INTRODUCTION
Combretum hypopilinum Diels [Syn: Combretum collinum Fresen. subsp. hypopilinum (Diels) Okafa; Combretaceae], locally referred to as “Pupiong” (Moba) or “Aléb’lé” (Lamba), is a small to medium tree. The plant is mainly found in woodlands, savannahs, and on termite mounds. It grows in semi-arid to moderate rainfall conditions and is tolerant to drought and frost at maturity (Burkill, 1985). In African folk medicine, the plant is used for the treatment of diarrhea, dysentery, stomachaches, ascaridiasis, cough, bronchitis, tuberculosis, jaundice, snake bites, ulcers, leprosy, headache, and malaria (Adjanohoun et al., 1986; Fyhrquist et al., 2004). Crushed leaves are used in baths to alleviate fatigue and rheumatism (Eloff, 1999). Furthermore, a decoction of root bark is used for epilepsy (McGaw et al., 2001). In Togolese folk medicine, the root bark and leaves are used in the treatment of jaundice, headache, inflammation, and malaria (Adjanohoun et al., 1986). Based on ethnobotanical report in the Savanes region of Togo, where C. hypopilinum is widely used for epileptic crisis, skin diseases, toothache, headache, diarrhea, dysentery, and wound healing and as anti-ulcer, the plant is considered as a “magic tree” because of its numerous medicinal properties. Previous phytochemical studies led to the isolation of stilbenoids compounds including combretastatins A and B as well as numerous phenanthrenes in the aerial parts of C. hypopilinum (Rogers and Coombes, 1999). A number of amino acids including aspartic acid, glycine, glutamic acid, and alanine have been isolated from the gum exudates (Anderson et al., 1987). Pharmacological investigations reported in vitro antifungal activities of different leaf extracts and antimicrobial activities of extracts from root and stem bark of C. hypopilinum (Fyhrquis et al., 2004). Recently, the hepatoprotective activity of the plant has been reported (Idoh et al., 2018). Although the above-mentioned studies suggested pharmacological activities of C. hypopilinum, there is still no experimental data on how it could modulate immune response against lipopolysaccharide (LPS)-induced inflammation. Therefore, this study is designed to evaluate the anti-inflammatory properties of C. hypopilinum in RAW 264.7 murine cells.
MATERIALS AND METHODS
Chemicals and reagents
Dimethyl sulfoxide (DMSO), 3-(4, 5-dimethylthiazol-2-y1)-2, 5-diphenyltetrazolium bromide (MTT), LPS from Escherichia coli O55:B5, Radioimmunoprecipitation assay (RIPA) buffer, phosphatase inhibitors, protease inhibitors, and Tri Reagent were purchased from Sigma-Aldrich Co. (St. Louis, MO); tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1 β), interleukin-6 (IL-6), and interleukin-10 (IL-10) ELISA kits were purchased from R&D Systems, Inc. (Minneapolis, MN). Caspase-3, -8, and -9 were purchased from BioVision Inc. (California, CA). All other reagents were of analytical grade.
Plant material and extraction
Combretum hypopilinum was collected from Dapaong (Savanes region of Togo) in March 2014. Botanical authentication was confirmed at the Department of Botany by Professor Kokou Kouami, University of Lomé, where a voucher specimen of C. hypopilinum was deposited in the herbarium under reference no. TOGO15209.
The dried root bark was coarsely reduced into powder (300 g) and extracted successively with dichloromethane, ethyl acetate, and absolute ethanol for 72 hours under intermittent stirring at room temperature. Extracts were filtered and respective solvents were evaporated with a rotary vacuum evaporator (Buchi, Switzerland) at 40°C. Dichloromethane extract (DmE), ethyl acetate extract (EaE) and ethanol extract (EtE) of C. hypopilinum which were represented yielding 0.56%, 1.17%, and 11.24% (w/w) were stored at 4°C for further utilization in experiments.
Cell culture
The RAW 264.7 murine macrophages used in this study are macrophage-like cells derived from BALB/c mice. Cells were purchased from the National Centre for Cell Science, Pune, India. RAW 264.7 cells were grown in Dulbecco’s Modified Eagle Medium supplemented with 10% of Fetal bovine serum (FBS), 100 units/ml of penicillin, and 100 μg/ml of streptomycin in a humidified atmosphere with 5% of CO2 and 95% of air, in a CO2 incubator at 37°C.
Cytotoxicity assay
Cytotoxicity effects of different extracts of C. hypopilinum on RAW 264.7 were determined using the reduction of MTT to purple formazan assay. Cells were plated in 96-well cell culture plates at a cell density of 2 × 104 cells/well. After 24 hours of incubation, the spent medium was replaced with fresh medium containing increasing concentrations of the DmE, EaE, and EtE of C. hypopilinum (10, 20, 40, and 80 µg/ml) in DMSO in the presence or absence of LPS. The final concentration of DMSO in each treatment did not exceed 0.01% in each well. Control cells were incubated with fresh medium containing DMSO. Both control and treated cells were incubated for a further 24 hours at 37°C; afterward 10 μl of MTT solution [5 mg/ml in phosphate-buffered saline (PBS)] was added and cells were incubated for an additional 4 hours. The supernatant was removed and 100 μl of DMSO was added to dissolve the formazan crystals. The absorbance (OD) was read at 570 nm and cell viability was calculated. The inhibition percentage in the presence or absence of LPS was calculated using the following formula:
% Inhibition = (OD of control − OD of sample/OD of control) × 100.
Results obtained from the cytotoxicity assay allowed choosing EaE for pharmacological investigations.
Measurement of nitric oxide (NO) production
RAW 264.7 macrophages were plated in a 12-well plate at 2 × 105 cells/well. After 24 hours of incubation, cells were treated with EaE at the concentrations 10, 20, 40, and 80 µg/ml for 1 hour and then activated with LPS (1 µg/ml) for an additional 24 hours (Xiong et al., 2014). Cell culture media supernatants were collected and frozen at −80°C for NO determination. The nitrite accumulated in supernatant was measured according to Griess reaction. In brief, 100 μl of supernatants were mixed with 100 μl of Griess reagent (1% sulfanilamide in 5% phosphoric acid, 1% α-naphthylamide in H2O) in a 96-well plate, incubated at room temperature for 10 minutes, and then read at 540 nm.
Determination of inflammatory cytokines and caspases-3, -8, and -9 estimation
RAW 264.7 macrophages were plated in a 6-well plate at 4 × 105 cells/well. After being incubated for 24 hours, cells were treated with EaE (10, 20, 40, and 80 µg/ml) for 1 hour before activation with LPS (1 µg/ml) for additional 24 hours (Xiong et al., 2014). Then each well was washed with ice-cold PBS twice followed by lysis with RIPA buffer containing phosphatase and protease inhibitor cocktail (Jaggi and Singh, 2010). The lysates were collected and centrifuged at 13,000 rpm for 15 minutes at 4°C. The concentrations of the cytokines such as TNF-α, IL-1β, IL-6, and IL-10 were measured using R&D Systems ELISA kit according to the manufacturer’s instructions (R&D Systems, Inc., Minneapolis, MN). The estimation of caspase-3, -8, and -9 levels was measured using the corresponding caspase kits, respectively, according to the manufacturer’s instructions (BioVision Inc. California, CA).
Statistical analysis
Results were presented as mean ± SEM. Data were collected and analyzed with ANOVA one-way statistical test followed by Dunnett’s multiple comparison testing. Results were considered to be statistically significant if p < 0.05. Statistical analyses were performed using GraphPad Prism version 5 (California, USA).
RESULTS AND DISCUSSION
Results
Effects of different extracts of C. hypopilinum on RAW 264.7 cells viability
Effects of C. hypopilinum on RAW 264.7 macrophage viability were assessed using MTT assay. As shown in Figure 1A, DmE significantly inhibited RAW 264.7 cells growth at 80 µg/ml (p < 0.05). The same extract did not show any toxicity at 10, 20, and 40 µg/ml. Results showed that the most toxic extract is EtE which showed cytotoxic effects at 20, 40, and 80 µg/ml (Fig. 1C). The less toxic extract is EaE which did not induce any significant toxicity at any concentration after treatment for 24 hours (Fig. 1B). Results from cell exposure to LPS showed that the most effective extract being able to inhibit LPS-induced inflammation is EaE (Fig. 2A). DmE and EtE showed some anti-inflammatory activity; however, their activity is less than that of EaE (Fig. 2A and C). Therefore, EaE has been chosen for further pharmacological investigation.
Effects of EaE of C. hypopilinum on inflammatory cytokines
Effects of C. hypopilinum EaE on pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 and anti-inflammatory cytokine IL-10 contents were estimated after treatment and LPS activation. Results presented in Table 1 show that LPS activation significantly elevated the cell content of TNF-α, IL-1β, and IL-6 (p < 0.05 or p < 0.01) in LPS-activated cells without treatment with the extract as compared to control cells. The cell content of IL-10 decreased significantly in LPS-stimulated RAW 264.7 cells as compared to control cells (p < 0.05). However, pretreatment of RAW 264.7 cells with EaE prior to exposure activation with LPS significantly decreased the production of TNF-α at 20, 40, and 80 µg/ml (p < 0.001) as compared to the production of TNF-α in LPS-activated cells without any treatment. Table 1 also shows that EaE significantly decreased the cell contents of IL-1β and IL-6 as compared to untreated cells. Hence, IL-1β level significantly decreased at 20–80 µg/ml (p < 0.01). When compared to untreated cells, EaE treatment significantly decreased the induction effect of LPS on production of IL-6 (p < 0.001 at 20 µg/ml and p < 0.01 at 40 and 80 µg/ml) but significantly increased LPS-induced production of IL-10 in RAW 264.7 cells (p < 0.05 at 10 µg/ml and p < 0.001 at 20, 40, and 80 µg/ml) as shown in Table 1.
EaE of C. hypopilinum inhibited the production of NO in LPS-stimulated RAW 264.7 cells
As shown in Figure 3, NO production significantly increased in cells following LPS activation without pre-treatment with the extract (p < 0.01). However, pre-treatment with various concentrations of EaE (20, 40, and 80 µg/ml) resulted in significant (p < 0.01) inhibition of the production of NO in LPS-stimulated macrophages.
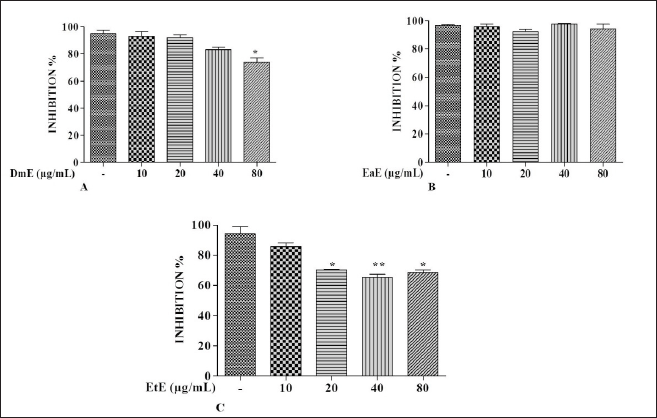 | Figure 1. Effects of C. hypopilinum’s different extracts on cell viability in the absence of LPS. RAW 264.7 cells were treated with DmE, EaE, and EtE extracts (10, 20, 40, and 80 μg/ml) and incubated for 24 hours. Values are expressed as mean ± SEM. *p < 0.05 and **p < 0.01 as compared to control cells. [Click here to view] |
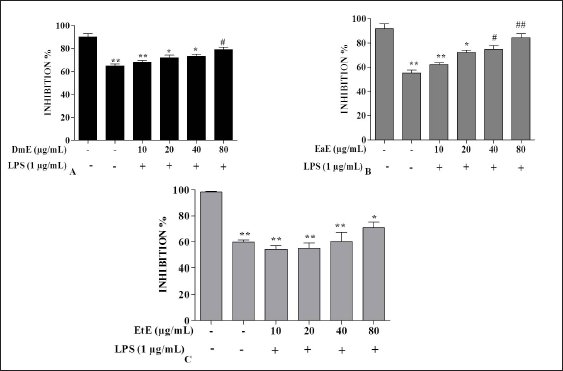 | Figure 2. Effects of C. hypopilinum’s different extracts on cell viability in the presence of LPS. RAW 264.7 cells were treated with DmE, EaE, and EtE extracts (10, 20, 40, and 80 μg/ml) and exposed to 1 μg/ml of LPS for 24 hours. Values are expressed as mean ± SEM. *p < 0.05, **p < 0.01 as compared to control cells; #p < 0.05 and ##p < 0.01 as compared to LPS-induced cells without treatment. [Click here to view] |
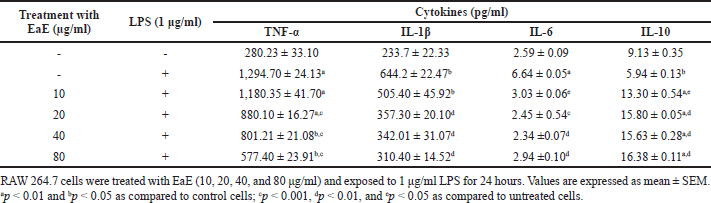 | Table 1. Effects of EaE of C. hypopilinum’s different fractions on inflammatory cytokines. [Click here to view] |
Effects of EaE of C. hypopilinum on caspases-3, -8, and -9 activities
To assess direct effect of C. hypopilinum EaE on LPS-induced apoptosis, caspase-3, -8, and -9 activities were determined in RAW 264.7 stimulated macrophages. Our results showed that LPS-induced inflammation markedly increased caspase-3,-8,an -9 activities in LPS-activated RAW 264.7 cells without any treatment with EaE (Fig. 4). However, treatment with EaE at 20,40, and 80 µg/ml (p < 0.05) and treatment with EaE at 80 µg/ml significantly (p < 0.05) decreased the activities of caspase-3 and -8, respectively, in treated cells as compared to untreated cells. The extract did not induce any significant change in the activity of caspase-9 (Fig. 4).
DISCUSSION
This study was designed to evaluate the potential anti-inflammatory activity of C. hypopilinum using LPS-activated RAW 264.7 macrophages’ in vitro model. The main results showed that EaE of C. hypopilinum showed anti-inflammatory properties through significant inhibition of the production of inflammatory cytokines such as TNF-α, IL-1β, and IL-6. EaE inhibited the release of NO and the activities of apoptotic caspase-3 and -8. Moreover, our results showed that EaE of C. hypopilinum increased the production of the anti-inflammatory IL-10. According to the authors, this study is the first to address the anti-inflammatory activity of C. hypopilinum through modulation of the immune system in RAW 264.7 murine macrophages.
In LPS-induced inflammation model, activated macrophages are capable of producing various inflammatory cytokines and mediators which play important role in the initiation and the maintenance of inflammatory responses. Hence, LPS activates RAW 264.7 macrophages and induces overproduction of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β (Fan et al., 2013). It has been reported that elevated level of TNF-α can induce the expression of inflammation mediators, such as cyclooxygenase and inducible NO synthase (iNOS) via activation of nuclear factor-kappa beta (Domitrovic et al., 2012). Moreover, NO is a highly reactive oxidant and inflammatory mediator which has various functions in inflammatory reaction. In normal physiological conditions, NO, synthesized by NO synthase, is an important mediator involved in the regulation of cellular activities. However, activation of inflammatory cells induces the overproduction of NO through activation of iNOS (Moncada et al., 1991). Therefore, inhibition of the production of pro-inflammatory cytokines and mediators such as TNF-α, IL-6, IL-1β, and NO should be very useful in the treatment of inflammatory conditions. In this study, exposure to LPS caused overproduction of both pro-inflammatory cytokines and NO reflecting the magnitude of the inflammatory reaction initiated by LPS in RAW 264.7 cells. However, ethyl acetate fraction of C. hypopilinum (EaE) significantly inhibited the production of TNF-α, IL-6, IL-1β, and NO. These results showed that C. hypopilinum can be used to alleviate pathological conditions including liver diseases and ulcer. Our results also showed that treatment with EaE significantly increased the production of the anti-inflammatory IL-10. Hence, inhibition of the production of pro-inflammation cytokines and the enhanced production of IL-10 suggest that C. hypopilinum exhibits anti-inflammatory activity against the LPS-induced inflammation. C. hypopilinum might modulate the immune response in RAW 264.7 cells.
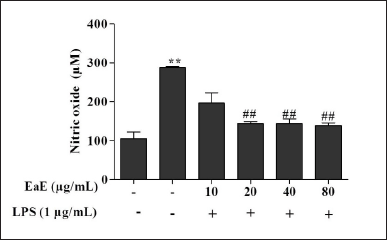 | Figure 3. Effects of EaE of C. hypopilinum on the production of NO in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with EaE (10, 20, 40, and 80 μg/ml) and exposed to 1 μg/ml of LPS for 24 hours. Values are expressed as mean ± SEM. **p < 0.01 as compared to control cells; ##p < 0.01 as compared to untreated cells. [Click here to view] |
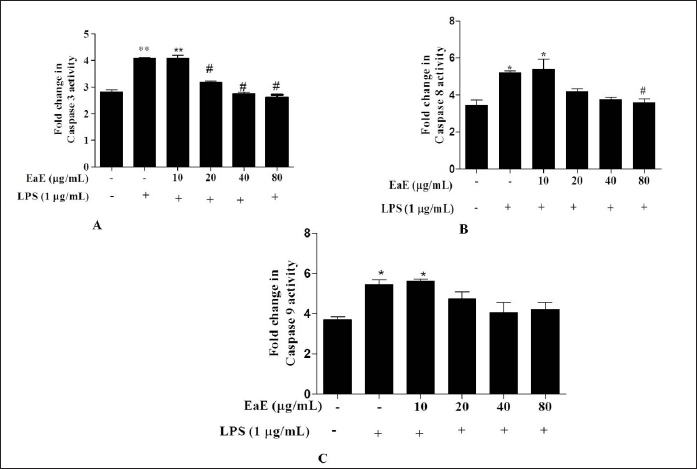 | Figure 4. Effects of EaE of C. hypopilinum on activities of caspases in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with EaE (10, 20, 40, and 80 μg/ml) and exposed to 1 μg/ml of LPS for 24 hours. Values are expressed as mean ± SEM. *p < 0.05 and **p < 0.01 as compared to control cells; #p < 0.05 as compared to untreated cells. [Click here to view] |
Several studies showed that LPS-activated cells undergo apoptosis through activation of caspase-8 and caspase-9 which are considered as apoptosis initiator caspases and caspase-3 which is considered as apoptosis process executioner (Green et al., 2009; Liu et al., 2009; Taylor et al., 2008; Tien et al., 2010). Therefore, the effects of EaE on the activities of caspase-3, caspase-8, and caspase-9 on LPS-activated RAW 264.7 macrophages were examined. Results suggested that EaE modulated the activities of capsapse-3 and caspase-8 by decreasing their activities in LPS-activated RAW 264.7 macrophages. However, the extract showed insignificant effect on the activity of caspase-9. These results suggest that EaE exhibited anti-apoptotic effect by inhibiting apoptotic cell death triggered by LPS. Caspases’ function is to cleave proteins and then to induce cell death. Hence, activated caspase-8 and caspase-9 may cleave numerous targets, including caspase-3 leading to rapid cell death by apoptosis process (Green et al., 2009). Our results suggest that EaE might inactivate caspase-8 and then the subsequent inhibition of executioner caspase-3 occurs.
CONCLUSION
Taken together, this investigation has shown that EaE of C. hypopilinum exerted anti-inflammatory activity on LPS-activated RAW 264.7 macrophages by inhibiting the production of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and the production of NO. The extract also inhibited LPS-induced apoptosis in RAW 264.7 cells by inhibiting the activation of caspase-3 and caspas-8. Therefore, C. hypopilinum might be considered as a potential source of anti-inflammatory molecules.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Science & Technology (DST) of India for the award of the prestigious CV Raman International Fellowship for African Researchers, program 2016, to Kokou Idoh. The authors are thankful to PSG & Sons’ Charities of India and Dr. Muthiah Ramanathan, principal of the PSG College of Pharmacy, for their support and help in carrying out this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adjanohoun EJ, Ahyi AMR, Ake AL, Akpagana K, Chibon P, El-Hadji A, Eyme J, Garba M, Gassita JN, Gbeassor M, Goudote E, Guinko S. Contribution aux études ethnobotaniques et floristiques au Togo. In Médecine traditionnelle et pharmacopée. Agence de Coopération Culturelle et Technique, Paris, France, pp 526–31, 1986.
Anderson D, Howlett J, McNab C. Amino acid composition of gum exudates from some African Combretum, Terminalia and Anogeissus species. Phytochemistry, 1987; 26(3):837–9.
Burkill H. The useful plants of West Tropical Africa. Royal Botanic Gardens, Kew, Richmond, UK, p 960, 1985.
Domitrovic R, Jakovac H, Blagojevic G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-alpha, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology, 2012; 280:33–43.
Eloff J. The antibacterial activity of 27 southern African members of the Combretaceae. South Afr J Sci, 2012; 95:148–52.
Fan G, Zhang Y, Jiang X, Zhu Y, Wang B, Su L, Cao W, Zhang H, Gao X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation, 2013; 36(6):1584–91.
Fyhrquist P, Mwasumbi L, Haeggstrom C, Vuorela H, Hitunen R, Vuorela P. Antifungal activity of selected species of Terminalia, Pteleopsis and Combretum (Combretaceae) collected in Tanzania. Pharm Biol, 2004; 42(4/5):308–17.
Green D, Ferguson T, Zitvogel L, Kroemer G. Immunogenic and tolerogenic cell death. Nat Rev Immunol, 2009; 9(5):353–63.
Idoh K, Dosseh K, Kpatcha T, Agbonon A, Gbeassor M. Protective effect of Combretum hypopilinum diels: root bark extract against CCl4-induced hepatotoxicity in wistar rats. Pharmacogn Res, 2018; 10(3):325–31.
Jaggi AS, Singh N. Differential effect of spironolactone in chronic constriction injury and vincristine-induced neuropathic pain in rats. Eur J Pharmacol, 2010; 648(1–3):102–9.
Liu C, Lo J, Kuo C, Chu C, Chen L, Tsai F, Tsai C, Tzang B, Kuo W, Huang C. Akt mediates 17 beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2 NFkappaB pathway. J Cell Mol Med, 2009; 13(9B):3655–67.
McGaw L, Rabe T, Sparg S, Jäger, A, Eloff J, van Staden J. An investigation on the biological activity of Combretum species. J Ethnopharmacol, 2001; 75:45–50.
Moncada S, Palmer RMJ, Higgs. Nitric oxide physiology, pathophysiology and pharmacology. Pharmacol Rev, 1991; 43:109–42.
Rogers C, Coombes P. Acidic triterpene glycosides in trichome secretions differentiate subspecies of Combretum collinum in South Africa. Biochem Syst Ecol, 1999; 27:321–3.
Taylor R, Cullen S, Martin S. Apoptosis: controlled demolition at the cellular level. Nat Rev, 2008; 9(3):231–41.
Tien Y, Lin J, Lai C, Kuo C, Lin W, Tsai C, Tsai F, Cheng Y, Peng W, Huang C. Carthamus tinctorius L. prevents LPS-induced TNF alpha signaling activation and cell apoptosis through JNK1/2-NFkappaB pathway inhibition in H9c2 cardiomyoblast cells. J Ethnopharmacol, 2010; 130(3):505–13.
Xiong H, Cheng Y, Zhang X, Zhang X. Effects of taraxasterol on iNOS and COX-2 expression in LPS-induced RAW 264.7 macrophages. J Ethnopharmacol, 2014; 155:753–7.