INTRODUCTION
Neurotrauma is one of the major causes of morbidity and mortality globally, more so in developing countries [1]. Taylor et al. [2] found that roughly 2%–3% of all patients admitted to a Level I trauma center have peripheral nerve injury (PNI). Recently, the PNI rate increased by over 15%, and less than 50% of such individuals undergo nerve repair surgery. Of those who do, only 40%–50% recover normal function [3,4]. PNIs usually occur in the young and entail significant socioeconomic hardships [5]. Despite clear progress in the understanding of the healing process of injured nervous tissue and the recent advancements in surgical nerve repair techniques, complete success still seems far off [6,7]. Recently, growth factors have come into vogue in the healing of nervous tissue, and the other recently identified members of the neurotrophin family have been used to heal the peripheral nerves [8–13]. The new neuronal growth (NNGF), a placenta-derived polypeptide now sequenced and laboratory synthesized, could heal iatrogenic divided sciatic nerves in small and large experimental animals [14,15]. Therefore, we conducted this study to examine pharmacokinetics and toxicology in rats and sheep to gather experimental evidence for the clinical application of an investigational new drug.
MATERIALS AND METHODS
The study was conducted in accordance with the basic & clinical pharmacology & toxicology policy for experimental and clinical studies [16] and as per the ARRIVE guidelines. The study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, after the approval of the Animal Welfare and Ethics Committee vide #2021-01-465 dated 12/12/2021. We acquired 90 Sprague Dawley adult male rats and 20 adult male sheep and acclimated them to the study environment for 15 days before dose administration. We housed the animals in individually ventilated cages and kept them on a 12/12 hours light/dark cycle except when interrupting it for study procedures. We maintained the average room temperature at 25°C, average relative humidity at 30%–70% and an average daily airflow >10 fresh air changes/hour. We fed the rats Lab Diet Certified Rodent Diet 5002 Meal ad libitum and gave them access to water ad libitum. As a standard practice, we kept the sheep separated in groups, not mixing them with other breeds to avoid stress for individual animals, which could have altered their physiology and stereotypic behaviors. We provided well-designed feed troughs with sufficient space for all animals to feed simultaneously with plenty of water.
The formula of NNGF is C47H77N11O11 (H-Pro-Lys-Val-Phe-Leu-Gln-Gln-Leu-OHis-OH) with a molecular weight of 1,223.49 g/mol. For pharmacokinetics, we used 70 skeletally mature Sprague Dawley rats and injected 5 mg/kg of body weight of NNGF intramuscularly [17] and we collected blood samples at 0, 30, 60, 120, 240, 360, and 480 minutes. We quantified the concentration of peptide present in the rat plasma samples using liquid chromatography and the standard curve method.
Pharmacokinetic studies
Chromatographic equipment and conditions
We used an high-performance liquid chromatography (HPLC) system from waters to quantify the NNGF (Waters®, USA). The instrument comprised an e2695 pump, an autosampler, and a UV/visible detector 2,489. We set the detector at a wavelength of 215 nm. The mobile phase (consisting of acetonitrile and water at a ratio of 76:24) was circulated through a waters symmetry RP-C18 analytical column (WAT045905) with dimensions of 5 μm, 4.6 × 150 mm, maintained at an ambient temperature, with a flow rate of 1 ml/minute. We used the isocratic mobile phase because selectivity does not change according to the column dimensions; it maintains a constant concentration in the mobile phase throughout the process. After each run, we rinsed the column for 15 minutes with water and methanol (90:10) to remove debris. The total run time was 4 minutes, and the retention time was 1.7 minutes. We controlled the HPLC system using Empower software.
Preparation of standard and samples
We prepared the master stock solution of 25 μM by dissolving 25 mg of lyophilized standard compound in 1 ml of deionized water and stored the stock at −20°C. We serially diluted the master stock to obtain the standard working stock solutions with concentrations of 12.5, 4.17, 1.39, 0.46, 0.15, 0.05, 0.017, and 0.00 μM.
We positioned a total of 12.5 μM of the standard samples at the beginning, followed by two levels of quality controls, 1.5 and 0.01 μM, and the rest of the standard samples throughout the analytical run. We based the optimization on the shorter retention time (1.7 minutes) and lower detection (STD 0.05 μM). We froze the collected blood samples at −20°C until analysis. We thawed the samples and deproteinized them using acetonitrile. We vortexed the sample vigorously for 30 seconds and then centrifuged it at 15,000 rpm for 15 minutes. After centrifugation, we injected 20 μl of supernatant directly into the HPLC system. We calculated the NNGF level and presented it as mean and standard deviation.
Specificity
Specificity is the ability of the analytical method to distinguish between the analyte(s) and the other components in the sample matrix. In the case of our HPLC method, it is assured by the complete separation of peak(s) of analyte(s) from other peaks originating from the sample matrix. Specificity is the ability to assess the exact component in a mixture, and retention time is an accurate way to specify components.
Recovery
The recovery is the ratio of the concentration of analyte found to that stated to be present. The acceptable range of recovery is between 70% and 120%.
Toxicology studies
For toxicology studies, we injected 100 mg/kg of body weight per day of NNGF intramuscularly for 7 consecutive days into 10 rats and 10 adult sheep. We used 10 rats and 10 sheep as control subjects. All animals underwent clinical observation daily and as necessary. After 2 weeks, we euthanized the animals and observed and weighed each animal’s organs, including the heart, kidney, liver, spleen, and brain. We stained the organs with hematoxylin and hematoxylin and then qualitatively analyzed them.
RESULTS
Weight and food residues in animals
We found no significant change in body weight in the control or study group or any change in the food residue.
Effects of NNGF injections
All animals withstood the study well, and no deaths occurred in any of the groups during the experiment. This result confirms that the animals could withstand the tests and adapted well to their environment.
Effects of NNGF injection on pharmacokinetics in rats
We detected NNGF in the plasma through liquid chromatography. Immediately after injection, the level was 0.108 ± 0.034 μM. It rose to 0.166 ± 0.024 μM at 30 minutes and peaked at 60 minutes at 0.362 ± 0.409 μM. At 120 minutes, the level started to decrease (0.202 ± 0.209 μM). After 240 minutes, it reached 0.086 ± 0.044 μM, and at 320 minutes, we detected no NNGF in the plasma (Fig. 1).
Effects of NNGF injection on the morphology of animal tissues
There was no difference between the weights of the animals and the organs tested (Table 1).
No abnormality was detected in the organs of all animals in comparison with the control group in sheep and rats (Figs. 2–6).
DISCUSSION
In this study on NNGF’s pharmacokinetics and toxicology, we used the standard dose of 5 mg/kg of body weight, which was used in two prior studies on rats and rabbits, and we found the drug efficacious. The regular clinical assessment and results of animal weight and lack of deaths in the animals demonstrated that the NNGF was well tolerated. The NNGF, an 8-amino-acid-chain peptide, as expected, had a shorter half-life in widespread distribution and reached its peak level in 60 minutes. In the histological studies on both animals, the toxic doses did not cause any damage or adverse effects on the organs tested.
The literature contains sparse data on the biodistribution of externally derived and delivered nerve growth factor (NGF). The available data came from studies on rats and cynomolgus monkeys. The administration route was intravenous and subcutaneous in rats, with a single injection of 35 mg/kg, a single dose or a continuous infusion with an osmotic mini-pump at a rate of 50–450 mg/pump. In both studies, maximum plasma concentrations (Tmax) occurred in 2–3 hours [18,19]. In our study, a single intramuscular injection of 5 mg/kg of body weight Tmax peaked in 60 minutes.
The use of NGF has immense potential in clinical situations in PNIs and general metabolic diseases. Several studies have suggested that the use of NGFs has potential beneficial effects. Further animal studies have shown that exogenous administration of NGFs can promote peripheral nerve growth and re-establish the functional activity of peripheral nerve fibers and damaged neurons [20–23]. NNGF was studied earlier in the iatrogenic division of sciatic nerves in rats and rabbits and showed promising results [14,15]. Successful animal studies have made some human trials possible regarding using NGF in diabetic neuropathy, diabetic ulcers, and vascular ulcers secondary to rheumatoid arthritis [24–28]. But in most of the studies, only topical application was used, leading to a successful outcome. The clinical trials of rhNGF were discontinued due to the reported side effects in clinical trials after intramuscular, intravenous, and subcutaneous injections, even though they proved to ameliorate symptoms effectively [29]. Studies are ongoing to overcome and limit the side effects of injectable NGF to accepted levels [30,31].
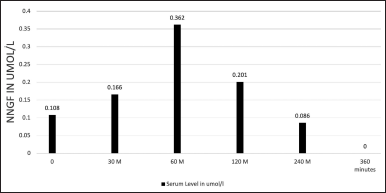 | Figure 1. Serum level of NNGF in Umol/l. [Click here to view] |
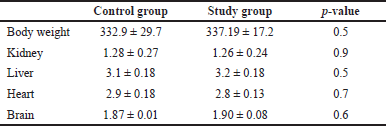 | Table 1. Body and organ weights (grams) of toxicology study in Sprague Dawley rats. [Click here to view] |
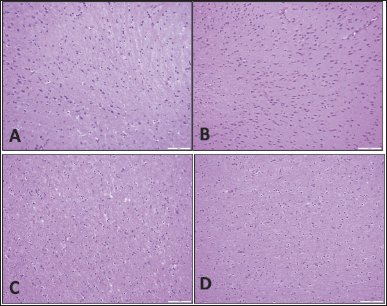 | Figure 2. Brain tissue. Specimens taken from the animals are comparable histologically to the controls; there is no gliosis, necrosis, inflammation, hemorrhage, or congestion. (A) Rat control, (B) rat study, (C) sheep control, and (D) sheep study (H&E; 20×). [Click here to view |
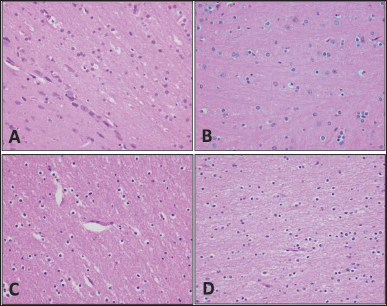 | Figure 3. Brain tissue. Specimens taken from the animals are comparable histologically to the controls; there is no gliosis, necrosis, inflammation, hemorrhage, or congestion. (A) Rat control, (B) rat subject, (C) sheep control, and (D) sheep subject (H&E; 40×). [Click here to view] |
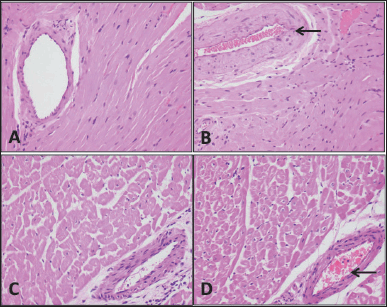 | Figure 4. Heart tissue. Specimens taken from the animals are comparable histologically to the controls; there is no fatty infiltration, necrosis, inflammation, hemorrhage, or congestion. The blood vessels (arrows) also appear normal without calcification in the wall or any element of vasculitis. (A) Rat control, (B) rat study, (C) sheep control, and (D) sheep study (H&E; 40×). [Click here to view] |
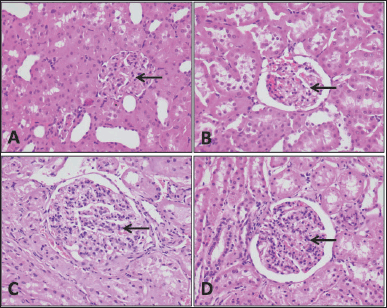 | Figure 5. Kidney tissue. Specimens taken from the animals are comparable histologically to the controls; there is no necrosis, inflammation, hemorrhage, or congestion. The glomeruli (arrows) also appear normal and the surrounding tubules do not show an element of acute tubular injury. (A) Rat control, (B) rat study, (C) sheep control, and (D) sheep control (H&E; 40×). [Click here to view] |
Our study has some limitations. First, we used small animals to assess serum levels after injecting a single dose, and we completed only one aspect of the pharmacokinetics study.
Second, we observed rats and sheep for 15 days after the toxic dose. A longer period of NNGF has to be given to check the toxic effect if any comes out. We need to study pharmacokinetics in large animals like sheep and look for chronic toxicity. Toxicity studies are also needed in different animal species and to monitor physiological effects and assess histological abnormalities. This study’s strength was that it was a single-dose study, which is generally more sensitive than multiple-dose studies in determining the release of a drug into the systemic circulation. Second, we examined toxicology in two animals. In conclusion, our pharmacokinetics and toxicology study of NNGF has shown that 5 mg/kg of body weight of intramuscular injection of NNGF and there are no acute toxic effects and the peptide is cleared out of the plasma after a peak of 60 minutes, which was expected. The results further give us credence for future research in human trials to confirm the efficacy and suitability to use in PNIs.
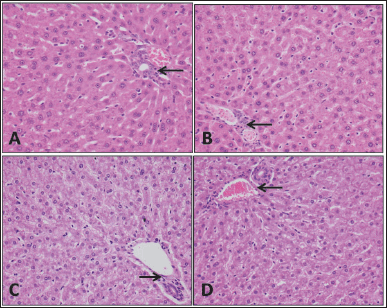 | Figure 6. Liver tissue. Specimens taken from the animals are comparable histologically to the controls; there is no apoptosis, necrosis, hemorrhage, or congestion. The portal tracts (arrows) also appear normal without inflammation. The hepatocytes do not show fatty change or ballooning degeneration. (A) Rat control, (B) rat study, (C) sheep control, and (D) sheep study (H&E; 40×). [Click here to view] |
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The study was supported by a special grant from the Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University, Dammam.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study was approved by the Institutional Review Board of Imam Abdulrahman Bin Faisal University, Dammam, after the approval of the Animal Welfare and Ethics Committee vide #2021-01-465 dated 12/12/2021.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Razmkon A. Priorities and concerns for research on neurotrauma in the developing world. Bull Emerg Trauma. 2013 Jan;1(1):5–6.
2. Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008 May;87(5):381–5.
3. Bozkurt A, Lassner F, O’Dey D, Deumens R, Böcker A, Schwendt T, et al. The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials. 2012 Feb;33(5):1363–75.
4. Visser PA, Hermreck AS, Pierce GE, Thomas JH, Hardin CA. Prognosis of nerve injuries incurred during acute trauma to peripheral arteries. Am J Surg. 1980 Nov;140(5):596–9.
5. Wojtkiewicz DM, Saunders J, Domeshek L, Novak CB, Kaskutas V, Mackinnon SE. Social impact of peripheral nerve injuries. Hand (N Y). 2015 Jun;10(2):161–7.
6. Houschyar KS, Momeni A, Pyles MN, Cha JY, Maan ZN, Duscher D, et al. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:4175293.
7. Carvalho CR, Oliveira JM, Reis RL. Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Front Bioeng Biotechnol. 2019 Nov 22;7:337.
8. Johnson EO, Charchanti A, Soucacos PN. Nerve repair: experimental and clinical evaluation of neurotrophic factors in peripheral nerve regeneration. Injury. 2008 Sep;39 Suppl 3:S37–42.
9. Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998;18(7):397–405.
10. Shakhbazau A, Martinez JA, Xu QG, Kawasoe J, van Minnen J, Midha R. Evidence for a systemic regulation of neurotrophin synthesis in response to peripheral nerve injury. J Neurochem. 2012 Aug;122(3):501–11.
11. Lopes CDF, Gonçalves NP, Gomes CP, Saraiva MJ, Pêgo AP. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials. 2017 Mar;121:83–96.
12. Cheng S, Tereshchenko J, Zimmer V, Vachey G, Pythoud C, Rey M, et al. Therapeutic efficacy of regulable GDNF expression for Huntington’s and Parkinson’s disease by a high-induction, background-free “GeneSwitch” vector. Exp Neurol. 2018 Nov;309:79–90.
13. Zhang R, Zhang Y, Yi S. Identification of critical growth factors for peripheral nerve regeneration. RSC Adv. 2019 Apr 5;9(19):10760–5.
14. Al-Dakheel DA, Sadat-Ali M, Azam MQ, El-Shawarby M. Effect of new neuronal growth factor on healing of sciatic nerve in rats. Neuropeptides. 2015 Dec;54:55–8.
15. Sadat-Ali M, AlDakheel DA, AlAbdali MN, AlJaafari DT, AlSulaiman AA, AlOmran AS et al. The effect of new neuronal growth factor (NNGF) in the healing of sciatic nerves in rabbits. Ann Afr Med. 2022; 21(4):361–5.
16. Tveden-Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J. BCPT policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2021;128:4–8.
17. Al-Habdan I, Sadat-Ali M, Safar Alghamdy M, Randhawa A, Chathoth S. Assessment of pharmacokinetics and toxicology of Sadat-Habdan mesenchymal stimulating peptide (SHMSP) in rats and goats. Int J Biomed Sci. 2014 Sep;10(3):167–71.
18. Nguyen CB, Harris L, Szönyi E, Baughman SA, Hale VG, Dybdal NO, et al. Tissue disposition and pharmacokinetics of recombinant human nerve growth factor after acute and chronic subcutaneous administration in monkeys. Drug Metab Dispos. 2000 May;28(5):598–607.
19. Tria MA, Fusco M, Vantini G, Mariot R. Pharmacokinetics of nerve growth factor (NGF) following different routes of administration to adult rats. Exp Neurol. 1994 Jun;127(2):178–83.
20. Hellweg R, Raivich G, Hartung HD, Hock C, Kreutzberg GW. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol. 1994 Nov;130(1):24–30.
21. Apfel SC, Kessler JA. Neurotrophic factors in the therapy of peripheral neuropathy. Baillieres Clin Neurol. 1995 Nov;4(3):593–606.
22. Riaz SS, Tomlinson DR. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog Neurobiol. 1996 Jun;49(2):125–43.
23. McMahon SB, Priestley JV. Peripheral neuropathies and neurotrophic factors: animal models and clinical perspectives. Curr Opin Neurobiol. 1995 Oct;5(5):616–24.
24. Lambiase A, Aloe L, Centofanti M, Parisi V, Báo SN, Mantelli F, et al. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: implications for glaucoma. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13469–74.
25. Lambiase A, Coassin M, Tirassa P, Mantelli F, Aloe L. Nerve growth factor eye drops improve visual acuity and electrofunctional activity in age-related macular degeneration: a case report. Ann Ist Super Sanita. 2009;45(4):439–42.
26. Generini S, Tuveri MA, Matucci Cerinic M, Mastinu F, Manni L, Aloe L. Topical application of nerve growth factor in human diabetic foot ulcers. A study of three cases. Exp Clin Endocrinol Diabetes. 2004 Oct;112(9):542–4.
27. Landi F, Aloe L, Russo A, Cesari M, Onder G, Bonini S, et al. Topical treatment of pressure ulcers with nerve growth factor: a randomized clinical trial. Ann Intern Med. 2003 Oct 21;139(8):635–41.
28. Tuveri M, Generini S, Matucci-Cerinic M, Aloe L. NGF, a useful tool in the treatment of chronic vasculitic ulcers in rheumatoid arthritis. Lancet. 2000 Nov 18;356(9243):1739–40.
29. Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413.
30. Bagal SK, Omoto K, Blakemore DC, Bungay PJ, Bilsland JG, Clarke PJ, et al. Discovery of allosteric, potent, subtype selective, and peripherally restricted TrkA kinase inhibitors. J Med Chem. 2019 Jan 10;62(1):247–65.
31. Carleton LA, Chakravarthy R, van der Sloot AM, Mnich K, Serrano L, Samali A, et al. Generation of rationally-designed nerve growth factor (NGF) variants with receptor specificity. Biochem Biophys Res Commun. 2018 Jan 1;495(1):700–5.