INTRODUCTION
Diltiazem is majorly prescribed to treat angina, supraventricular arrhythmias, and hypertension (Chaffman and Brogden, 1985; Pool, 1996; Weir, 1995). It undergoes an extensive presystemic metabolism because it is a substrate of cytochrome P-450 3A4 (CYP3A4) and P-glycoprotein (P-gp) (Lefebvre et al., 1996; Buckley et al., 1990). The bioavailability of diltiazem is approximately 40% and was found to be metabolized mainly into N-demethyldiltiazem in humans and dogs. The most common metabolites in rabbits and rats were desacetyldiltiazem and O-diacetyl-N-monomethyl diltiazem, correspondingly (Yeung et al., 1998). CYP3A4 is the major human variant of diltiazem N-demethylation in liver microsomes, and it’s also located in the gut (Pichard et al., 1990). The metabolism of diltiazem can be more in the proximal segment of the small intestine than in the distal section (Kolars et al., 1992; Watkins et al., 1987). The P-gp and CYP34A might decrease the oral absorption of diltiazem. The calcium channel blockers verapamil, nicardipine, and diltiazem compete to suppress P-gp multidrug resistance, according to Yusa and Tsuruo (1989).
Diltiazem is not only an Multidrug Resistance modulator but also a precursor for CYP 3A4 and the P-gp efflux transporter Wacher et al. (2001). Flavonoids are produced by a huge variety of plants in large quantities (Dixon and Steele 1999). Hesperetin is a flavanone found naturally in citrus and grapefruits that have been shown to have anti-cancer, antioxidant, anti-inflammatory, anti-hypertensive, anti-atherogenic, hepatoprotective, and neuroprotective properties (Choi and Ahn 2008). Hesperetin can pass the blood-brain barrier. Furthermore, earlier research has shown that hesperetin and naringenin are CYP enzyme and P-gp inhibitors (Sridhar et al., 2014; Surya et al., 2014). The present study aimed to investigate the influence of hesperetin on the diltiazem pharmacokinetics (PK) in rats.
MATERIALS AND METHODS
Drugs and chemicals
Throughout the investigation, analytical grade compounds were employed. Hesperetin was furnished by Sigma Aldrich. Diltiazem and ritonavir were provided as complimentary samples by Sipra Labs in Hyderabad, India. Finisar Chemicals Ltd. of Ahmadabad, India, provided acetonitrile for high-performance liquid chromatography (HPLC).
Laboratory animals
Wister rats (Male) weighing 160–200 g were procured from CPCSEA registered breeder. Animals were quarantined, acclimatized for at least 1 week, housed six per cage. The approved animal experiments protocol was KVSRSCOPS/11-03-14-008.
Study design
The current investigation involves two experiments, acute (single dose) and sub-acute (once daily for 15 days) administration of diltiazem and hesperetin, as previously reported (Challa et al., 2013).
In vivo pharmacokinetic studies
Male Wistar rats were used throughout the study and randomly assigned to four groups of six animals each per study. After overnight fasting, the animals in group 1 was treated with 15 mg/kg (oral) diltiazem in 1% Sodium Carboxymethylcellulose (SCMC) while the other groups viz., groups 2, 3, & 4 were administered with 12.5, 25, and 50 mg/kg hesperetin in 2% SCMC p.o, respectively, followed by diltiazem, 15 mg/kg p.o in single-dose PK study (SDS). Similarly, in multiple dosing PK studies (MDS), the animals were given the same drugs once a day for 15 days.
Everted rat gut sac study
Preparation of gut sac
The rat-everted gut sac model, a simple and effective in vitro model for evaluating drug absorption and processes by assessing drug content in the colon and conveying it via intestinal tissue, is used to assess diltiazem transport across the intestine (Barthe et al., 1999). Babu et al. (2013) presented a modified method for preparing everted sacs of rat ileum. The ileum of a rat was removed and flushed with ice-cold saline multiple times (0.9%). Under phenobarbitone (40 mg/kg) anesthesia, the distal part of the ileum from the rat intestines (approximately 15 cm each) was removed from an over day fasted male Wistar rat weighing around 180–220 g, everted after removal of fat and mesenteric connectors, and fused with a silk incision to make into sacs (Capraro et al., 2011).
Effect of hesperetin on diltiazem transport across gut sac
The everted sacs were supplied with a mixture containing diltiazem 50 µg/ml in the presence or absence of ritonavir 50 µg/ml, conventional CYP3A4, and P-gp blocker (Kharasch et al., 2008; Kumar et al., 1996; Li et al., 2012), and hesperetin 50, 100, and 200 µg/ml. Diltiazem travel from the serosal to the mucosal side of the everted sac was measured by collecting 1 ml of the outer medium [replaced by 1 ml Krebs–Ringer bicarbonate buffer (KRB) buffer] at 10, 20, 30, and 60 minutes from an Erlenmeyer flask comprising 30mL of oxygenated (O2/CO2; 95:5) KRB and incubating in a shaker bath at 37°C for 60 minutes. Each experiment was repeated three times.
Analytical methods
Diltiazem plasma concentrations were measured with changes using a technique published by Li et al., 2003). Briefly, a Shimadzu HPLC system with a pump (LC-20AT VP), a C18 column (Kromasil 150 × 4.6 mm) with a particle size of 5 µm and a dual-wavelength ultraviolet (UV) visible detector (SPD-10A VP) was used. liquid chromatography solution software was used to gather and process the data. The mobile phase consisted of 0.2% formic acid solution in acetonitrile and water (80:20 v/v) that was ultrasonically degassed and filtered through a 0.45 µm membrane filter. The effluent was monitored at 235 nm with a UV detector at a flow rate of 1 ml/minute. The total run time was 5.0 minutes and the diltiazem eluted at 4.8 minutes (Fig. 1). The analysis was performed at room temperature.
Extraction of diltiazem from plasma
Diltiazem was extracted from rat plasma using the liquid-liquid extraction method (Kallem et al., 2013). 1.5 ml tert-butyl methyl ether was added to a 50 µl plasma aliquot, vortexed, and centrifuged at 6,000 rpm for 5 minutes in each step. The residue (1.2 ml) was dried in a moderate nitrogen stream at 40°C. The dried residue was reconstituted and used for chromatography (Fig. 1).
Calculation of PK parameters
Thermo Kinetica was used to perform a non-compartmental PK analysis of each rat’s plasma concentrations versus time data.
Statistical analysis
GraphPad Prism software was used for data analysis and p-value < 0.05 was considered significant.
RESULT
Influence of hesperetin on the PK of diltiazem in SDS
Diltiazem plasma concentrations versus time curves after oral administration of diltiazem alone and pre-treatment with hesperetin 12.5, 25, and 50 mg/kg in SDS are shown in Figure 2. Except for Tmax, all PK parameters were logarithmically converted and compared using one-way ANOVA and Dunnett’s multiple comparisons test. The mean plasma PK parameters are shown in Table 1. Hesperetin raised the Cmax, area under the curve (AUC) 0-24, AUC0-, t1/2, and Mean Residence Time (MRT) of diltiazem and lowered the clearance and volume of distribution of diltiazem considerably (p < 0.001). The Cmax of diltiazem was changed from 39.273 ± 4.265 to 49.715 ± 6.855 and 39.273 ± 4.265 to 89.946 ± 7.222 ng/ml at a dose of hesperetin 25, 50 mg/kg correspondingly. The AUC0-24 of Diltiazem was significantly increased from 454.722 ± 52.362 to 593.578 ± 42.841 and 454.722 ± 52.362 to 665.135 ± 84.250 and 454.722 ± 52.362 to 1373.784 ± 142.545 ng/ml/hour at the dose of hesperetin 12.5, 25, 50 mg/kg respectively. The AUC0-∞ of diltiazem was increased from 818.206 ± 65.241 to 993.643 ± 74.325 and 818.206 ± 65.241 to 1472.611 ± 121.471 and 818.206 ± 65.241 to 2613.662 ± 187.266 ng/hour/ml at a dose of hesperetin 12.5, 25, 50 mg/kg, individually. The tmax does not change from 0.5 ± 0.1 hour. The t1/2 of diltiazem was raised from 18.471 ± 1.584 to 30.463 ± 3.674 and 18.471 ± 1.584 to 24.886 ± 4.278 and 18.471 ± 1.584 to 23.090 ± 3.625 hour at a dose of hesperetin 12.5, 25, 50 mg/kg correspondingly. The MRT of Diltiazem was increased from 28.128 ± 2.639 to 28.450 ± 3.485 and 28.128 ± 2.639 to 37.702 ± 5.475 and 28.128 ± 2.639 to 32.588 ± 4.556 hour at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The clearance of diltiazem was decreased from 3.000 ± 0.341 to 0.009 ± 0.0001 and 3.000 ± 0.341 to 0.002 ± 0.0001 and 3.000 ± 0.341 to 0.001 ± 0.0001 l/hour/kg at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The volume of distribution of diltiazem was decreased from 0.111 ± 0.01 to 0.007 ± 0.0001 and 0.111 ± 0.01 to 0.083 ± 0.001 and 0.111 ± 0.01 to 0.040 ± 0.001 ml/kg at a dose of hesperetin 12.5, 25, 50 mg/kg respectively.
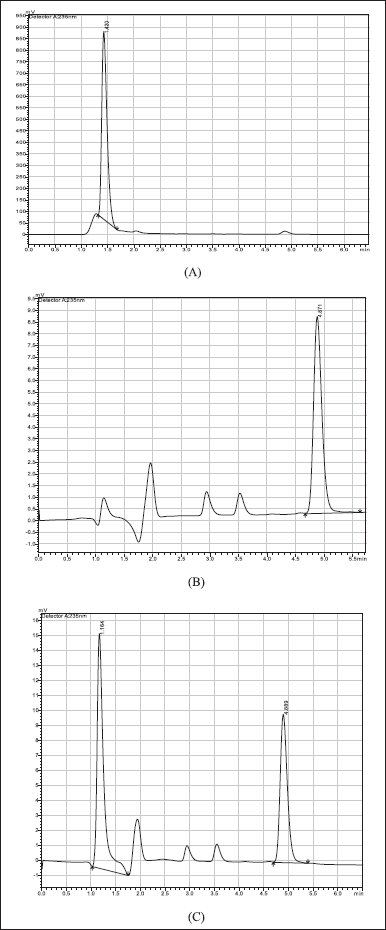 | Figure 1. Chromatograms: (A) Blank plasma; (B) Diltiazem hydrochloride (2 μg/ml); (C) Plasma + Diltiazem hydrochloride 2 μg/ml. [Click here to view] |
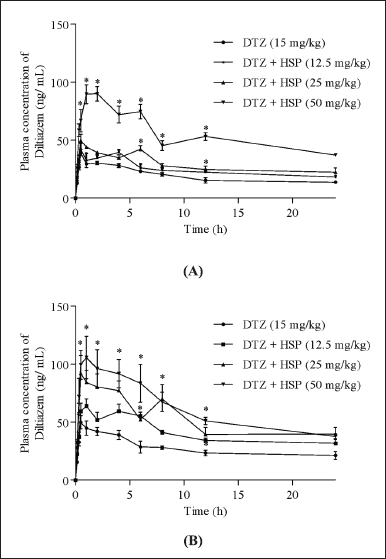 | Figure 2. After oral administration of diltiazem (15 mg/kg), plasma concentration-time curves in rats treated with or without hesperetin. (A) on the first day; (B) on the fifteenth day. HSP, Hesperetin; DTZ, Diltiazem. *p < 0.05 compared to diltiazem control group. [Click here to view] |
Effect of hesperetin on the PK of diltiazem in MDS
Diltiazem plasma concentrations versus time curves in MDS patients after an oral dose of Diltiazem alone and pretreatment with hesperetin 12.5, 25, and 50 mg/kg are also shown in Figure 2. Except for Tmax, all PK parameters were logarithmically converted and compared using one-way ANOVA and Dunnett’s multiple comparisons test. The mean plasma PK parameters are shown in Table 2. Hesperetin raised the Cmax, AUC0-24, AUC0-, t1/2, and MRT of Diltiazem and lowered the clearance and volume of distribution of Diltiazem in studies (p < 0.001). The Cmax of Diltiazem was increased from 49.822 ± 4.368 to 69.213 ± 5.477 and 49.822 ± 4.368 to 92.000 ± 7.254 and 49.822 ± 4.368 to 105.662 ± 9.571 ng/ml at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The AUC0-24 of Diltiazem was increased from 654.148 ± 45.265 to 987.381 ± 57.248 and 654.148 ± 45.265 to 1509.941 ± 85.479 and 654.148 ± 45.265 to 1736.974 ± 98.246 ng. hour/ml at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The AUC0-∞ of Diltiazem was increased from 1,965.421 ± 94.258 to 2,882.222 ± 158.695 and 1,965.421 ± 94.258 to 3,670.254 ± 241.587 and 1,965.421 ± 94.258 to 4,038.111 ± 271.328 ng. hour/ml at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The tmax does not change from 0.5 ± 0.100 hour. The t1/2 of Diltiazem was increased from 22.651 ± 5.374 to 23.591 ± 2.565 and 22.651 ± 5.374 to 30.083 ± 2.478 and 22.651 ± 5.374 to 27.715 ± 2.851 hour at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The MRT of Diltiazem was increased from 30.562 ± 2.555 to 30.311 ± 2.652 and 30.562 ± 2.555 to 44.191 ± 4.274 and 30.562 ± 2.555 to 41.202 ± 3.625 hour at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The clearance of diltiazem was decreased from 1.000 ± 0.1 to 1.000 ± 0.1 and 1.000 ± 0.1 to 0.800 ± 0.2 and 1.000 ± 0.1 to 0.800 ± 0.2 l/hour/kg at a dose of hesperetin 12.5, 25, 50 mg/kg respectively. The volume of distribution of diltiazem was decreased from 0.1001 ± 0.1 to 0.052 ± 0.005 and 0.1001 ± 0.1 to 0.038 ± 0.005 and 0.1001 ± 0.1 to 0.033 ± 0.005 ml/kg at a dose of hesperetin 12.5, 25, 50 mg/kg respectively.
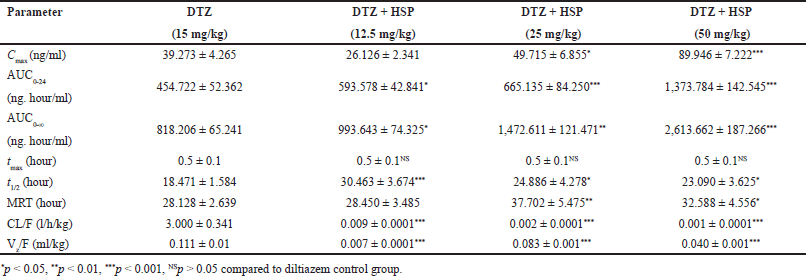 | Table 1. PK parameters of diltiazem on 1st day. [Click here to view] |
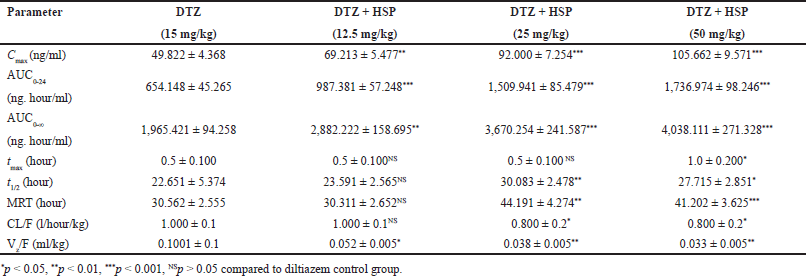 | Table 2. PK parameters of diltiazem on the 15th day. [Click here to view] |
Hesperetin’s effect on P-gp-mediated diltiazem transport
P-gp operates as a barrier in the gut, lowering net absorption of xenobiotics and medicines into the intraluminal space, which can have a substantial influence on P-gp substrate bioavailability and therapeutic uses. Diltiazem bowel absorption was assessed using everted gut sacs from the mucosal to the serosal compartments. Hesperetin increased diltiazem absorption in a concentration-dependent manner (Table 3). When administered alone, the transport of Diltiazem was found to be 11.026 ± 1.811 at a concentration of 50 µg/ml over a time interval of 60 minutes. The transport of Diltiazem increased from 11.026 ± 1.811 to 12.235 ± 1.854 and 11.026 ± 1.811 to 16.541 ± 2.203 and 11.026 ± 1.811 to 17.425 ± 2.820 when pretreated with hesperetin at concentrations of 25, 50, 100 μg/ml at a time interval of 60 minutes. To validate the role of P-gp in diltiazem transport, the studies were performed in the presence of 50 µg/ml ritonavir, a P-up blocker. When administered in the presence of ritonavir, the amount of Diltiazem was increased from 11.026 ± 1.811 to 18.362 ± 3.652 at a concentration of 50 μg/ml at a time interval of 60 minutes. The findings show that ritonavir increased absorption at the incubation time.
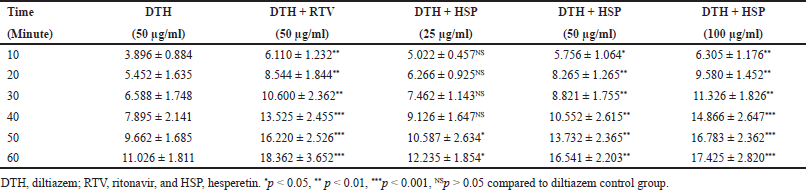 | Table 3. Transport of diltiazem (n = 3). [Click here to view] |
DISCUSSION
In the current investigation, Hesperetin dramatically changed the PK of Diltiazem in rats owing to an inhibition of CYP3A4 and P-gp. These results are consistent with previous study reports. Morin, a flavonoid, significantly increased diltiazem oral exposure. The Cmax and AUC have significantly increased from 173 ± 41.5 to 374 ± 55.2 ng/ml and 358 ± 56.9 to 642 ± 76.6 ng. hour/ml respectively at a dose of 7.5 mg/kg. the T1/2 of Diltiazem was decreased from 13 ± 2.9 to 11 ± 3.2 hour and clearance was decreased from 710 ± 93.4 to 393 ± 38.5 ml/minute. Kg at a dose of morin 7.5 mg/kg. The increased oral absorption of Diltiazem is due to the inhibition of the CYP3A4-mediated metabolism of Diltiazem (Choi et al., 2005a). The bioavailability of diltiazem increased considerably in rabbits pretreated with quercetin compared to the control, but not in rabbits co-administered with quercetin. The Cmax and AUC significantly increased from 94.2 ± 23.5 to 99.3 ± 24.8 ng/ml and 232 ± 58 to 287 ± 71 ng/ml hour respectively at a dose of 2 mg/kg. The t1/2 of the Diltiazem was increased from 11.3 ± 2.8 to 12.3 ± 2.9 hour at a dose of 2 mg/kg. The increased bioavailability of Diltiazem in rabbits treated with quercetin could be attributed to quercetin’s inhibition of the efflux pump P-gp and the first-pass metabolizing enzyme CYP 3A4 (Choi et al., 2005b).
The concomitant use of hesperetin significantly enhanced the oral exposure of Diltiazem in rats. The Cmax and AUC of Diltiazem were raised from 173 ± 41.5 to 375 ± 61.1 ng/ml and 358 ± 56.9 to 682 ± 54.8 ng/ml hour respectively at a dose of naringenin 15 mg/kg. There is no significant change in T1/2 and Tmax (Choi et al., 2005c). The concurrent use of fluvastatin significantly enhanced the oral exposure of Diltiazem in rats. At a dosage of fluvastatin 2 mg/kg, the Cmax and AUC of Diltiazem rose from 174 ± 35.8 to 310 ± 62.1 ng/ml and 363 ± 63.9 to 628 ± 130 ng. hour/l, correspondingly, when fluvastatin was used concurrently. Diltiazem bioavailability increased considerably in rabbits pretreated with quercetin compared to the control, but not in rabbits co-administered with quercetin. Fluvastatin may inhibit presystemic metabolism during intestinal absorption, according to these studies. According to Lee et al. (1991) Diltiazem extraction ratios in the small intestine and liver were about 85% and 63%, significantly, following oral therapy of rats, suggesting that Diltiazem is widely extracted in both the small intestine and the liver . In conclusion, simultaneous fluvastatin medication may contribute, at least in part, to the increased oral exposure to Diltiazem by reducing both intestinal and hepatic extraction (Choi et al., 2006; Lee et al., 1991).
Simvastatin enhanced Diltiazem’s oral absorption. At a dosage of 1 mg/kg, Diltiazem’s Cmax and AUC were raised from 182 ± 33 to 246 ± 44 ng/ml and 270 ± 51 to 392 ± 74 ng. hour/ml, accordingly. Tmax and t1/2 were similarly elevated, although the differences were not important. Increased absorption in the small intestine due to P-gp inhibition and reduced first-pass metabolism of Diltiazem due to CYP3A subfamily inhibition in the small intestine and/or liver, rather than renal elimination of Diltiazem by simvastatin, could explain the rise in Diltiazem oral bioavailability (Choi et al., 2011). The resveratrol had increased the bioavailability of Diltiazem. At a dosage of resveratrol 10 mg/kg, the Cmax and AUC of Diltiazem were considerably enhanced from 165 ± 37.8 to 259 ± 60.6 ng/ml and 342 ± 80.2 to 547 ± 131 ng/ml, correspondingly. Resveratrol did not alter Tmax. The resveratrol significantly increased the bioavailability of Diltiazem due to the inhibition of both the CYP3A4-mediated metabolism and the P-gp in the intestine and or liver (Hong et al., 2008).
The addition of lovastatin increased Diltiazem’s systemic bioavailability. The AUC0-∞ of Diltiazem was increased from 342 ± 69 to 508 ± 107 ng hour/ml at a 1 mg/kg dose of lovastatin. The Cmax of Diltiazem was increased from 165 ± 35 to 234 ± 53 ng/ml at a dosage of lovastatin 1 mg/kg. The Tmax of Diltiazem was decreased from 0.33 ± 0.13 to 0.29 ± 0.10 hour at a dosage of lovastatin 1 mg/kg. The volume of distribution of Diltiazem was reduced from 52.2 ± 14.9 to 42.4 ± 12.2 ml/kg at a dose of lovastatin 1 mg/kg. The clearance of Diltiazem was decreased from 45.2 ± 13.8 to 38.0 ± 9.9 ml/minute per kg at a dose of lovastatin 1 mg/kg. The increased bioavailability of Diltiazem in the presence of lovastatin may be due to lovastatin’s suppression of the P-gp mediated efflux pump in the bowel and/or suppression of CYP3A4-mediated metabolic in the gut and/or liver (Hong et al., 2011). Rasagiline’s concentration in the brain rose when it was coupled with hesperetin and significant naringenin. In the presence of hesperetin or naringenin, rasagiline transport from the mucosal to the serosal side did not change significantly ex vivo (rat-everted gut sacs used). Hesperetin and naringenin enhanced rasagiline systemic exposure via CYP1A2 inhibition but not P-gp suppression, according to our findings Ravindra et al., 2016). In vitro studies demonstrated that hesperetin enhanced felodipine intestinal absorption. Concurrent usage of hesperetin changed the PK of felodipine, resulting in increased systemic exposure (Sridhar et al., 2014).
CONCLUSION
Due to P-gp and CYP3A4 inhibition, hesperetin significantly increased the plasma concentration, AUC, t1/2, MRT, and greatly lowered the clearance, Vz/F, of diltiazem in rats. According to in vitro study results, diltiazem transport was significantly increased in the presence of hesperetin and ritonavir owing to P-gp and CYP3A4 suppression.
ACKNOWLEDGMENTS
We are very grateful to the management of SVKM’s NMIMS and Siddhartha College for necessary provided facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study was approved by the Institutional Animal Care. The approved protocol number was KVSRSCOPS/11-03-14-008.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Babu PR, Babu KN, Peter PL. Influence of quercetin on the pharmacokinetics of ranolazine in rats and in vitro models. Drug Dev Ind Pharm, 2013; 39:873–9.
Barthe L, Woodley J, Houin G. Gastrointestinal absorption of drugs: methods and studies. Fundam Clin Pharmacol, 1999; 13:154–68.
Buckley MMT, Grant SM, Goa KL, McTabish D, Sorkin EM. Diltiazem: a reappraisal of its pharmacological properties and therapeutic use. Drugs, 1990; 39:757–806.
Capraro J, Clemente A, Rubio LA, Magni C, Scarafoni A, Duranti M. Assessment of the lupin seed glucose-lowering protein intestinal absorption by using in vitro and ex vivo models. Food Chem, 2011; 125:1279–83.
Chaffman M, Brogden RN. Diltiazem: a review of its pharmacological properties and therapeutic efficacy. Drugs, 1985; 29:387–454.
Challa VR, Babu PR, Challa SR, Johnson B, Maheswari C. Pharmacokinetic interaction study between quercetin and valsartan in rats and in vitro models. Drug Dev Ind Pharm, 2013; 39:865–72.
Choi EJ, Ahn WS. Neuroprotective effects of chronic hesperetin administration in mice. Arch Pharm Res, 2008; 31:1457–62.
Choi DH, Choi JS, Li C, Choi JS. Effects of simvastatin on the pharmacokinetics of diltiazem and its main metabolite, desacetyldiltiazem, after oral and intravenous administration in rats: possible role of P-glycoprotein and CYP3A4 inhibition by simvastatin. Pharmacol Rep, 2011; 63:1574–82.
Choi JS, Han HK. Pharmacokinetic interaction between diltiazem and morin, a flavonoid, in rats. Pharmacol Res, 2005a; 52:386–91.
Choi JS, Han HK. Enhanced oral exposure of diltiazem by the concomitant use of naringin in rats. Int J Pharm, 2005b; 305:122–8.
Choi JS, Li X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int J Pharm, 2005c; 297:1–8.
Choi JS, Piao YJ, Han HK. Pharmacokinetic interaction between fluvastatin and diltiazem in rats. Biopharm Drug Dispos, 2006; 27:437–41.
Dixon RA, Steele CL. Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends Plant Sci, 1999; 4:394–400.
Homsy W, Caille G, du Souich P. The site of absorption in the small intestine determines diltiazem bioavailability in the rabbit. Pharm Res, 1995a; 12:1722–6.
Homsy W, Lefebvre M, Caille G, du Souich P. Metabolism of diltiazem in hepatic and extrahepatic tissues of rabbits: in vitro studies. Pharm Res, 1995b; 12:609–14.
Hong SP, Choi DH, Choi JS. Effects of resveratrol on the pharmacokinetics of diltiazem and its major metabolite, desacetyldiltiazem, in Rats. Cardiovasc Ther, 2008; 26:269–75.
Hong SP, Yang JS, Han JY, Ha SI, Chung JW, Koh YY, Chang KS, Choi DH. Effects of lovastatin on the pharmacokinetics of diltiazem and its main metabolite, desacetyldiltiazem, in rats: possible role of cytochrome P450 3A4 and P-glycoprotein inhibition by lovastatin. J Pharm Pharmacol, 2011; 63:129–35
Kallem RR, Ramesh M, Seshagirirao JVLN. Validated LC-ESI-MS/ MS method for simultaneous quantitation of felodipine and metoprolol in rat plasma: application to a pharmacokinetic study in rats. Biomed Chromatogr, 2013; 27:784–91.
Kharasch ED, Bedynek PS, Walker A. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. ritonavir effects on CYP3A and pglycoprotein activities. Clin Pharmacol Ther, 2008; 84:506–12.
Kolars JC, Schmiedlin RP, Dobbins WO, Schuetz J, Wrighton SA, Watkins PB. Heterogeneity of cytochrome P450IIIA expression in rat gut epithelia. Gastroenterology, 1992; 102:1186–98.
Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450 – mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT – 538) in human liver microsomes. J Pharmacol Exp Ther, 1996; 277:423–31.
Lee YH, Lee MH, Shim CK. Pharmacokinetics of diltiazem and deacetyldiltiazem in rats. Int J Pharm, 1991; 76:71–6.
Lefebvre M, Homsy W, Caille G, du Souich P. First-pass metabolism of diltiazem in anesthetized rabbits: role of extrahepatic organs. Pharm Res, 1996; 13:124–8.
Li F, Lu J, Ma X. CPY3A4-mediated lopinavir bioactivation and its inhibition by ritonavir. Drug Metab Dispos, 2012; 40:18–24.
Li K, Zhang X, Zhao F. HPLC determination of diltiazem in human plasma and its application to pharmacokinetics in humans. Biomed Chromatogr, 2003; 17(8):522–5.
Pichard LG, Gillet G, Fabre I, Dalet-Beluche I, Bonfils C, Thenot JP, Maurel P. Identification of the rabbit and human cytochromes P-450IIIA as the major enzymes involved in the Ndemethylation of diltiazem. Drug Metab Dispos, 1990; 18:711–9.
Pool PE. Diltiazem. In: Messerli FH (ed.). Cardiovascular drug therapy. 2nd edition, Saunders, Philadelphia, PA, pp 931–71, 1996.
Ravindra BP, Vemulapalli S, Mullapudi SS, Nuthakki S, Pendyala S, Kilaru N. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Dev Ind Pharm, 2016; 42(7):1110–7.
Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. P-glycoprotein- mediated transcellular transport of MDR-reversing agents. FEBS Lett, 1993; 324:99–102.
Sridhar V, Surya Sandeep M, Ravindra BP, Naveen BK. Evaluation of first-pass cytochrome P4503A (CYP3A) and P-glycoprotein activities using felodipine and hesperetin in combination in Wistar rats and everted rat gut sacs in vitro. Phytother Res, 2014; 28:699–705.
Surya SM, Sridhar V, Puneeth Y. Enhanced oral bioavailability of felodipine by naringenin in Wistar rats and inhibition of P-glycoprotein in everted rat gut sacs in vitro. Drug Dev Ind Pharm, 2014; 40:1371–7.
Wacher VJ, Salphati L, Benet LZ. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev, 2001; 46:89–102.
Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest, 1987; 80:1029–36.
Weir MR. Diltiazem: ten years of clinical experience in the treatment of hypertension. J Clin Pharmacol, 1995; 35:220–32.
Yeung PK, Feng JDZ, Buckley SJ. Pharmacokinetics and hypotensive effect of diltiazem in rabbits: comparison of diltiazem with its major metabolites. J Pharm Pharmacol, 1998; 50:1247–53.
Yusa K, Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res, 1989; 49:5002–6.