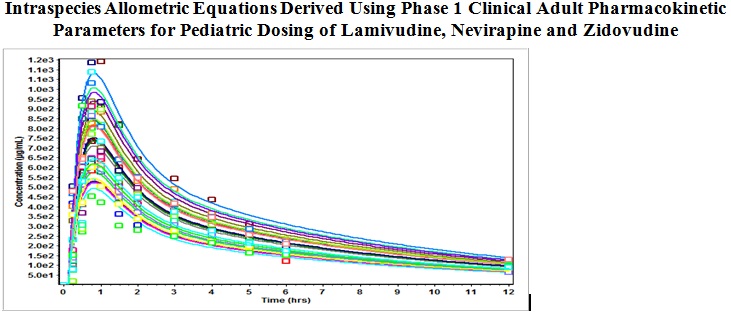
Intraspecies allometric equations derived using phase 1 clinical adult pharmacokinetic parameters for pediatric dosing of lamivudine, nevirapine, and zidovudine
Amusa Sarafadeen Adebayo, Ravikiran Panakanti
DOI: 10.7324/JAPS.2022.120804Pages: 026-035
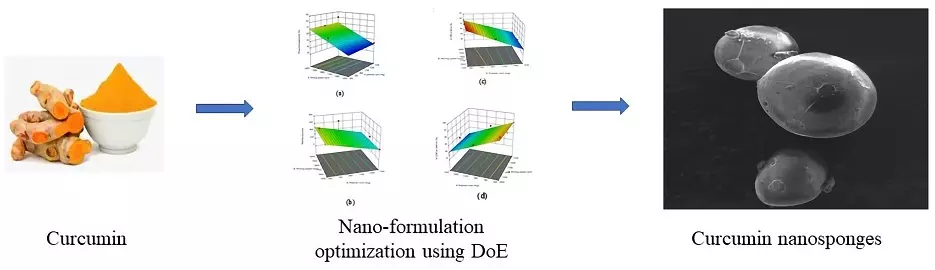
Design, formulation, and evaluation of curcumin-loaded nanosponges for the effective management of colitis
Praharsh Kumar Mandadhi Rajendra, Karthik Ganesan, Bala Sai Souith Nidamanuri, Jawahar Natarajan, Nirmala Puttaswamy
DOI: 10.7324/JAPS.2022.121207Pages: 059-071
Development and validation of LC-MS/MS method for alpelisib quantification in human plasma: Application to pharmacokinetics in healthy rabbits
Tandrima Majumder, Shiva Kumar Gubbiyappa
DOI: 10.7324/JAPS.2023.75269Pages: 089-096

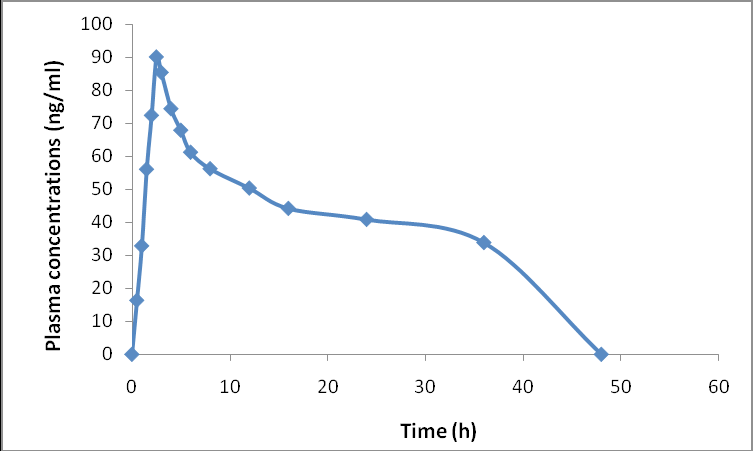
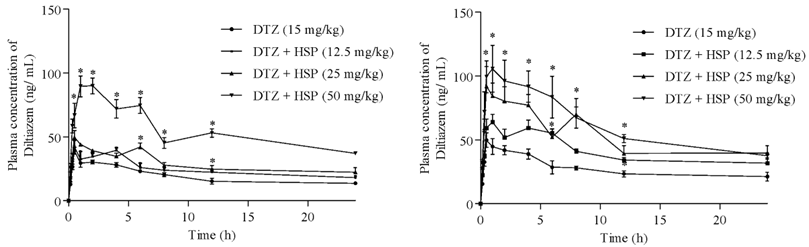
Influence of hesperetin on the pharmacokinetics of diltiazem in rats
Ravindra Babu Pingili, Surya Sandeep Mullapudi, Sridhar Vemulapalli, Naveen Babu Kilaru
DOI: 10.7324/JAPS.2023.118669Pages: 093-099
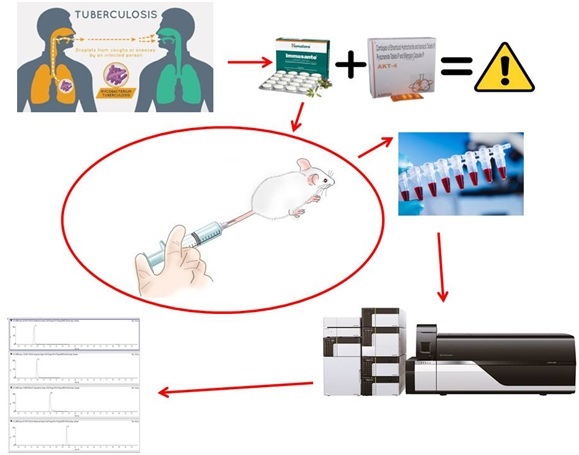
A validated LC-MS/MS method for simultaneous quantification of antitubercular drugs in rat plasma and its application for a pharmacokinetic interaction study with Immusante®
Lakavalli Mohankumar Sharath Kumar, Mohammed Mukhram Azeemuddin, Krishna Chaitanya Routhu, Keerthi Priya, Uddagiri Venkanna Babu, Sreedhara Ranganath Pai
DOI: 10.7324/JAPS.2023.118956Pages: 151-158
A validated LC-MS/MS method for studying the pharmacokinetic interaction of Immusante® and antiviral drugs in rats
Lakavalli Mohankumar Sharath Kumar, Mohammed Mukhram Azeemuddin, Tirumalai Ramanujam Tulasi, Madhavan Vijayakumar, Uddagiri Venkanna Babu, K Sreedhara Ranganath Pai
DOI: 10.7324/JAPS.2023.127117Pages: 125-131
_.jpg)
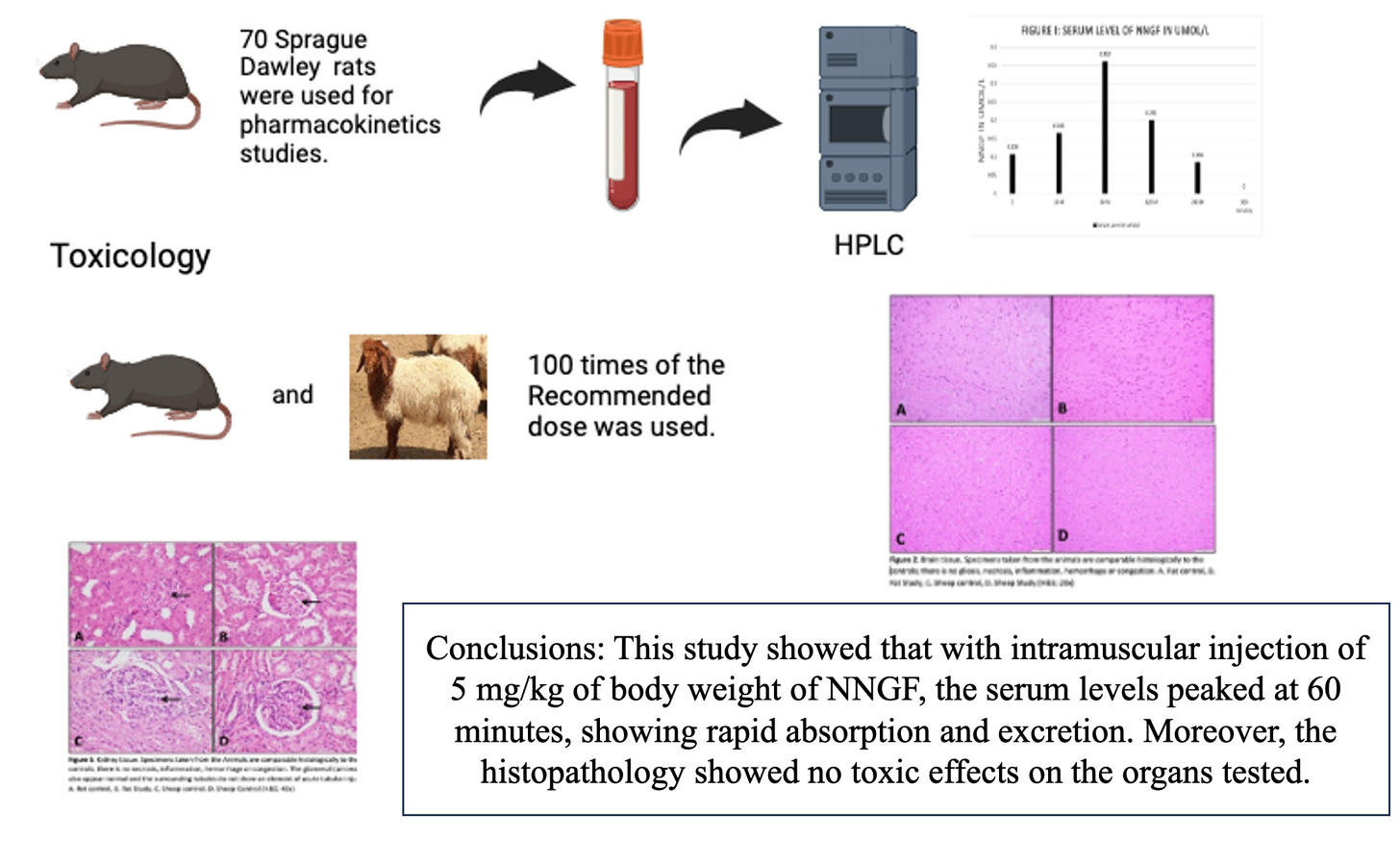
Pharmacokinetics and toxicology studies of new neuronal growth factor
Mir Sadat-Ali, Dakheel A Al-Dakheel, Khulood S. Al Ghamdi, Shahanas Chathoth, Fadel A. Al-Omar, Hassan A. Al Saad, Ayesha Ahmed
DOI: 10.7324/JAPS.2024.112811Pages: 120-124
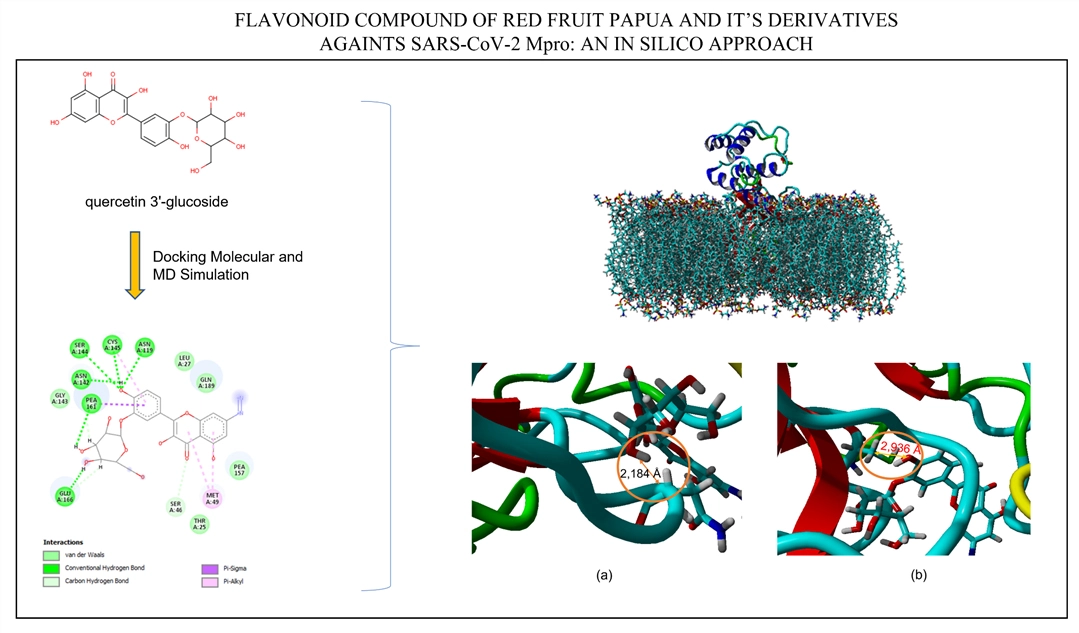
Flavonoid compound of red fruit papua and its derivatives against sars-cov-2 mpro: An in silico approach
Agus Dwi Ananto, Harno Dwi Pranowo, Winarto Haryadi, Niko Prasetyo
DOI: 10.7324/JAPS.2024.177392Pages: 090-097
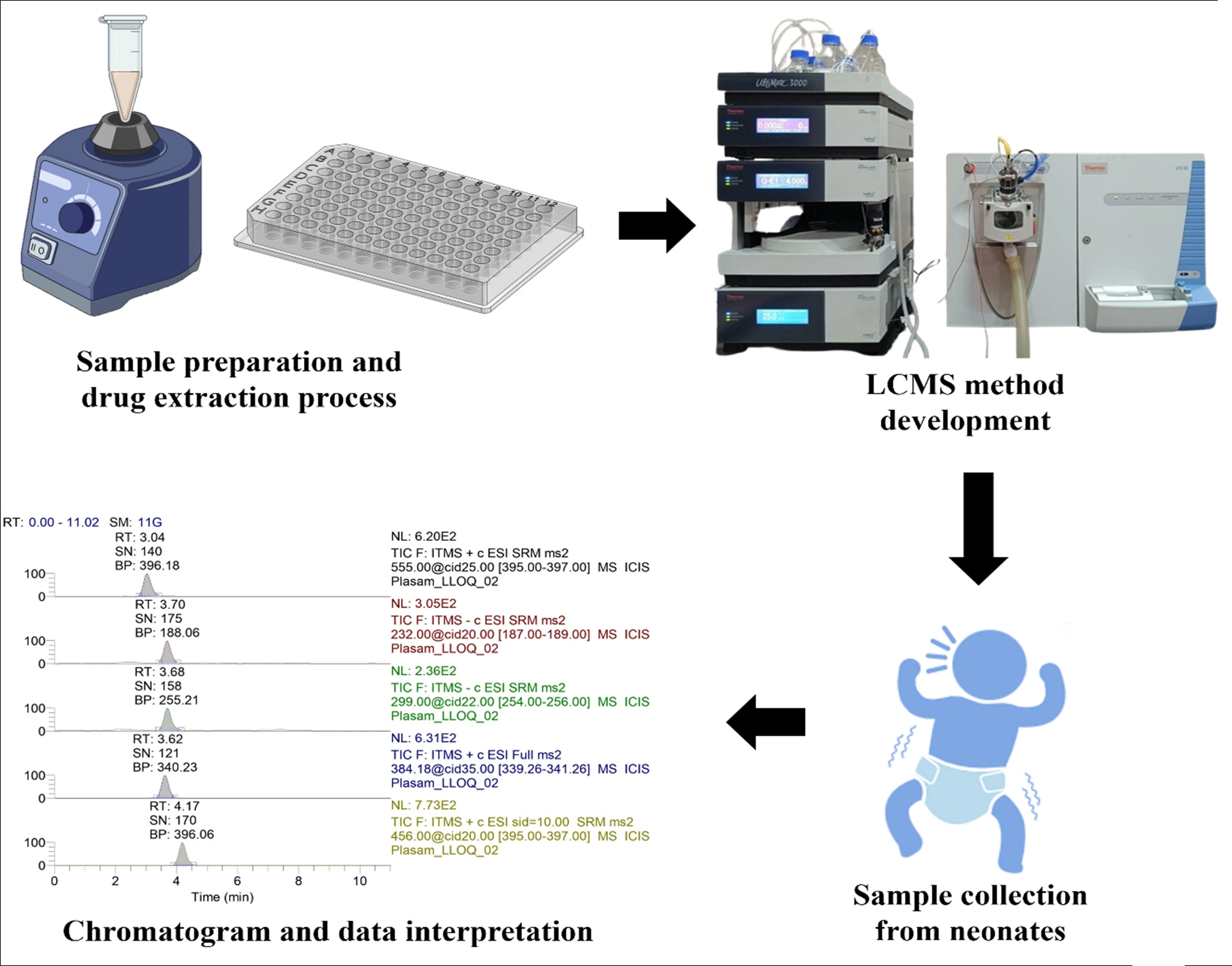
A comparative evaluation of the plasma and DBS-based LC-MS/MS methods for the simultaneous analysis of nine antibiotics for application to pharmacokinetic evaluations and precision dosing in neonates
Bhim Bahadur Chaudhari, Leslie E. Lewis, M. Surulivel Rajan, Ashutosh Gupta, Moumita Saha, Shivani Kunkalienkar, Sudheer Moorkoth
DOI: 10.7324/JAPS.2025.214611Pages: 129-142
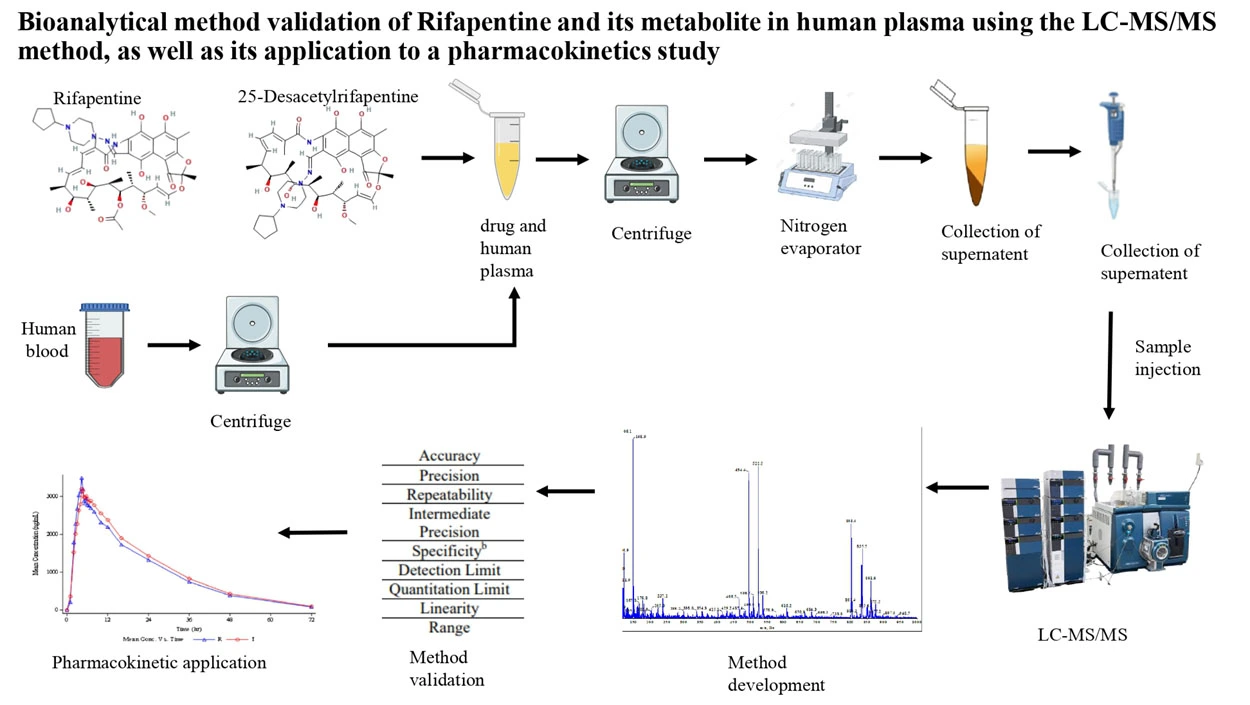
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201
Development and validation of an LC-MS/MS method for pharmacokinetic assessment of tucatinib in rat plasma
Bandaru Venkata Ramarao, Anand Solomon Kamalakaran
DOI: 10.7324/JAPS.2025.202389Pages: 225-233
_.jpg)
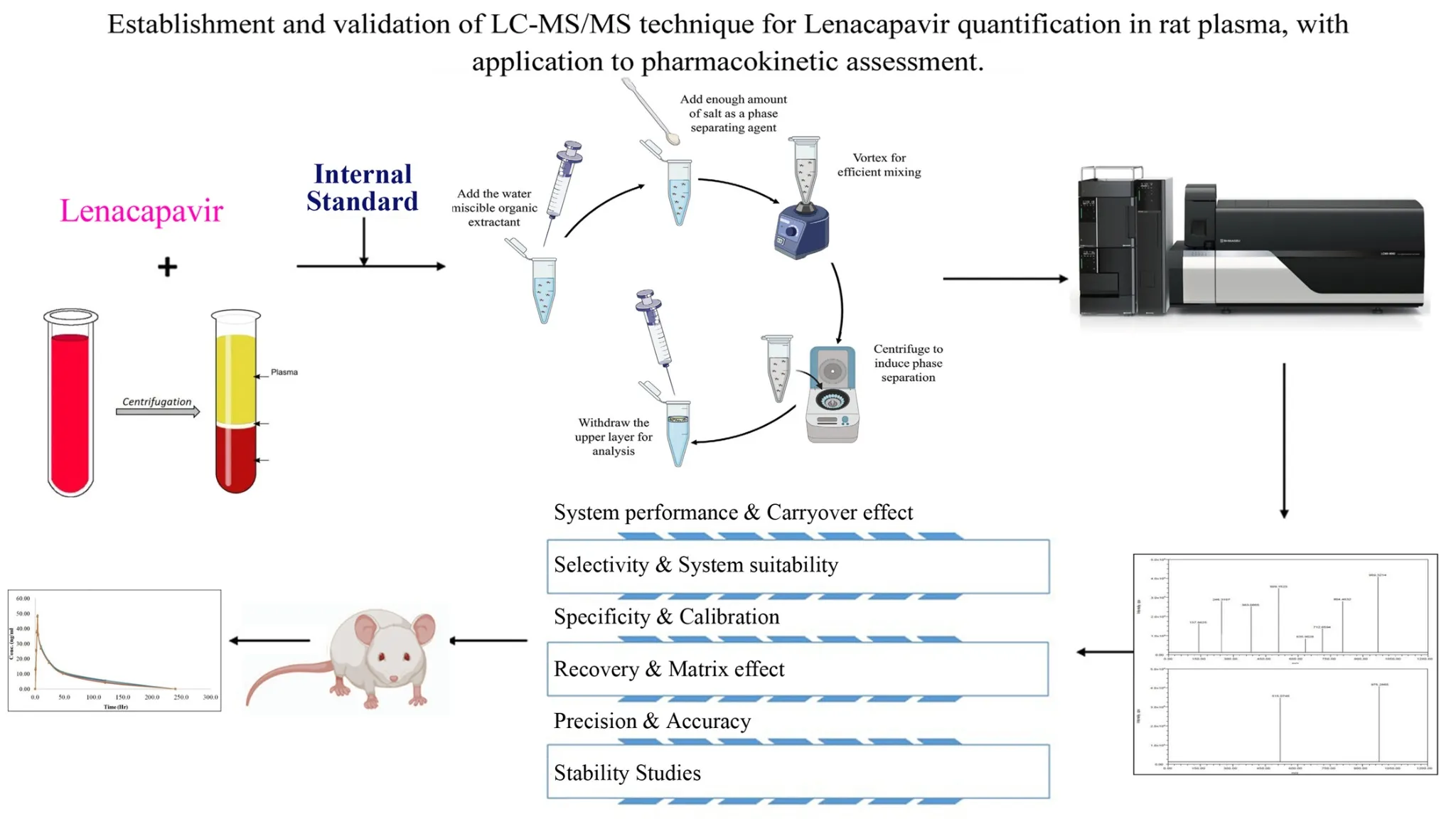
Establishment and validation of LC-MS/MS technique for Lenacapavir quantification in rat plasma, with application to pharmacokinetic assessment
Edward Raju Gope, Srikanth Pottendla, Suneetha Yaparthi
DOI: 10.7324/JAPS.2025.229006Pages: 112-120